Abstract
To explore novel therapeutic targets, develop a gene signature and construct a prognostic nomogram of bladder cancer (BCa). Transcriptome data and clinical traits of BCa were downloaded from UCSC Xena database and Gene Expression Omnibus (GEO) database. We then used the method of Single sample Gene Set Enrichment analysis (ssGSEA) to calculate the infiltration abundances of 24 immune cells in eligible BCa samples. By weighted correlation network analysis (WGCNA), we identified turquoise module with strong and significant association with the infiltration abundance of immune cells which were associated with overall survival of BCa patients. Subsequently, we developed an immune cell infiltration-related gene signature based on the module genes (MGs) and immune-related genes (IRGs) from the Immunology Database and Analysis Portal (ImmPort). Then, we tested the prognostic power and performance of the signature in both discovery and external validation datasets. A nomogram integrated with signature and clinical features were ultimately constructed and tested. Five prognostic immune cell infiltration-related module genes (PIRMGs), namely FPR1, CIITA, KLRC1, TNFRSF6B, and WFIKKN1, were identified and used for gene signature development. And the signature showed independent and stable prognosis predictive power. Ultimately, a nomogram consisting of signature, age and tumor stage was constructed, and it showed good and stable predictive ability on prognosis. Our prognostic signature and nomogram provided prognostic indicators and potential immunotherapeutic targets for BCa. Further researches are needed to verify the clinical effectiveness of this nomogram and these biomarkers.
Similar content being viewed by others
Introduction
Bladder cancer (BCa) is one of the most common urinary malignancies and there are around ten thousand newly diagnosed BCa per year worldwide1. Approximately 3/4 BCa are non- muscle-invasive bladder cancer (NMIBC) at the initial time of being diagnosed2. Treatment strategies for NMIBC include transurethral resection of bladder tumor with or without intavesical chemotherapy or immunotherapy, while the primary treatment option for muscle-invasive bladder cancer (MIBC) is radical cystectomy combined with intavesical medicine therapy3. The patients of BCa can have a favorable prognosis if the cancerous cells are confined to sub-mucosal connective tissues. Disappointingly, the 5-year overall survival rate can be declined to lower than 15% from 80% once the lesions progress beyond the lining, into the surrounding bladder muscle and even other parts of the body4. Moreover, the conventional therapeutics cannot make a satisfactory outcome for patients of late-stage BCa5. In such conditions, development of new strategies and efficient therapeutics are urgently needed to improve the prognosis for patients with BCa.
Recently, the explosive growth of researches on immunotherapy made historic breakthroughs in various types of malignancies including prostate cancer, renal clear cell carcinoma, etc.6,7. Li et al. have confirmed the critical role of tumor microenvironment in the progression of BCa8. And significant heterogeneities have been found in genome, transcriptome and biological process among groups with different immune-infiltration abundances in BCa9. These works suggest the importance and necessity of exploration of the immune-related molecular mechanisms in BCa.
This study intended to quantify the immune cells’ infiltration abundances in BCa by single sample gene set enrichment analysis (ssGSEA), and found genes correlated with the prognostic immune cells by weighted correlation network analysis (WGCNA). Then, a signature based on hub genes was constructed to calculate the survival risk of patient of BCa. Additionally, we built a nomogram to predict the overall survival rate at 1-, 3- and 5-year follow up, based on the signature and clinicopathological parameters. The results of our study provided several potential immunotargets and evidences for further research in immunotherapy of BCa.
Results
Identification of shared prognostic immune cells
As listed in Table 1, five shared immune cells related to prognosis of BCa were identified, including cytotoxic cells, CD8+ T cells, T helper cells, T follicular helper cells (TFH), and Dendritic Cells (DC). The KM curves of the five common prognostic cells were presented in Supplementary Fig. 1a, which showed that high infiltration levels tumor samples had favorable prognosis.
