Abstract
Anaplasma species, which are distributed worldwide, are gram-negative obligate intracellular tick-borne bacteria that pose a threat to human and animal health. Haemaphysalis longicornis ticks play a vital role as vectors in the transmission of Anaplasma pathogens. However, the Anaplasma species carried by H. longicornis in China are yet to be characterized. In this study, 1074 H. longicornis specimens were collected from goats in four provinces of China from 2018 to 2019 and divided into 371 sample pools. All tick sample pools were examined for the presence of Anaplasma species via nested PCR amplification of 16S ribosomal RNA, major surface protein 4 (msp4), or citric acid synthase (gltA) genes, which were sequenced to determine the molecular and phylogenetic characteristics of the isolates. The overall Anaplasma spp-positive rate of H. longicornis was determined to be 26.68% (99/371). The percentage prevalence of A. phagocytophilum-like1, A. bovis, A. ovis, A. marginale, and A. capra were 1.08% (4/371), 13.21% (49/371), 13.21% (49/371), 1.35% (5/371), and 10.24% (38/371), respectively, and the co-infection rate of two or more types of Anaplasma was 6.47% (24/371). Phylogenetic analyses led to the classification of A. phagocytophilum into an A. phagocytophilum-like1 (Anaplasma sp. Japan) group. Anaplasma bovis sequences obtained in this study were 99.8–100% identical to those of an earlier strain isolated from a Chinese tick (GenBank accession no. KP314251). Anaplasma ovis sequences showed 99.3–99.6% identity to an A. ovis human strain identified from a Cypriot patient (GenBank accession no. FJ460443). Only one msp4 sequence of A. marginale was detected and was grouped with those of other A. marginale isolates, and these A. capra isolates obtained in this present study may be zoonotic. The detection and characterization of four Anaplasma species in H. longicornis in this study have added to the current knowledge of the parasite and provided data on multiple Anaplasma species with veterinary and medical significance from four provinces of China.
Similar content being viewed by others
Introduction
The genus Anaplasma includes obligate intracellular parasitic pathogens transmitted by ticks, some of which are zoonotic and cause anaplasmosis in humans and animals1. At present, the genus mainly comprises A. phagocytophilum, A. ovis, A. bovis, A. marginale, A. platys, A. centrale, and the recently discovered A. capra2,3. Among the Anaplasma species, A. phagocytophilum not only infects neutrophil granulocytes of rodents and ruminants, such as sheep, goats, cattle and deer, but also humans4,5. Anaplasma phagocytophilum reportedly causes human granulocytic anaplasmosis (HGA) with symptoms of fever, headache, discomfort, myalgia, leukopenia, and thrombocytopenia6 and have been identified in North America, Europe, and Asia7. More recently, strains genetically related to A. phagocytophilum, i.e., A. phagocytophilum-like1: Anaplasma sp. and A. phagocytophilum-like2: Anaplasma sp., were identified in Japan and China, respectively8,9,10. Anaplasma bovis usually parasitizes monocytes and causes disease in small mammals and ruminants with symptoms of fever, weight loss, and ultimately, the possible death of cattle11. This pathogenic bacterium has mainly been identified in African and Asian countries, including Tunisia, China, and Japan1. Anaplasma ovis, a pathogen that parasitizes erythrocytes, is globally distributed and considered the most common cause of anaplasmosis in small ruminants, inducing fever, fatigue, anorexia, reduced milk production, and abortion, although related mortality rates are relatively low12. In addition, A. ovis is a potential human pathogen, and so far the only known human anaplasmosis case associated with this species was identified in 2007 in a Cypriot patient, who presented with fever, lymphadenopathy, and hepatosplenomegaly6. Anaplasma marginale are erythrocytic parasites causing bovine anaplasmosis that are transmitted by Ixodes sp., Dermacentor sp., Rhipicephalus sp., and Haemaphysalis sp., ticks, and clinical signs may include fever, weight loss, abortion, lethargy, and icterus13,14,15. Recently discovered in China, Anaplasma capra is a novel tick-transmitted pathogen, and its vectors and target cell types remain to be elucidated3. The pathogen infects several ruminants, such as goats, sheep, and deer (Hydropotes inermis argyropus), as well as humans16.
