Abstract
Tamarindus indica is one of the tropical medicinal plants that has been attributed curative potential of numerous diseases by many rural dwellers. This study was designed to evaluate the antioxidant, antibacterial activities and also to determine the various chemical constituents responsible for its pharmacological activities. The methanol extract of Tamarindus indica fruit pulp was analyzed by Gas Chromatography/Mass Spectrometer to determine the volatile compounds present. The antioxidant activities were performed using DPPH and FRAP method and the antibacterial activity was tested against some common pathogens by macro broth dilution method. The GCMS analysis shows the presence of 37 compounds, out of which 14 had their peak area percentages ≥ 1% and only two compounds had no reported pharmacological activities. Most of the bioactive compounds including 5-Hydroxymethylfurfural (31.06%)-3-O-Methyl-d-glucose (16.31%), 1,6-anhydro-β-D-Glucopyranose (9.95%), 5-methyl-Furancarboxaldehyde (3.2%), Triethylenediamine (1.17%), 1-(2-furanyl)-1-Propcanone (2.18%), Methyl 2-furoate (3.14%), Levoglucosenone (3.21%), methyl ester-Hepta-2,4-dienoic acid, (8.85%), 2,3-dihydro-3,5-dihydrox-4H-Pyran-4-one (3.4%), O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl-α-D-Glucopyranoside (2.18%), n-Hexadecanoic acid (1.38%), 2-Heptanol, acetate (1.29%), 5-[(5-methyl-2-fur-2-Furancarboxaldehyde (1.08%), 3-Methyl-2-furoic acid (1.05%) and cis-Vaccenic acid (2.85%)have been reported with different activities such as antibacterial, antifungal, antitubercular, anticancer, antioxidant and other prophylactic activities. The extract demonstrated inhibitory potential against all tested pathogen. However, Plesiomonas shigellosis ATCC 15903 and Bacillus pumillus ATCC 14884 are more sensitive with the MIC of 0.22 and 0.44 mg/ml respectively. The antioxidant activity was relatively low due to the low phenolic content of the extract. This shows that there is a strong correlation between antioxidant activities and phenolic content. GC–MS analysis revealed the presence of bioactive phytoconstituents with various biological activities and this justifies the rationale behind its usage as a curative therapy by many local dwellers.
Similar content being viewed by others
Introduction
Plants play imperative roles in human existence and they are the bedrock of traditional medicine1. Unlike the synthetic drugs used for the treatment of various infections, plants are effective, safe, affordable, and with fewer side effects2. A larger percentage of these plants are capable of producing numerous categories of secondary metabolites which are the major reason why they are effective for therapeutic purposes even since prehistorical days3. Many compounds isolated from these plants have been used as drugs either in their natural form or in semi-synthetic form4. While the secondary metabolites are structurally diverse chemical compounds effective against pathogens and environment constraints5, these bioactive compounds have been shown to contain great medicinal activities such as antibacterial, antioxidant, antifungal, anti-allergic, anti-inflammatory, antiparasitic, anticancer, and antihypertensive activities6,7. They have been utilized for the therapy of mild to chronic ailments such as inflammation, cancer, diabetes, and stomach ulcer8,9. Oladeji10 reported that 25% of synthetic drugs are produced from plants originally used by orthodox medicine while Welz et al.11 indicated that the usage of herbal drugs as complementary or alternate treatment is on the increase globally and many medicines are benefitting greatly from natural products12.
Tamarindus indica Linn., commonly called Tamarind, is a tropical leguminous evergreen tree, family Fabaceae, subfamily Caesalpiniaceae, found throughout Africa and Southern Asia. The plant is made up of about 30–50% pulp, 11–30% shell, and 25–40% seeds13. It is one of the plants highly utilized medicinally due to its healing potential in numerous pharmacopeias14. The British and American pharmacopeias indicated that the pulp has anti-pyretic, antiscorbutic, purgative, and relief properties for nausea and bile illness15. The leaves possess antihelmintic and vermifuge properties destroying intestinal parasites16 and are extensively used ethnobotanically in Africa, Asia, and Latin America as antimicrobial and antiseptics17,18. The seeds have been used as a therapy for diabetes, fevers, and gastrointestinal infections in traditional settings19. The pharmacological activities of the various parts of this plant have been associated with the presence of several phytochemicals such as flavonoids, saponin, alkaloids, tannins, polyphenols, and steroids20. While the therapeutic potential of the T. indica plant is attributed to the presence of bioactive phytoconstituent available in every part of this plant21, its several economic values and health benefits is highly commercialized throughout the world22. However, there is a scarcity of information on the bioactive compounds of its methanol extract and its antioxidant and antibacterial activities. Since more awareness has been drawn to the search of novel drugs originating from natural products through innovative technology such as high-throughput selection23, the present study investigated the bioactive phytoconstituents available in the methanolic extract of Tamarindus indica fruit through the use of GC–MS techniques and indicated the antibacterial and antioxidant activities of the extract.
Methods
Sample collection
Mature and dried Tamarind fruits were obtained from the plants growing in its natural habit in Yola, Adamawa state, North East, Nigeria (9.2035° N, 12.4954° E). The fruits were collected in accordance with relevant guidelines and minimum number of fruits required for the accomplishment of the study was collected after permission was taken from the indigenes in whose locality the plant was found. The pulp was removed from the seeds by scrapping with the hand. It was ethno-botanically authenticated by a taxonomist (Dr. Nodaz George) from the University of Lagos herbarium with voucher No. LUH: 8771 and was deposited at the herbarium. Before the analyses, all visible contaminants and infested pulp were removed to ensure healthy and qualitative dried Tamarind fruits.