The results of significant relationship between clinicopathological factors and prognostic cells were demonstrated in Supplementary Fig. 1b. The infiltration abundance of T helper cells was significantly lower in samples of elder patients, high grade, late stage (stage III and IV) and MIBC subtype. TFH also showed lower infiltration level in samples of late stage. The infiltration abundance of CD8 T cells, however, was significantly higher in MIBC, which may be influenced by the basis of small sample size of NMIBC.
Development of a co-expression network and identification of the key module
Firstly, we constructed the sample dendrogram and trait heatmap with no outlier sample detected. Then, under the soft-thresholding of 3, the gene cluster dendrogram was produced with the high similarity of feature genes into the same module. Next, we created a module-trait heatmap to demonstrate the correlation between the infiltration levels of the five prognostic immune cells and different modules. As showed in Fig. 1, turquoise module showed the highest correlation coefficient with CD8 T cells (cor = 0.54), TFH (cor = 0.43), cytotoxic cells (cor = 0.75) and DC (cor = 0.55) simultaneously. Additionally, this module had marginally significant and weak correlation with the survival time compared to other modules. Taking together, this module was regarded as the key module and genes within the module were the most relevant to tumor prognosis. We then extracted all genes in this module for next studies.
Identification of key module associated with the prognostic immune cells through WGNCA by module-trait heatmap. Each cell contains correlation and p value. Each column represents a trait. The module name is shown to the left side of each cell. Each module represents a cluster of densely interconnected genes in terms of co-expression.
Identify IRMGs and perform enrichment analysis
As shown in Fig. 2a, a total of 259 shared genes of IRGs and MGs were identified and considered as immune-related module genes (IRMGs).
The top 20 enriched terms by the online tool Metascape were listed in Fig. 2b. The highest-levels of enriched clusters in the four categories were cytokine-cytokine receptor interaction in KEGG pathway, lymphocyte activation in biological process of GO, Adaptive Immune System in Reactome Gene Sets, and PID IL12 2PATHWAY (IL12-mediated signaling events) in Canonical pathways.
PIRMGs identification, mutation analysis and TF regulatory network construction
376 samples were randomly and equally divided into training and testing cohorts. We then performed univariate Cox regression analysis on the IRMGs in training cohort and identified 12 IRMGs related to the prognosis of BCa (Fig. 3a). Subsequently, to clarify the molecular characteristics of the PIRMGs, we used the online tool cBioportal to perform the gene alternations analysis and found that the most common types were amplification and mutation (Fig. 3b).
Regulatory network analysis of PIRMGs and module-related TFs. (a) A forest plot demonstrated the 12 IRMGs identified by univariate Cox regression analysis (p-value < 0.05). Right panel illustrated the hazard ratio of 12 IRMGs, in which red box indicated HR > 1 and light-blue indicated HR < 1. (b) Alteration analysis of PIRMGs by online database cBioPortal. The “+” and “−” symbols indicated that whether the alterations in DNA levels can be detected or not in corresponding dataset. (c) Identification of 15 module-related TFs (overlapping area) by intersection of 1017 MGs (circle at left) and 318 TFs (circle at right). (d) A regulatory network of the significant correlations of PIRMGs (circle) and module-related TFs (triangle). Lines indicated correlations. Red or light-blue indicated positive or negative correlation, respectively.
We then distinguished the TFs from the turquoise module and constructed a regulatory network based on the 12 PIRMGs and 15 module-related TFs (Fig. 3c,d).
Develop a signature in the training cohort
The prognostic signature was developed with five PIRMGs, namely FPR1, CIITA, KLRC1, TNFRSF6B, and WFIKKN1 (Table 2). The risk score calculator was: Risk score = 0.148502 × Exp.FPR1 − 0.23798 × Exp. CIITA − 0.1929 × Exp. KLRC1 + 0.190133 × Exp. TNFRSF6B − 0.24818 × Exp. WFIKKN1.