Haemaphysalis longicornis is distributed throughout China, with reports of infection in a variety of host animals, including dogs, goats, cattle, and sheep17. Some pathogens have been detected in H. longicornis, for instance, Anaplasma spp., Rickettsia conorii, Babesia ovata, and Ehrlichia canis18,19. However, limited information is available about Anaplasma app in H. longicornis. The main goal of the study was to identify the Anaplasma species carried by H. longicornis in China with a view to generating further information and enriching the available data on these pathogens, which may provide the basis for prevention and control strategies.
Materials and methods
Tick collection and identification
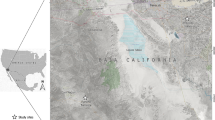
Our study was conducted at four localities in China (Fig. 1). Shaanxi (34° 40′ N; 107° 27′ E), Shanxi (34° 49′N, 111° 15′ E), and Henan (34° 20′ N; 111° 48′ E) have warm temperate climates with 500–800 mm average annual precipitation, while Guizhou (26° 44′ N; 106° 27′ E) has a subtropical humid and mild climate and average annual precipitation of 1129 mm. Between 2018 and 2019, 39, 3, 4 and 36 goats were randomly selected from Shaanxi, Shanxi, Henan and Guizhou, respectively. Tweezers were used to collect 10–15 adult ticks from the ears, face, and neck of each goat (once per goat) and were placed in a 10-mL centrifuge tube and stored in 70% ethanol until identification. Informed consent was obtained from the animal owners for the collection of tick sample. Family, genus, developmental stage, and species of all collected ticks were identified based on morphology20,21. Tick identity was further confirmed by analyzing 400 bp of the 16S rRNA gene using 16S − 1 and 16S + 1 primers22. All the ticks collected from goats were grouped by species, sex, and sampling site into pools23 and the 1074 H. longicornis specimens were divided into 371 sample pools. All sample pools were individually placed into tubes containing 70% ethanol and stored at − 4 °C prior to DNA extraction.
Geographic map of the sampling locations of China. The figure was originally designed by the authors under the software ArcGIS 10.2. The original vector diagram imported in ArcGIS was adapted from Natural Earth (http://www.naturalearthdata.com).
DNA extraction
First, ticks were washed sequentially with 30%, 50%, and 70% absolute ethanol and distilled water for 5 min each time and dried on sterile filter paper. Next, they were ground in a grinder with liquid nitrogen. DNA was extracted according to the manufacturer’s instructions for the Universal Genomic DNA Kit (CwBio, Beijing, China) and eluted in a final volume of 100 μL. Extracted DNA was stored at − 20 °C until experimental use.
PCR amplification
To assess whether the H. longicornis specimens were infected with Anaplasma species, nested PCR assays targeting the 16S rRNA gene of A. bovis, A. phagocytophilum and related strains, the major surface protein 4 (msp4) gene of A. ovis and A. marginale, and the citric acid synthase (gltA) gene of A. capra were performed for all H. longicornis sample pools. The first PCR reaction system contained 4 µL dNTP mixture (2.5 mmol L−1), 2.5 μL 10 × LA PCR Buffer (Mg2+ Plus), 0.5 μL forward and reverse primers (20 pmol each), 1.25 U LA Taq DNA Polymerase (5 U μL−1) (TaKaRa, Dalian, China), 1 μL raw DNA template, and 16.25 µL distilled water. The final amplification reaction was conducted in a 25 μL volume containing 2.0 μL PCR products, 0.75 U Taq DNA polymerase (5 U μL−1) (TaKaRa, Dalian, China), 2.5 μL 10 × PCR buffer (Mg2+ Plus), 2.0 μL dNTPs (concentration of 2.5 mM), 0.5 μL primers (20 pmol each), and 17.35 μL distilled water. All PCR reactions were conducted using an ABI 2720 thermal cycler instrument (Life Technologies Holdings Pte Ltd., Singapore). The primers and amplification conditions used are listed in Table 1. In each PCR assay, DNA samples that had been sequenced and kept in the laboratory from sheep positive for A. phagocytophilum, A. bovis, A. ovis, A. capra and cattle positive for A. marginale, were run as the positive controls and distilled water as the negative control. PCR products (5 μL) were separated via electrophoresis on a 1.0% agarose gel and visualized via UV transillumination following ethidium bromide staining.