Chemical reagents
Only analytical grade chemical reagents and solvents were used for this investigation. They were obtained from Germany, produced by Merck KGaA, with the product name Sigma-Aldrich.
Extraction of tamarind pulp
The extraction process was carried out by soaking 80 g of scrapped pulp in 640 ml of 70% methanol at room temperature for 72 h and agitated intermittently for proper digestion. Whatman No. 1 filter paper was later used to filter the mixture and the residue was discarded. The solvent was evaporated from the filtrate in a rotary vacuum evaporator (Laborota 4000-efficient, Heidolph city Germany) at 40 °C under pressure until a semisolid concentrate was obtained. The crude extract was allowed to cool down and air-dried at ambient temperature prior to storage in a refrigerator at 4 °C for further use.
GC–MS analysis
The bioactive phytoconstituents in the extract were analyzed with the aid of Gas Chromatography-Mass Spectrometry (GC–MS) equipment (QP 2010 Plus SHIMADZU)24. The GC–MS was equipped with a flame ionization detector. Instrument conditions: injector temperature − 250 °C, detector temperature − 250 °C, oven temperature − 60 °C (Isothermal), flow rate − 2.0 mL/min, split ratio − 10:1, injection volume − 0.5 µL and 24 min run time. The compounds eluted in the methanol extract of T. indica were identified by comparing the spectrum of unidentified compounds with those of identified compounds in the NIST MS 2.0 structural library to discover their nomenclatures, molecular weight, and structure25.
Test organisms
Bacteria isolates used in this study included Escherichia coli ATCC 8739, Klebsiella pneumoniae ATCC 10031, Pseudomonas aeruginosa ATCC 19582, Acinetobacter calcaoceuticus UP and Plesiomonas shigelloides ATCC 15903 for Gram-negative and Bacillus cereus ATCC 10702, Staphylococcus aureus ATCC 6558, Bacillus pumilus ATCC 14884, Staphylococcus aureus NCT 6571 and Staphylococcus aureus ATCC 6558 for Gram-positive. All bacteria used were collected from the Department of Microbiology, Babcock University, Ilisan Remo, Ogun State Nigeria. All isolates were aseptically introduced into a nutrient broth for resuscitation purposes and incubated at 37 °C for 24 h prior to the antibacterial activity test.
Preparation and standardization of inoculums
The bacteria isolates were subculture in a broth for a period of 24 h at optimal temperature (37 °C) and a suspension equivalent to a cell density of 1 × 108 CFU/ml was prepared according to McFarland standard for each isolate. Extra dilution was carried out until the cell density reduces to 1 × 106 CFU/ml which was confirmed with the aid of a UV visible spectrophotometer (Thermo electron corporation USA) at an absorbance of 625 nm. The standardization was sustained throughout the experimental period26.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) is a technique used to calculate the sample with the least concentration that could inhibit the growth of microorganisms. It was performed using the macro broth dilution method. Prior to the analysis, 1% dimethylsulfoxide (DMSO) was used to dissolve the extract and the concentration varies from 0.1 to 14.04 mg/ml was prepared in the extracting solvent. The reconstituted extract was assessed for sterility by dispensing 1 mL of the extract into 9 mL of sterile nutrient broth before incubating at 37 °C for 24 h. Briefly, the crude extract, the antibiotics (positive control), and saline water (negative control) were serially diluted each in twofold Mueller Hinton broth in the different test tubes to obtain different concentrations of the antibacterial agents. In addition, 100 µl of standardized overnight cultured organisms were inoculated into all the test tubes except the control. The test tubes were incubated for 24 h at 37 °C and observed for any visible growth or turbidity27.
Determination of antioxidant activity
Two different antioxidant assays which include DPPH and FRAP were performed to assess the antioxidant potential of the extracted fruit pulp.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method
Five different concentrations of the extract were thoroughly mixed with 0.2 mM of DPPH prepared with ethanol. The absorbance of each concentration was determined at 517 nm after an incubation period of 30 min in a dark room. The standard used was gallic acid while methanol serves as control28. The radical scavenging activity of the extract was calculated by applying the formulae below.
\({\text{DPPH scavenging activity }}\left( \% \right) \, = \, \left[ {\left( {{\text{Abs control }}{-}{\text{ Abs sample}}} \right)} \right]/\left( {\text{Abs control}} \right)] \times {1}00;\;\) where; Abs control is the absorbance of DPPH + methanol; Abs sample is the absorbance of DPPH radical + sample (sample or standard).
Ferric reducing antioxidant power (FRAP)
The FRAP assay focus on the ferric reducing ability of the extract. The reduction of ferric ion (Fe3+) to the ferrous ion (Fe2+) is visible by the formation of the blue complex (Fe2+/TPTZ). Briefly, in the preparation of the working reagent, 100 mL of acetate buffer at 30 mM with 10 mL of a 10 mM TPTZ [2,4,6-tripyridyl-s-triazine] in 40 mM HCL was added to 10 mL of FeCl3.6H2O at 20 mM. Afterward, 3 ml of the freshly prepared FRAP solution was vigorously mixed with 100 µl of the crude extract (100–500 mg/mL). This resulted in the formation of a blue color complex indicating ferric tripyridyl triazine (Fe3+ TPTZ) complex was turned to ferrous (Fe2+) ion after 30 min of incubation at 37 °C. The absorbance was then determined at 593 nm. Freshly prepared working solutions of FeSO4 were used for calibration29. All determinates were indicated as gallic acid equivalents (GAE) in mg per gram dry weight.