Next, 188 BCa samples in the training cohort were divided into high- and low-risk groups according to the median risk score of 1.018. Figure 4a–c demonstrated the risk score distribution, survival status and the five PIRMGs expression patterns between two groups. To assess the prognostic value of the signature, we performed KM survival analysis and found that patients in high-risk group had worse prognosis compared to those in low-risk group (Fig. 4d). We then produced time‐dependent ROC curves to estimate the performance of the signature, and found that area under curve (AUC) for 1‐, 3- and 5-year survival prediction was 0.798, 0.748 and 0.656, respectively (Fig. 4e).
Evaluation of the prognosis prediction power of the five PIRMGs signature in the training group. (a) Distribution of risk score. (b) Survival status of BCa patients. (c) The expression profiles of the five PIRMGs. (d) KM survival analysis of the high- and low-risk groups. (e) Time-dependent ROC curve of the signature.
Signature validation in internal and external validation datasets
Based on the risk score calculator proposed in the training cohort, the risk score of each BCa sample of both internal and external validation datasets were obtained and these samples were further divided into high- and low-risk groups according to the median risk score in training group.
In internal validation dataset, the results were similar to those of the training cohort (Supplementary Fig. 2). Additionally, the results of the external validation datasets also suggested that BCa patients in high-risk group also suffered from worse prognosis than those in low-risk group (Fig. 5). Moreover, the signature presented stable performance in all cohorts.
In conclusion, the five PIRMGs signature can divided BCa patients into two risk-level groups with significant differences in overall prognosis.
Independent analysis of the signature and clinicopathological characteristics
As shown in Table 3, the signature showed independent prognostic prediction ability in three cohorts of TCGA dataset, simultaneously. Besides, age and stage were considered as two independent clinicopathological predictors of BCa.
The results of clinical features relationship analysis demonstrated that patients of high grade, late stage, MIBC subtype, and older age had significant higher risk scores (Fig. 6a).
Stratified analysis was further carried out between the signature and age and stage. And the results showed that the signature had significant prognostic value and reliability for BCa patients with the same age and late stage (Fig. 6b–i).
In short, these results suggested the relationship between signature and progression of BCa.
Construction of a nomogram
Based on the five PIRMGs signature and age and stage, we constructed a nomogram to predict the 1-, 3-, and 5-year overall survival of BCa (Fig. 7a). Time-dependent ROC curves showed the adequate discrimination of the nomogram with an AUC of 0.774, 0.724, and 0.709 at 1-, 3-, and 5-year follow up (Fig. 7b). Additionally, we plotted the calibration plots to demonstrated the good predictive effect of this nomogram on overall survival (Fig. 7c).
Building and evaluating the predictive power of the nomogram. (a) A nomogram constructed based on the five PIRMGs signature, age and stage. (b) Time-dependent ROC curves of the nomogram in the prediction of prognosis at 1-, 3-, and 5-year time points. (c) Calibration plots of the nomogram in 1-, 3-, and 5-year survival. The predicted and the actual probabilities of survival are plotted using blue and black solid lines, respectively.
In traditional clinical practices, some clinical variables such as age, tumor stage and grade were widely used in evaluating the survival probability of patients of BCa. Hence, to further test the clinical utility of our signature, we designed a full model composed of risk score and clinical variables, and a base model only integrating clinical variables. As illustrated in Supplementary Fig. 3, the full model demonstrated superiority to the based model in 1-, 3-, and 5-year time point. Moreover, we compared the AUC value of the nomogram to those of other clinical variables by ROC curves (Supplementary Fig. 4). In a word, the signature combined with traditional clinical variables has potential clinical utility.
Discussion
In this study, we identified five PIRMGs (FPR1, CIITA, KLRC1, TNFRSF6B, and WFIKKN1) from the turquoise module which showed positive and significant correlations with several immune cells including CD8+ T cells. A prognostic signature was then constructed to divide patients of BCa into two distinct risk groups. Ultimately, a nomogram composing of signature, age and tumor stage was developed to calculate the total score of each BCa and return a quantitative survival probability. Two GEO datasets were obtained to validate the significant predictive power and good performance of the signature and nomogram, respectively. Although there have been several immune-related signatures proposed in literature, we firstly proposed a novel immune cell infiltration abundance-related prognostic signature of BCa through WGCNA.