Sequence and phylogenetic analyses
PCR products were sequenced by a commercial company (TSINGKE, Beijing, China). Sequence accuracy was verified via bidirectional sequencing, and sequences were identified and analyzed using BLASTN (http://www.ncbi.nlm.nih.gov/BLAST) and CLUSTALW 2.0.10 (https://www.ebi.ac.uk/Tools/msa/clustalo/) programs. To ascertain the phylogenetic placement of Anaplasma spp. identified in this study, a phylogenetic tree was constructed based on the sequence distance method using the Maximum likelihood method with the best evolutionary model of MEGA 7.0 (http://www.megasoftware.net). Confidence values for each branch of the resulting tree were confirmed by bootstrap analysis with 1000 replicates.
Statistical analysis
The infection rates of Anaplasma in sample pools of H. longicornis from different sites and different sexes were compared using the chi-square test in SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA). Data were considered significant at P < 0.05.
Nucleotide sequence accession numbers
The GenBank accession numbers obtained in this study were as follows: MN097866 and MN097867 for A. phagocytophilum-like1, MK991952 to MK991955 for A. bovis, MK991961 to MK991963 for A. ovis, MW772454 for A. marginale, MK991956 to MK991960 for A. capra, and the accession numbers MN956525 to MN956527 for H. longicornis.
Ethics statement
This study was carried out in accordance with the Chinese Laboratory Animal Administration Act (1988) after it was reviewed and its protocol was approved by the Research Ethics Committee of Henan Agricultural University. Appropriate permission was gained from the animal owners before the collection of ticks.
Results
Rates of positivity for Anaplasma species
All ticks taken from goats were identified as H. longicornis. As shown in Table 2, A. phagocytophilum-like1, A. bovis, A. bovis, A. marginale, and A. capra were detected in H. longicornis collected from Shaanxi, Shanxi, Guizhou, and Henan provinces. From the 371 sample pools tested, 99 (26.68%) were positive for Anaplasma species. The infection percentages of A. phagocytophilum-like1, A. bovis, A. ovis, A. marginale, and A. capra were 1.08% (4/371), 13.21% (49/371), 13.21% (49/371), 1.35% (5/371), and 10.24% (38/371), respectively. Anaplasma infection percentages in H. longicornis collected at different sampling sites varied from 0% (0/9) to 43.59% (68/156) and were significantly different between sites (P < 0.05).
We additionally observed a significant difference in Anaplasma infection between the sexes (0.01 < P < 0.05), specifically, 25.31% (61/241) and 36.92% (48/130) in female and male ticks, respectively. Moreover, the 13.21% infection rates determined for A. bovis and A. ovis were significantly higher than those for A. phagocytophilum-like1 (1.08%) (0.01 < P < 0.05) and A. marginale (1.35%) (0.01 < P < 0.05) but only slightly higher than that of A. capra (10.24%) (P > 0.05). As shown in Table 3, 24 samples were infected with two or more types of Anaplasma concurrently, and the co-infection rate of Anaplasma was 6.47% (24/371).
Phylogenetic analysis
Phylogenetic analysis was performed by aligning sequences obtained in this study with sequences available in GenBank from selected ticks and Anaplasma spp. isolated from ticks, ruminants and humans. The three H. longicornis 16S rRNA sequences displayed 98.5–99.8% identity to each other and showed 98.8–100% identity to H. longicornis collected in China (MH024508).
The genotypes (MN097866, MN097867) obtained in this study were classified as strains genetically related to the A. phagocytophilum (Anaplasma sp. Japan) cluster (Fig. 2). Sequencing the 16S rRNA gene showed that the two strains from H. longicornis, which were genetically related to A. phagocytophilum, displayed 98.7% identity to each other. The 16S rRNA gene sequences from the four A. bovis found in H. longicornis displayed 99.6–100% identity to each other and 99.8–100% identity to a tick strain (KP314251) found in China. Furthermore, they belonged to the same clade as isolates from Chinese cattle, giraffe (Giraffa camelopardalis giraffa), and tick (KF055358, KU870666, KP314251, respectively) (Fig. 2).
Phylogenetic relationship between 16S rRNA gene sequences of Anaplasma spp. Phylogenetic tree constructed based on partial 16S rRNA gene sequences of A. phagocytophilum and A. bovis using the Maximum likelihood method with the best evolutionary model and bootstrap analysis of 1000 replicates. The A. phagocytophilum like strain and A. bovis sequences obtained in this study are indicated by black diamonds and Black triangles, respectively. Values lower than 50% are hidden.