Determination of total phenolics
The total phenolic content (TPC) was evaluated using the Folin-Ciocalteu method. One milliliter of the methanol extract was dropped in a test tube containing 1 ml of Folin-Ciocalteu reagent, whose concentration has been initially reduced to 60% by the addition of water. The mixture was thoroughly mixed by vigorous shaking of the tubes. The introduction of 2 ml 20% (w/v) of sodium carbonate was next and the mixture was left in a dark place for 30 min and absorbance was determined by the use of a UV–Vis spectrophotometer (Jascov-530) at a wavelength of 765 nm. The results obtained were compared with the standardized gallic acid results. The assay results were expressed as milligrams of gallic acid/gram of the dried extract30.
Determination of the total flavonoid content
The total flavonoid content of the methanolic extract was quantified by the aluminum chloride colorimetric assay for quantitative determination. Briefly, 1 ml of the extract was added to 2.8 mL of distilled water and mixed with 0.1 mL of 1 mg/mL potassium acetate solution. Then, 0.1 mL of 10% aluminum chloride was added to this solution. After 30 min of incubation, the absorbance was monitored and measured at 415 nm by using a UV–visible spectrophotometer31. Total flavonoid content was expressed as gallic acid equivalents (GAE) in milligram/gram dry weight.
Statistical analysis
All the antioxidant analyses, total phenolic, and flavonoid content were performed in triplicate, and average values and their standard derivations of the results were presented. The relationship among DPPH, FRAP assay, TPC, and TFC was evaluated by the use of correlation.
Results
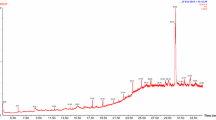
Gas chromatography-mass spectrometry (GC–MS) was used to analyze the methanol extract to identify the bioactive compounds present in the fraction. The GC–MS chromatogram of these bioactive compounds is presented in Fig. 1 and their peak area percent, chemical structure as well as biological activities are represented in Table 1. From the GC–MS analysis, 37 bioactive compounds were identified and expressed as percentages of the peak area relative to the total peak area. Bioactive compounds with greater than or equal to 1.0% peak areas were identified as the prominent bioactive compounds. The percentages of bioactive compounds > 1% are 5-Hydroxymethylfurfural (31.06%), -3-O-Methyl-d-glucose (16.31%), 1,6-anhydro-β-D-Glucopyranose (9.95%), 5-methyl-Furancarboxaldehyde (3.2%), Triethylenediamine (1.17%), 1-(2-furanyl)-1-Propcanone (2.18%), Methyl 2-furoate (3.14%), Levoglucosenone (3.21%), methyl ester-Hepta-2,4-dienoic acid, (8.85%), 2,3-dihydro-3,5-dihydroxy-4H-Pyran-4-one (3.4%), O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl-α-D-Glucopyranoside (2.18%), n-Hexadecanoic acid (1.38%), 2-Heptanol, acetate (1.29%), 5-[(5-methyl-2-fur-2-Furancarboxaldehyde (1.08%), 3-Methyl-2-furoic acid (1.05%) and cis-Vaccenic acid (2.85%). The pharmacological activities of Hydroxymethylfurfural with the highest peak area percentage and those of other bioactive compounds have been reported as being of therapeutic importance while those of 2-ethyl-2-Butenal (0.3%) and 3-(hydroxymethyl)-6-2-Cyclohexene-1-one (0.42%) with lower peak area percentages, having no reported pharmacological activities, were as shown in Table 1.
The methanol extract of T. indica Linn fruit pulp was evaluated for its antimicrobial potential against the test isolates using the macro tube dilution method. The ability of this extract to prevent the growth of the tested organisms was in Table 2. The result displayed the ability of the extract to suppress the growth of the bacterial isolates at varying concentrations. P. shigelloides ATCC 15903 was the most susceptible at MIC as low as 0.22 mg/mL while B. cereus ATCC 10702 and K. pneumoniae ATCC 10031 had the same MIC of 3.51 mg/mL. A. calcoaceuticus UP, S. aureus ATTC 6558, and S. aureus NCTC 6571 showed some level of resistance with MIC greater than 7.02 mg/mL though the minimum inhibition concentrations of the bacterial isolates varied from 0.0195 and 1.25 µg/ml for ciprofloxacin used as control.
The IC50 values of the extract for each antioxidant parameter assayed are 5.34, 18.67, and 7.34 for DPPH, FRAP, and Gallic acid, respectively. In the DPPH assay, the extract exhibited a concentration-dependent radical scavenging activity and this activity increases significantly with increase concentration. The results obtained from different concentrations are compared to the Gallic acid (ρ < 0.05) as shown in Fig. 2.
FRAP analysis was carried out to ascertain the antioxidant properties of the methanolic extract focusing on its potential to reduce ferric (III) to ferrous (II). The mean scavenging activities with different superscripts at the same concentration of the extract are notably different (ρ < 0.05) as shown in Fig. 3. The assessment of FRAP activity using gallic acid was remarkably higher than those of the extract from the T. indica fruit.
The gross phenolic and flavonoid content of the methanol extract was also assessed and compared with that of the standard (Gallic acid). The overall quantity of the flavonoid content of the fruit extract was higher than the phenolic content as shown in Fig. 4.