Though correlation analysis between TFs of the turquoise module and PIRMGs, three key TFs (STAT4, IKZF1 and STAT1) were identified from the network. STAT4 and IKZF1 have been proved as essential factors in immune cell development and immune response10. STAT1 can affect the cellular survival and response to pathogens due to its critical roles in gene expression.
Cytotoxic cells are composed of CD8+ T cells, gamma delta T cells (Tγδ) and natural killer (NK) cells, participating in the innate immune system. The definite function of cytotoxic cells is eliminating intracellular pathogens and abnormal cells. Unlike CD8 T cells with strict major histocompatibility complex (MHC) restriction, both Tγδ and NK cells have the ability to recognize and kill infected cells in the absence of antibodies and MHC, and secret a large number of cytokines, allowing for a rapid immune response11. As a critical role in the adaptive immune system, T helper cells regulate the proliferation of B cells and participate in pathogen clearance, and autoimmunity through specific coordinate effector functions. There are two major subsets of T helper cells (Th1 and Th2 cells) once the proliferating T helper cells develop into effector T cells. Th1 cells mainly lead to an activated cell-mediated response, while Th2 cells favor a predominantly humoral response12. TFH, a subset CD4+ T cells, are found in B cell follicles and germinal centers. TFH play an important role in regulating the selection and survival of B cells that can differentiate into plasma cells and memory B cells. More importantly, TFH are believed to have the ability to decrease the repertoire of potentially autoimmune-causing mutated B cells within the germinal center. In general, the functions of, and mediation between, the cytokine, immune cells, and immune systems are critical for anti-tumor immunity, and profound understanding in these molecular interactions can promote the breakthroughs in tumor immunotherapies.
WFIKKN1, encodes large extracellular multidomain proteins, were mainly explored in cell growth and metabolism13, while, the biological behavior of WFIKKN1 was poorly studied in tumors.
As reported in previous studies, the expression of peptide-loaded HLA class I molecule (HLA-E) in tumor cells can negatively mediate the anti-tumor activity of NK cell, by ligation of the NK inhibitory receptor CD94/NKG2A (KLRC1)14. This key molecular mechanism in tumor resistance to immune cells was further explored by Kamiya et al. via establishing NKG2Anull and NKG2A + NK cells. And the results showed that NKG2A downregulation was associated with the higher cytotoxicity of NK cell in decreasing HLA-E-expressing tumor cells15. The consistent result was also reported in the study by Chen et al. who further found that NKG2A + CD8+ T cells form the predominant subset of NKG2A+ cells in lung cancer tissue but not NK cells and NKG2A blockade could promote anti-tumor immunity by reducing dysfunctional CD8+ T cells16. Surprisingly, these findings were contrary to the results of our study in which high expression of NKG2A was associated with favorable prognosis. Further studies are required to explore the role and molecular mechanism of NKG2A in BCa.
The critical role of CIITA in immune response to tumor cells was well established in literature. Mortara et al. reported that MHC class II expression in breast cancer was dependent on CIITA. And they found that CIITA-induced MHC class II expression on tumor cells had the ability of triggering an adaptive and protective immunity by presenting tumor antigen to Th cells, antitumor polarization, and establishment of antitumor immune memory17. Accolla et al. proposed an innovative method for construction of optimal anti-tumor vaccine, based on the biological functions of CIITA-induced MHC class II. The reason why traditional tumor-specific MHC-I-bound peptides had limited anti-tumor efficacy, as they analyzed, was that Th cell was inadequate triggered to maintain the proliferation of all the immune effector cells. Considering this drawback, they conducted a in vivo in mice assay and found that CIITA-driven MHC class II expression in tumor cells revealed strong inhabitation of tumor growth18. The protective role of CIITA in tumor was also proved by Lee et al.19. Based on these findings, novel anti-tumor vaccination protocols will be established and translated in clinics to provide a favorable prognosis for patients with tumors.