Discussion
The global increase in demand for plant-derived products for therapeutic and nutraceutical purposes have stimulated the quest to identify the chemical compounds present in each plant and their various pharmacological activities. In addition, the need for researchers to search for safer antioxidants from natural sources over synthetic ones such as BHT, BHA, propyl gallate, and tertbutyl-hydro quinine which are known to be carcinogenic has increased over the years as well67. Thus, the consumption of natural products such as fruits and vegetables showing strong antioxidant activities in preventing heart diseases and several cancerous ailments becomes necessary68. The GCMS analysis showed that the pulp has potential novel compounds that could be isolated for therapeutic purposes and irrespective of the percentages of the identified compounds, scientific reports showed that each of the compounds possessed significant therapeutic potentials.
Although the pharmacological activities of the major and minor bioactive compounds of plants are rarely reported and pharmacological activities of plants are mostly attributed to the flavonoids, alkaloids and phenolic compounds, Shapla et al.69 indicated that 5-Hydroxymethylfurfural (HMF) is an organic compound that possesses several beneficial potentials including antioxidant, anti-allergic, antiproliferative, anti-sickling, anti-hypoxic and anti-hyperuricemic impacts while Rajkumari et al.49 documented its antibiofilm activity. Its mechanism of antimicrobial action was related to growth or proliferation inhibition. Similarly, these inhibitory activities of 5-Hydroxymethylfurfural were reported by Palchykov et al.70 as the major compound of Punica granatum peel extract with deleterious effect on bacteria, protists, and nematodes. This is in agreement with an earlier study by Ahmed and Ayoub71. Their studies affirmed 5-Hydroxymethylfurfural as one of the main compounds in Tamarindus indica pulp extract with over 30% of the extract component. While 3-O-Methyl-d-glucose (16.31%), identified also as a component of the Tamarindus indica pulp extract, have been implicated in preservative activities as well as antitumor and anti-inflammatory potentials57,58, other bioactive components of the extract with their various pharmacological activities have been reported in many studies as bactericidal, fungicidal, nematocidal and antioxidant agents48. Supaphon and Preedanon56 also reported on the anti-convulsant, antioxidant, antitumor, and the anti-bacterial potential of O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosylα-D-Glucopyranoside. That 2-ethyl-2-Butenal and 3-(hydroxymethyl)-6-2-Cyclohexen-1-one had no pharmacological activities reported from the literature search could mean that these are new compounds that may not have been previously identified in medicinal plants.
Furthermore, in this study, the test organisms could have been inhibited by this extract due to the presence of β-sitosterol, cis-Vaccenic acid and other compounds earlier reported to have antimicrobial potentials though their real mode of action on microorganisms is not clearly understood. The minimum inhibitory concentration of the extract against E. coli ATCC 8739 (1.75 mg/ml), Pseudomonas aeruginosa ATCC 19582 (0.88 mg/ml) and P. shigelloides ATCC 15903 (0.22 mg/ml) showed the extract has stronger antimicrobial activities against the Gram-negative bacteria. This supports the findings of Abukakar et al.72, Adeola et al.73, and Bhadoriya et al.74 that showed that T. indica extract was capable of suppressing the growth of the test organisms. This implies that the extract would be an effective therapy for infections such as wounds, dysentery, diarrhea, and food poisoning in which the test organisms have been implicated75,76,77.
Since some metabolic and age-related ailments are intimately linked with oxidative activities, therefore the exploitation of herbs and spices as a natural origin of antioxidants to prevent oxidation deserves more awareness78. While Luengthanaphol et al.79 reported that ethanol extract of tamarind seed coat exhibited antioxidant activity, this study indicated that methanol extract of the pulp has strong antioxidant properties. Although a good correlation has been recognized between antioxidant capacity and ferric reducing potential of the extract, the radical scavenging and ferric reducing potentials were relatively low when compared with that of the standard. This is in agreement with Atawodi et al.80 and Reis et al.81 indicating that extracts of T. indica displayed high antioxidant activities and Ugwuona and Onweluzo82 reported that Tamarind pulp possesses high antioxidant activities at elevated extraction temperature. Many reports have shown strong interdependence between antioxidant activities, phenolic and flavonoid content of plant extracts83,84, a strong positive correlation was also noticed between the total phenolic (r = 0.9912, p ˃ 0.05) and antioxidant activities evaluated by DPPH (r = 0.8938, p ˂ 0.05) and FRAP (r = 0.9808, p ˂ 0.05) assays.
Conclusion
In conclusion, the bioactive compounds, antibacterial and antioxidant activities of methanol extract of T. indica fruit pulp were investigated in vitro and the various pharmacological activities of each bioactive compound in this extract were identified. The pulp extract showed effective antibacterial and antioxidant activities while the pharmacological activities of the extract could be attributed to the bioactive compounds identified in the pulp extract and justify more reasons for the numerous usages of the plant in ethnomedicine.
Data availability
All data generated or analysed during this study are included in this published article.
References
Salim, A. A., Chin, Y. W. & Kinghorn, A. D. Drug discovery from plants. In Bioactive Molecules and Medicinal Plants (eds Ramawat, K. G. & Merillon, J. M.) 1–24 (Springer, 2008).
Sam, S. Importance and effectiveness of herbal medicines. J. Pharmacogn. Phytochem. 8(2), 354–357 (2019).
Egbuna, C., Nadia, S. & Jabeen, N. S. Pharmacognosy and prehistoric uses of medicinal plants. In Phytochemistry (eds Egbuna, C. et al.) 3–16 (Apple Academic Press, 2018).
Subramani, R., Narayanasamy, M. & Feussner, K. D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 7(3), 172. https://doi.org/10.1007/s13205-017-0848-9 (2017).
Olajuyigbe, O. O., Ijeyan, F. O. & Adeoye-Isijola, M. O. GC-MS analysis and in vitro antimicrobial effects of methanol stem bark extracts of Trilepisium madagascriense DC. Int. J. Sci. 6(8), 34–45. https://doi.org/10.18483/ijSci.1366 (2017).
Ribeiro, V. R. et al. Bringing together Saccharomyces cerevisiae and bioactive compounds from plants: a new function for a well-known biosorbent. J. Funct. Foods 60, 103433. https://doi.org/10.1016/j.jff.2019.103433 (2019).
Adeoye-Isijola, M. O., Jonathan, S. G., Coopoosamy, R. M. & Olajuyigbe, O. O. Molecularcharacterization, GCMS analysis, phytochemical screening, and Insecticidal activities of ethanol extract of Lentinus squarrosulus against Aedes aegypti. Mol. Biol. Rep. https://doi.org/10.1007/s11033-020-06119-6 (2021).
Boadu, A. A. & Asase, A. Documentation of herbal medicines used for the treatment and management of human diseases by some communities in southern Ghana. Evidence-Based Complement. Altern. Med. https://doi.org/10.1155/2017/3043061 (2017).
Aswad, M. et al. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 67(1), 67–75. https://doi.org/10.1007/s00011-017-1096-5 (2018).
Oladeji, O. The characteristics and roles of medicinal plants: Some important medicinal plants in Nigeria. Indian J. Nat. Prod. 12(3), 102 (2016).
Welz, A. N., Emberger-Klein, A. & Menrad, K. Why people use herbal medicine: insights from a focus-group study in Germany. BMC Complement. Altern. Med. 18(1), 92. https://doi.org/10.1186/s12906-018-2160-6 (2018).
Joo, Y. E. Natural product-derived drugs for the treatment of inflammatory bowel diseases. Intest. Res. 12(2), 103–109. https://doi.org/10.5217/ir.2014.12.2.103 (2014).
El-Siddig, K., Gunasena, H. P. M., Prasa, B. A., Pushpakumara, D. K. N. G., Ramana, K. V. R., Vijayan, P. & Williams, J. T. Tamarind – Tamarindus indica L. Fruits for the future 1, Southampton Centre for Underutilized Crops, Southampton, UK, 188p. (2006).
Menezes, P. P. A., Trevisan, C. C. S., Barbalho, S. M. & Guiguer, L. E. Tamarindus indica L. a plant with multiple medicinal purposes. J. Pharmacogn. Phytochem. 5(3), 50–4 (2016).
Raimondi, L. et al. The polysaccharide from Tamarindus indica (TS-polysaccharide) protects cultured corneal-derived cells (SIRC cells) from ultraviolet rays. J. Pharm. Pharmacol. 55(3), 333–338. https://doi.org/10.1211/002235702630 (2003).
Pamplona-Roger, G. D. Encyclopedia of Medicinal Plants Education and Health Library; Madrid, Spain: 2536 (1999).
Muthu, S. E., Nandakumar, S. & Rao, U. A. The effect of methanolic extract of Tamarindus indica Linn. on the growth of clinical isolates of Burkholderia pseudomallei. Indian J. Med. Res. 122, 525–8 (2005).
Lans, C. Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J. Ethnobiol. Ethnomed. https://doi.org/10.1186/1746-4269-3-3 (2007).
Gupta, C., Prakash, D. & Gupta, S. Studies on the antimicrobial activity of Tamarind (Tamarindus indica) and its potential as a food bio-preservative. Int. Food Res. J. 21(6), 2437–2441 (2014).
Komakech, R., Kim, Y. G., Matsabisa, G. M. & Kang, Y. Anti-inflammatory and analgesic potential of Tamarindus indica Linn. (Fabaceae): a narrative review. Integrat. Med. Res. 8(3), 181–186. https://doi.org/10.1016/j.imr.2019.07.002 (2019).
Kaur, R., Mudgal, R., Jose, J., Kumar, P. & Tomar, S. Glycan-dependent chikungunya viral infection divulged by the antiviral activity of NAG-specific chi-like lectin. Virology 526, 91–98. https://doi.org/10.1016/j.virol.2018.10.009 (2019).
Lakor, J., Elepu, G., Buyinza, M. J. & Nyeko, P. Analysis of Tamarindus (Tamarindus indica L.) value chain in Uganda: identification of opportunities and constraints to its commercialization and domestication. J. Agric. Environ. Sci. 5(1), 101–12. https://doi.org/10.15640/jaes.v5n1a11 (2016).
Ngo, L. T., Okogun, J. I. & Folk, W. R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 30, 584–592. https://doi.org/10.1039/c3np20120a (2013).
Hussein, H. J., Hadi, M. Y. & Hameed, I. H. Study of the chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 8(3), 60–89. https://doi.org/10.5897/JPP2015.0372 (2016).
Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry 804 (Allured Publishing, 2007).
Ayaz, M. et al. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res. Thessalon. 22(1), 4. https://doi.org/10.1186/s40709-015-0028-1 (2015).
Banothu, V., Neelagiri, C., Adepally, U., Lingam, J. & Bommareddy, K. Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plant Albizia odoratissima. Pharm. Biol. 55(1), 1155–1161. https://doi.org/10.1080/13880209.2017.1291694 (2017).