FPR1 functions as a key part of the innate immune system mainly expressed in the phagocytic and blood leukocyte cells, and mediates the response to pathogens invasion. Jiang et al. reported that overexpression of FPR1 was associated with drug-resistant BCa and may deteriorate the overall condition of drug-resistant BCa20. The biological behaviors of FPR1 were also explored in ovarian cancer21, cervical cancer22, and lung cancer23 as a risk factor related to poor prognosis, advanced stage and metastasis. However, Prevete et al. found that FPR1 acted as a tumor suppressor in human gastric cancer by counteract angiogenesis24.
TNFRSF6B belongs to the tumor necrosis factor receptor superfamily and acts as inhibiting Fas ligand-induced apoptosis25. Upregulated TNFRSF6B was identified in several human cancers including colon and lung cancers, and functioned as predictor of tumor invasion26. Tseng and colleagues found that overexpression of TNFRSF6B was also associated with the progression of chronic kidney disease27. Zekri et al. studied the differentially expressed genes in metastasis advanced Egyptian BCa and found that TNFRSF6B was downregulated in BCa samples, which was contrary to our findings28. This study, however, did not explore the molecular mechanism of TNFRSF6B in depth.
Together, we identified five PIRMGs, including FPR1, CIITA, KLRC1, TNFRSF6B, and WFIKKN1, which might play a vital role in tumorigenesis of BCa and serve as potential targets of immunotherapy. Besides, we developed a prognosis signature based on a series of analyses, which had good prognosis prediction ability in BCa. Ultimately, a stable prognostic predictive nomogram of signature, age, and stage, was developed to predict the 1-, 3- and 5-year survival probability of patient with BCa. Further researches are needed to verify the clinical effectiveness of this nomogram.
Methods
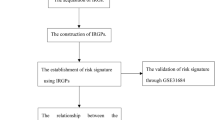
Figure 8 presents the work flow of our study.
Work flow of this study. TF transcription factors, IRG immune-related gene, ssGSEA single sample gene set enrichment analysis, WGCNA weighted correlation network analysis, IRMG immune-related module gene, PIRMG prognostic IRMG, LASSO Least Absolute Shrinkage and Selector Operation, multiCox multivariate Cox.
Data acquisition
We downloaded the transcriptome data (log2 transformed RSEM normalized count) and clinical data of BCa from the TCGA Hub in the UCSC Xena database (https://tcga.xenahubs.net). GSE32548 and GSE32894 were downloaded from GEO database (https://www.ncbi.nlm.nih.gov/gds/). The exclusive criteria of BCa samples were: (1) BCa with follow up < 90 days and, (2) BCa with missing survival data. Finally, 376, 126 and 224 BCa samples in TCGA and GSE32548 and GSE32894 datasets were obtained, respectively. Samples of TCGA dataset were divided into training and testing cohorts. The training cohort was used for signature construction, and the testing cohort and entire TCGA dataset were used for internal validations. Tow GEO datasets were used for external validations. Ethics approval statement is not needed because the BCa samples were obtained from the public databases.
A total of 2498 immune-related genes (IRGs) were obtained from the Immunology Database and Analysis Portal (ImmPort) database (https://www.immport.org/)29. 318 transcription factors (TFs) were downloaded from the Cancer database (http://cistrome.org)30 for constructing a regulatory network.
Infiltration levels of 24 immune cells in BCa samples
Firstly, we quantified the infiltration abundances of the 24 immune cells (reported in previous studies) in BCa samples by ssGSEA31,32. After the infiltration levels and the survival data of the BCa samples were obtained and merged, univariate Cox regression analysis and Kaplan–Meier survival analysis were performed to screen the shared prognostic immune cells. KM curves were produced to illustrate the results of survival analyses.