Dhanani, T., Shah, S., Gajbhiye, N. A. & Kumar, S. Effect of extraction methods on yield, phytochemical constituents, and antioxidant activity of Withania somnifera. Arab. J. Chem. 10(1), S1193–S1199. https://doi.org/10.1016/j.arabjc.2013.02.015 (2017).
Nair, V. D. P. et al. Investigation of the antioxidant activity of African potato (Hypoxis hemerocallidea). J. Agric. Food Chem. 55, 1707–1711. https://doi.org/10.1021/jf0619838 (2007).
Johari, M. A. & Khong, H. Y. Total phenolic content and antioxidant and antibacterial activities of Pereskia bleo. Adv. Pharmacol. Sci. https://doi.org/10.1155/2019/7428593 (2019).
Mathur, R. & Vijayvergia, R. Determination of total flavonoid and phenol content in Mimusops elengi Linn.. Int. J. Pharm. Sci. Res. 8(12), 5282–5285. https://doi.org/10.13040/IJPSR.0975-8232.8(12).5282-85 (2017).
Tian, J. et al. Regional variationin components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod. 59, 69–79. https://doi.org/10.1016/j.indcrop.2014.04.048 (2014).
Sharafutdinov, I. S. et al. Unraveling the molecular mechanism of selectiveantimicrobial activity of 2 (5H)-furanone derivative against Staphylococcus aureus. Int. J. Mol. Sci. 20(3), 694. https://doi.org/10.3390/ijms20030694 (2019).
Effenberger, I., Hoffmann, T., Jonczyk, R. & Schwab, W. Novel biotechnological glucosylation of high-impact aroma chemicals, 3 (2H)-and 2 (5H)-furanone. Sci. Rep. 9(1), 1–9. https://doi.org/10.1038/s41598-019-47514-9 (2019).
Pandey, S. & Srivastava, R. S. Synthesis and characterization of some heterocyclic Schiffbases: potential anticonvulsant agents. Med. Chem. Res. 20(7), 1091–1101. https://doi.org/10.1007/s00044-010-9441-z (2011).
Mutalib, M. A., Rahmat, A., Ali, F., Othman, F. & Ramasamy, R. Nutritional compositions and antiproliferative activities of different solvent fractions from ethanol extract of Cyphomandra betacea (Tamarillo) fruit. Malays. J. Med. Sci. 24(5), 19–32. https://doi.org/10.21315/mjms2017.24.5.3 (2017).
Evjen, S. et al. Degradative behavior and toxicity of alkylated imidazoles. Ind. Eng. Chem. Res. 59(2), 587–595. https://doi.org/10.1021/acs.iecr.9b05100 (2019).
Lokesh, B. V. S., Prasad, Y. R. & Shaik, A. B. Synthesis and biological activity of novel 2, 5-dichloro-3-acetylthiophene chalcone derivatives. Indian J. Pharm. Educ. Res. 51(4S), s679–s690. https://doi.org/10.5530/ijper.51.4s.99 (2017).
Omran, B. A., Fatthalah, N. A., El-Gendy, N. S., El-Shatoury, E. H. & Abouzeid, M. A. Green biocides against sulfate-reducing bacteria and macrofouling organisms. J. Pure Appl. Microbiol. 7(3), 2219–2232 (2013).
Casimir, O. A., Martin, D. K., Philippe, E. K., Augustin, A. A. & Parfait, K. E. J. Chemical composition, antioxidant and antimicrobial activities of Capsicum annuum var. annuum concentrated extract obtained by reverse osmosis. GSC Biol. Pharm. Sci. 5(2), 116–125. https://doi.org/10.30574/gscbps.2018.5.2.0123 (2018).
Zaky, M. F., Sabbah, I., Negm, N. A. & Hendawy, M. Biocidal activity and corrosion inhibition of dome cationic surfactants derived from thiol polyurethane. Egypt. J. Chem. 61(1), 45–60. https://doi.org/10.21608/ejchem.2017.2119.1169 (2018).
Api, A. M. et al. RIFM fragrance ingredient safety assessment, benzene acetaldehyde, 3, 4-dimethyl-, CAS Registry Number 68844–97-3. Food Chem. Toxicol. 122(1), S664–S669. https://doi.org/10.1016/j.fct.2018.11.008 (2018).
Gomes, A. A. et al. Simplicillium coffeanum, a new endophytic species from Brazilian coffee plants, emit antimicrobial volatiles. Phytotaxa 333(2), 188–198. https://doi.org/10.11646/phytotaxa.333.2.2 (2018).
Maurya, S., Kushwaha, A. K. & Flamini, G. A study of physicochemical properties, volatile component analysis, and antioxidative properties of honey. Int. J. Res. Dev. Pharm. L. Sci. 4(6), 1852–1860 (2015).
Tsai, Y. H. et al. Synthesis of triazole derivatives of Levoglucosenone as promising anticancer agents: effective exploration of the chemical space through retro-aza-Michael//aza-Michael isomerizations. J. Org. Chem. 83(7), 3516–3528. https://doi.org/10.1021/acs.joc.7b03141 (2018).
Uguzlar, H., Maltas, E. & Yildiz, S. Antioxidant activity and fatty acid compositions of Arum Dioscorides extracts. Biosci. Biotechnol. Res. Asia 8(1), 75–82 (2016).
Shukla, R., Banerjee, S. & Tripathi, Y. B. Antioxidant and antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. on streptozotocin-induced diabetic nephropathy in rats. BMC Complement. Altern. Med. 18(1), 156. https://doi.org/10.1186/s12906-018-2221-x (2018).