Then, with an attempt to further clarify the possible association between the shared prognostic cells and tumor progression, we analyzed the relationship between clinicopathological factors (age, grade, stage and subtypes) and these cells.
Construction of a weighted co-expression network
The input data files of WGCNA were: (1) transcriptome data of the 376 BCa samples of TCGA dataset and (2) the phenotype matrix which consisted of the survival data and the infiltration levels of the identified prognostic immune cells.
Firstly, the variability of gene expressions across the 376 samples was measured by a robust method called median absolute deviation (MAD). And the top 5000 MAD genes were identified for following analysis. A sample dendrogram with phenotype heatmap was constructed.
Subsequently, we calculated the best soft-thresholding, named β, by Soft Threshold function. A weighted adjacency matrix was then constructed based on the β value. And the adjacency matrix was further transformed into a topological overlapping matrix (TOM). Finally, genes with similar expressions were clustered into one module called co-expression module. p‑values and correlation coefficients were calculated to identify the association between a co-expression module and the phenotype. Finally, the key module with significant correlation with phenotype was identified, and all genes within the module (module genes, MGs) were extracted for next studies.
Identification of immune-related module genes (IRMGs)
IRMGs were considered as the shared genes of MGs and IRGs. A Venn plot by “VennDiagram” package in R was generated to show the analytic results33. To reveal the functional mechanism of the IRMGs, we then performed enrichment analysis by online tool Metascape (https://metascape.org/) which is designed to provide a comprehensive gene list annotation and analysis resource for researchers34.
Identification of prognostic IRMGs (PIRMGs)
The 376 BCa samples of TCGA dataset were randomly divided into training cohort and testing cohort in the ratio of 1:1. Then, we performed univariate Cox regression analysis in the training cohort to screen the IRMGs associated with prognosis of BCa, and IRMGs with p-values lower than 0.05 were considered as PIRMGs. The online tool cBioportal (http://www.cbioportal.org/) was then used to analyze the alterations of the PIRMGs35.
Next, we identified TFs in the key module by “VennDiagram” package and analyzed their correlations with PIRMGs by Pearson correlation coefficient. We further selected significant correlations under the cutoff values: correlation score > 0.4 and p-value < 0.001. A regulatory network of the significant correlations was ultimately constructed to further explore the molecular mechanisms of these PIRMGs. Software Cytoscape 3.8.0 was employed to visualize the network36.
Development of a prognostic signature
A prognostic signature was developed by Least Absolute Shrinkage and Selector Operation (LASSO) analysis and multivariate Cox regression analysis. Risk score of each BCa was calculated using the formula: Risk score = ExpGene1 × CoefGene1 + ExpGene2 × CoefGene2 + … ExpGene(n) × CoefGene(n). In this equation, “ExpGene” represented gene expression and “CoefGene” was the regression coefficient.
Then, BCa samples in training cohort were divided into high- and low-risk groups depending on the median value of the risk score. KM survival curve by log-rank test and time‐dependent receiver operating characteristic (ROC) curve were produced to assess the prognostic ability and performance of the signature. Further, the stability and reliability of the signature was verified in the internal and external validation group by the same methods.
Furthermore, the independent prognostic ability of signature was assumed by univariate and multivariate Cox regression analysis. Stratified analysis was also employed to assess the performance of signature in the same clinicopathological characteristics.
Development and evaluation of a nomogram
With an attempt to predict the 1-, 3- and 5-year survival probability of each BCa patient, we constructed a nomogram based on the risk score and independent clinicopathological characteristics. The performance of the nomogram was validated using time‐dependent ROC curves and calibration plots.
Additionally, to further asses the clinical utility of the signature for prognosis, we compared a full model including risk score and clinical variables (age, stage and grade) to a base model including only clinical variables through time-dependent ROC curves. AUC represents the performance of each model at specific time point.