Taechowisan, T., Sarakoat, P. & Phutdhawong, W. S. Major chemical composition of fruit extracts of Morinda citrifolia L. and their antibacterial, antioxidant, and cytotoxicity properties. J. Appl. Sci. 19(5), 366–375. https://doi.org/10.3923/jas.2019.366.375 (2019).
Rajkumari, J. et al. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: insights from in vitro, in vivo, and silico studies. Microbiol. Res. 226, 19–26. https://doi.org/10.1016/j.micres.2019.05.001 (2019).
Abdellaoui, K., Acheuk, F., Miladi, M., Boughattas, I. & Omri, G. Phytochemistry, biochemical and insecticidal activities of Ruta chalepensis essential oils on Tribolium confusum. Agric. For. 64(3), 31–45. https://doi.org/10.17707/AgricultForest.64.3.03 (2018).
Kumar, V., Anwar, F., Verma, A. & Mujeeb, M. The therapeutic effect of umbelliferon-α-Dglucopyranosyl-(2 I→ 1 II)-α-D-glucopyranoside on adjuvant-induced arthritic rats. J. Food Sci. Tech. 52(6), 3402–3411. https://doi.org/10.1007/s13197-014-1403-x (2015).
Zhang, J., Onakpoya, I. J., Posadzki, P. & Eddouks, M. The safety of herbal medicine: from prejudice to evidence. Evidence-Based Complement. Altern. Med. https://doi.org/10.1155/2015/316706 (2015).
Moustafa, A. H. et al. Pyrazoles and isoxazoles based sulfanilamide and phenazone as antimicrobial agents: synthesis and biological activity. Russ. J. Gen. Chem. 89(11), 2314–2320. https://doi.org/10.1134/S1070363219110240 (2019).
Anthonia, E. E. & Philip, H. S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 3(2), 42–47. https://doi.org/10.14419/ijac.v3i2.5048 (2015).
Yoo, Y. C. et al. Isolation of fatty acids with anticancer activity from Protaetia breviaries Larva.. Arch. Pharm. Res. 30, 361–365. https://doi.org/10.1007/BF02977619 (2007).
Supaphon, P. & Preedanon, S. Evaluation of in vitro alpha-glucosidase inhibitory, antimicrobial, and cytotoxic activities of secondary metabolites from the endophytic fungus, Nigrospora sphaerica, isolated from Helianthus annuus. Ann. Microbiol. 69, 1397–1406. https://doi.org/10.1007/s13213-019-01523-1 (2019).
Hussein, H. M., Ubaid, J. M. & Hameed, I. H. Insecticidal activity of methanolic seeds extracts of Ricinus communis on an adult of Callosobruchus maculatus (coleopteran: branchiae) and analysis of its phytochemical composition. Int. J. Pharmacog. Phytochem. Res. 8(8), 1385–1397 (2016).
Mickymaray, S., Al Aboody, M. S., Rath, P. K., Annamalai, P. & Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 6(3), 185–191. https://doi.org/10.1016/j.apjtb.2015.12.005 (2016).
Khan, K., Rasheed, M., Nadir, M., Firdous, S. & Faizi, S. GC-MS & preliminary screening profile of Cordia Sinensis leaves–antiglycation, antifungal, and insecticidal agents. Nat. Prod. Res. 35(7), 1212–1216. https://doi.org/10.3109/13880209.2016.1172320 (2021).
Somers-Edgar, T. J. et al. Mechanisms for the activity of heterocyclic cyclohexanone curcumin derivatives in estrogen receptor-negative human breast cancer cell lines. Invest. New Drugs 29(1), 87–97. https://doi.org/10.1007/s10637-009-9339-0 (2011).
Krishnaveni, M., Dhanalakshmi, R. & Nandhini, N. GC-MS analysis of phytochemicals, fatty acid profile, antimicrobial activity of Gossypium seeds. Int. J. Pharm. Sci. Rev. Res. 27(1), 273–276 (2014).
Hamazaki, K. et al. Is vaccenic acid (18:1t n-7) associated with an increased incidence of hip fracture? An explanation for the calcium paradox. Prostaglandins Leukot. Essent. Fatty Acids 109, 8–12. https://doi.org/10.1016/j.plefa.2016.04.001 (2016).
Pu, Z.-H. et al. Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3, 4 diyl ester from neem oil. Agric. Sci. China 9(8), 1236–1240. https://doi.org/10.1016/S1671-2927(09)60212-1 (2010).
Arora, S., Kumar, G. & Meena, S. GC-MS analysis of bioactive compounds from the whole plant hexane extract of Cenchrus setigerus Vahl.. Pharm. Sci. Monit. 8(4), 137–146. https://doi.org/10.1007/s13197-013-1105-9 (2017).
Zahid, M., Arif, M., Rahman, M. A., Singh, K. & Mujahid, M. Solvent extraction and gas chromatography-mass spectrometry analysis of Annona squamosa L. seeds for determination of bioactive, fatty acid/fatty oil composition, and antioxidant activity. J. Diet. Suppl. 15(5), 613–623. https://doi.org/10.1080/19390211.2017.1366388 (2018).
Bharanishankar, S. et al. Effects of beta-sitosterol on inflammatory cytokines in high-fat diet-fed type-2 diabetic rats. Drug Invent. Today 12(5), 906–909 (2019).
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. G. & Lightfoot, D. A. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6(4), 42. https://doi.org/10.3390/plants6040042 (2017).
World Health Organization (WHO). Food and Agriculture Organization (2003) Diet nutrition and the prevention of chronic diseases: report of a Joint WHO/FAO Expert Consultation. WHO technical report series, 916 (2016).