Data availability
The data underlying this study are freely available from the TCGA Hub at Xena datasets (https://tcga.xenahubs.net) and the GEO database with accession number of GSE32548 and GSE32894 (http://www.ncbi.nlm.nih.gov/geo/).
Abbreviations
- BCa:
-
Bladder cancer
- NMIBC:
-
Non- muscle-invasive bladder cancer
- MIBC:
-
Muscle-invasive bladder cancer
- ssGSEA:
-
Single sample gene set enrichment analysis
- WGCNA:
-
Weighted correlation network analysis
- IRGs:
-
Immune-related genes
- TFs:
-
Transcription factors
- GSEA:
-
Gene set enrichment analysis
- MAD:
-
Median absolute deviation
- MGs:
-
Module genes
- IRMGs:
-
Immune-related module genes
- PIRMGs:
-
Prognostic IRMGs
- LASSO:
-
Least absolute shrinkage, and selector operation
- AUC:
-
Area under curve
- ROC:
-
Receiver operating characteristic
- MHC:
-
Major histocompatibility complex
References
Saginala, K. et al. Epidemiology of bladder cancer. Med. Sci. (Basel) 8, 15. https://doi.org/10.3390/medsci8010015 (2020).
Seidl, C. Targets for therapy of bladder cancer. Semin. Nucl. Med. 50, 162–170. https://doi.org/10.1053/j.semnuclmed.2020.02.006 (2020).
Stenzl, A. et al. Treatment of muscle-invasive and metastatic bladder cancer: Update of the EAU guidelines. Eur. Urol. 59, 1009–1018 (2011).
Soukup, V. et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization Grading classification systems in non-muscle-invasive bladder cancer: A European Association of Urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur. Urol. 72, 801–813 (2017).
Kouznetsova, V. L., Kim, E., Romm, E. L., Zhu, A. & Tsigelny, I. F. Recognition of early and late stages of bladder cancer using metabolites and machine learning. Metabolomics 15, 94 (2019).
Smith, C. C. et al. Endogenous retroviral signatures predict immunotherapy response in clear cell renal cell carcinoma. J. Clin. Investig. 128, 4804–4820 (2019).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Li, F., Guo, H., Wang, Y., Liu, B. & Zhou, H. Profiles of tumor-infiltrating immune cells and prognostic genes associated with the microenvironment of bladder cancer. Int. Immunopharmacol. 85, 106641 (2020).
Jiang, W., Zhu, D., Wang, C. & Zhu, Y. An immune relevant signature for predicting prognoses and immunotherapeutic responses in patients with muscle-invasive bladder cancer (MIBC). Cancer Med. 9, 2774–2790 (2020).
Yamamoto, K. et al. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol. Cell. Biol. 14, 4342–4349 (1994).
Zhang, N. & Bevan, M. J. CD8(+) T cells: Foot soldiers of the immune system. Immunity 35, 161–168. https://doi.org/10.1016/j.immuni.2011.07.010 (2011).
Knutson, K. L. & Disis, M. L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 54, 721–728. https://doi.org/10.1007/s00262-004-0653-2 (2005).
Szláma, G., Kondás, K., Trexler, M. & Patthy, L. WFIKKN1 and WFIKKN2 bind growth factors TGFβ1, BMP2 and BMP4 but do not inhibit their signalling activity. FEBS J. 277, 5040–5050. https://doi.org/10.1111/j.1742-4658.2010.07909.x (2010).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765 (2017).
Kamiya, T., Seow, S. V., Wong, D., Robinson, M. & Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 129, 2094–2106. https://doi.org/10.1172/jci123955 (2019).
Chen, Y. et al. CD8+ T cells form the predominant subset of NKG2A+ cells in human lung cancer. Front. Immunol. https://doi.org/10.3389/fimmu.2019.03002 (2020).
Mortara, L. et al. CIITA-induced MHC class II expression in mammary adenocarcinoma leads to a Th1 polarization of the tumor microenvironment, tumor rejection, and specific antitumor memory. Clin. Cancer Res. 12, 3435–3443 (2006).