Shapla, U. M., Solayma, M., Alam, N., Khalil, M. I. & Gan, S. H. 5 Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chem. Cent. J. 12, 35. https://doi.org/10.1186/s13065-018-0408-3 (2018).
Palchykov, V. A. et al. Bactericidal, protistocidal, nematicidal properties and chemical composition of ethanol extract of Punica granatum peel. Biosyst. Divers. 27(3), 300–306 (2019).
Ahmed, A. O. E. E. & Ayoub, S. M. H. Chemical composition and antimalarial activity of extracts of Sudanese Tamarindus indica L. (Fabaceae). The Pharm. Innov. J. 4(4), 9–93 (2015).
Abukakar, M. G., Ukwuani, A. N. & Shehu, R. A. Phytochemical screening and antibacterial activity of Tamarindus indica pulp extract. Asian J. Biochem. 3(2), 134–138. https://doi.org/10.3923/ajb.2008.134.138 (2008).
Adeola, A. A., Adeola, O. O. & Dosumu, O. O. Comparative analyses of phytochemicals and antimicrobial properties of extracts of wild Tamarindus indica pulps. Afr. J. Microbiol. Res. 4(24), 2769–2779 (2010).
Bhadoriya, S. S., Ganeshpurkar, A., Narwaria, J., Rai, G. & Jain, A. P. Tamarindus indica: extent of explored potential. Pharmacogn. Rev. 5(9), 73–81. https://doi.org/10.4103/0973-7847.79102 (2011).
Kim, D. H. et al. Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Korean J. Food Sci. Anim. Resour. 36(6), 787–790. https://doi.org/10.5851/kosfa.2016.36.6.787 (2016).
Aman, M. J. Superantigens of a superbug: major culprits of Staphylococcus aureus disease?. Virulence 8(6), 607–610. https://doi.org/10.1080/21505594.2016.1255399 (2017).
Dinev, T. et al. Antimicrobial activity of Lactobacillus Plantarum against pathogenic and food spoilage microorganisms: a review. Bulg. J. Vet. Med. 21(3), 1–16. https://doi.org/10.15547/BJVM.1084 (2018).
Tapsell, L. C. et al. Role of herbs and spices: the past, the present, the future. Med. J. Aust. 185(S4), S1–S24. https://doi.org/10.5694/j.1326-5377.2006.tb00548.x (2006).
Luengthanaphol, S. et al. Extraction of antioxidants from sweet Thai tamarind seed coat: preliminary experiments. J. Food Eng. 63(3), 247–252. https://doi.org/10.1016/j.jfoodeng.2003.07.006 (2004).
Atawodi, S. E., Liman, M. L., Ottu, J. O. & Iliemene, U. D. Total polyphenols, flavonoids, and antioxidant properties of different parts of Tamarindus indica Linn. of Nigerian origin. Ann. Res. Rev. Biol. 4(24), 4273–4283. https://doi.org/10.9734/ARRB/2014/8602 (2014).
Reis, P. M. C. L., Dariva, C., Vieira, G. A. B. & Hense, H. Extraction and evaluation of the antioxidant potential of the extracts obtained from tamarind seeds (Tamarindus indica), sweet variety. J. Food Eng. 173, 116–123. https://doi.org/10.1016/j.jfoodeng.2015.11.001 (2016).
Ugwuona, F. U. & Onweluzo, J. C. Assessment of antioxidant properties of tamarind fruit pulp and its effect on the storage stability of African breadfruit seed dhal and flour. Niger. Food J. 31(2), 41–47. https://doi.org/10.1016/S0189-7241(15)30075-8 (2013).
Hatamnia, A. A. et al. Antioxidant activity of different parts of Pistacia khinjuk Stocks fruit and its correlation to phenolic composition. Nat. Prod. Res. 30(12), 1445–1450. https://doi.org/10.1080/14786419.2015.1060593 (2016).
Aryal, S. et al. Total phenolic content, flavonoid content, and antioxidant potential of wild vegetables from Western Nepal. Plants 8(4), 96. https://doi.org/10.3390/plants8040096 (2019).
Funding
The research did not have financial support from any organization.
Author information
Authors and Affiliations
Contributions
K.O.F, M.O.A. and O.O.O. designed the experiments. K.O.F., M.O.A., D.A.A. and O.O.O. performed the experiment. K.O.F., M.O.A., K.K.N., R.M.C. and O.O.O. analyzed the data. K.O.F., M.O.A., K.K.N. and O.O.O. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fagbemi, K.O., Aina, D.A., Adeoye-Isijola, M.O. et al. Bioactive compounds, antibacterial and antioxidant activities of methanol extract of Tamarindus indica Linn.. Sci Rep 12, 9432 (2022). https://doi.org/10.1038/s41598-022-13716-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13716-x
- Springer Nature Limited
This article is cited by
-
In vitro antibacterial activity of fruit pulp extracts of Tamarindus indica against Staphylococcus aureus and Klebsiella pneumoniae
BMC Complementary Medicine and Therapies (2024)
-
Proanthocyanidins supplemented diet alter anti-aging-markers and improved lifespan in Drosophila melanogaster model
Beni-Suef University Journal of Basic and Applied Sciences (2024)
-
Synthesis, in silico guided DNA-interaction analysis, in vitro anti-oxidant evaluation, and antibacterial assay of 4-amino-5-(2-benzylidenehydrazinyl)-2H-1,2,4-triazole-3(4H)-thiones
Journal of the Iranian Chemical Society (2024)