Accolla, R. S., Ramia, E., Tedeschi, A. & Forlani, G. CIITA-driven MHC class II expressing tumor cells as antigen presenting cell performers: Toward the construction of an optimal anti-tumor vaccine. Front. Immunol. 10, 1806 (2019).
Lee, Y. S., Kim, S. H., Cho, J. A. & Kim, C. W. Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp. Mol. Med. 43, 281–290 (2011).
Jiang, X., Lei, T. & Zhang, M. Expression and functions of formyl peptide receptor 1 in drug-resistant bladder cancer. Technol. Cancer Res. Treat. 17, 1533034618769413. https://doi.org/10.1177/1533034618769413 (2018).
Minopoli, M. et al. Targeting the Formyl Peptide Receptor type 1 to prevent the adhesion of ovarian cancer cells onto mesothelium and subsequent invasion. J. Exp. Clin. Cancer Res. 38, 459. https://doi.org/10.1186/s13046-019-1465-8 (2019).
Cao, G. & Zhang, Z. FPR1 mediates the tumorigenicity of human cervical cancer cells. Cancer Manag. Res. 10, 5855 (2018).
Morris, S. et al. Whole blood FPR1 mRNA expression predicts both non-small cell and small cell lung cancer. Int. J. Cancer 142, 2355–2362 (2018).
Prevete, N. et al. The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene 34, 3826–3838. https://doi.org/10.1038/onc.2014.309 (2015).
Pitti, R. M. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396, 699–703 (1998).
Migone, T.-S. et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16, 479–492 (2002).
Tseng, W.-C., Yang, W.-C., Yang, A.-H., Hsieh, S.-L. & Tarng, D.-C. Expression of TNFRSF6B in kidneys is a novel predictor for progression of chronic kidney disease. Mod. Pathol. 26, 984–994. https://doi.org/10.1038/modpathol.2013.29 (2013).
Zekri, A. et al. Differentially expressed genes in metastatic advanced Egyptian bladder cancer. Asian Pac. J. Cancer Prev. 16, 3543–3549 (2015).
Bhattacharya, S. et al. ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci. Data 5, 180015 (2018).
Liu, T. et al. Cistrome: An integrative platform for transcriptional regulation studies. Genome Biol. 12, R83. https://doi.org/10.1186/gb-2011-12-8-r83 (2011).
Chen, X. et al. Bioinformatics analysis finds immune gene markers related to the prognosis of bladder cancer. Front. Genet. 11, 607 (2020).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795. https://doi.org/10.1016/j.immuni.2013.10.003 (2013).
Chen, H. & Boutros, P. C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 12, 35. https://doi.org/10.1186/1471-2105-12-35 (2011).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523. https://doi.org/10.1038/s41467-019-09234-6 (2019).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1. https://doi.org/10.1126/scisignal.2004088 (2013).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
We appreciate the works by the Cancer Genome Atlas database (TCGA) and Gene Expression Omnibus (GEO) datasets for the source of data used in our study.
Funding
This work was supported by Chongqing Science and Technology Commission (cstc2015shmszx120067).
Author information
Authors and Affiliations
Contributions
C.H.L. and C.G.: conceptualization. C.H.L., P.Y. and J.X.X.: data curation and methodology. P.Y. and J.X.X.: data analysis and visualization. C.H.L.: writing review & editing. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, H., Pan, Y., Jin, X. et al. An immune cell infiltration-related gene signature predicts prognosis for bladder cancer. Sci Rep 11, 16679 (2021). https://doi.org/10.1038/s41598-021-96373-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96373-w
- Springer Nature Limited
This article is cited by
-
Decreased TLR7 expression was associated with airway eosinophilic inflammation and lung function in asthma: evidence from machine learning approaches and experimental validation
European Journal of Medical Research (2024)
-
mRNA–miRNA bipartite networks reconstruction in different tissues of bladder cancer based on gene co-expression network analysis
Scientific Reports (2022)