The three A. ovis msp4 sequences were 98.9–99.3% identical to each other and 99.3–99.6% identical to a human A. ovis strain from Cyprus (FJ460443) grouped within the same clade (Fig. 3). The only msp4 sequence of A. marginale identified had a homology of 98.9–99.9% with other A. marginale isolates and was therefore placed in the same group (Fig. 3). The five A. capra gltA sequences identified in H. longicornis were 98.4–100% identical to a human isolate from China (KM206274). Phylogenetic analysis showed the genotypes obtained in this study were classified in A. capra cluster. (Fig. 4).
Phylogenetic relationships between gene sequences of Anaplasma spp. Phylogenetic tree constructed based on partial msp4 gene sequences of A. ovis and A. marginale using the Maximum likelihood method with the best evolutionary model and bootstrap analysis of 1000 replicates. The A. ovis and A. marginale sequences obtained in this study are indicated by black squares and Black triangle, respectively. Values lower than 50% are hidden.
Phylogenetic relationships between gltA gene sequences of Anaplasma spp. Phylogenetic tree based on partial gltA gene sequences of A. capra by using the Maximum likelihood method with the best evolutionary model and bootstrap analysis of 1000 replicates. Black circles indicate the sequences obtained in this study.
Discussion
Previous studies have demonstrated that different kinds of ticks, such as Haemaphysalis qinghaiensis, Dermacentor silvarum, D. nuttalli and so on, existed different degrees of infection of Anaplasma pathogen29,30. However, a limited number of investigations into these pathogens and their mechanisms of infection have been conducted in China. In this study, we identified and characterized five Anaplasma species that may present a potential threat to human and animal health, A. phagocytophilum, A. bovis, A. ovis, A. marginale, and A. capra, isolated from H. longicornis sampled in four provinces of China.
In recent years, two variants genetically related to A. phagocytophilum have been independently detected in surveys undertaken in Asia. For instance, A. phagocytophilum-like 1 (Anaplasma sp.-Japan) has been discovered in sika deer (Cervus nippon yesoensis), cattle, and ticks infesting ruminants in Japan (Ixodes persulcatus, I. ovatus, Hyalomma megaspinosa)16,31. while A. phagocytophilum-like 2 (Anaplasma sp.-China) has been recently detected in cattle, goats, and H. asiaticum ticks infesting ruminants in China9,32,33. In this study, A. phagocytophilum-like1 (Anaplasma sp.-Japan) has been identified in H. longicornis (Fig. 2), and the H. longicornis infection percentages of this variant in Henan, Shaanxi, Shanxi, and Guizhou provinces were determined to be 3.2% (1/31), 0.58% (1/171), 11.1% (1/9), and 0.65% (1/155), respectively. This suggests that H. longicornis may be a common transmission medium for A. phagocytophilum-like strains in these four Chinese provinces. Therefore, in the future, further studies into the distribution of A. phagocytophilum and A. phagocytophilum-like strains in tick species and infested ruminants or other animals should be carried out in parts of China.
Anaplasma bovis, which was first reported in cattle, has been shown to infect circulating monocytes and tissue macrophages34,35. To date, A. bovis has been isolated from dogs, sheep, goats, and wild deer (Cervus nippon nippon) in addition to vector ticks, including H. longicornis, Rhipicephalus appendiculatus, and H. qinghaiensis, indicating they have a broad host range30,36,37. The A. bovis infection rate in H. longicornis was 13.39% in our study, and related studies have also been detected in Japan (12.0%), Korea (0.4%), Shenyang (0.6%), Heilongjiang province (0.7%)25,38,39,40. These findings signify that H. longicornis may be a vector for A. bovis, both in China and many other parts of the world. Moreover, the A. bovis infection incidence of H. longicornis ticks was significantly higher in Guizhou than the other sampling sites (P < 0.05), possibly due to the different geographical conditions. Guizhou, where Anaplasma bacteria are widespread, is the natural focal area of anaplasmosis41; moreover, it is a subtropical region with rich vegetation, which is suitable for a variety of host animals of Anaplasma. However, there was no A. bovis DNA in ticks from Shanxi and Henan in the present study. The putative absence of A. bovis in ticks at the two sampling localities is probably a reflection of the small numbers of ticks tested. The A. bovis isolates obtained in this study were 99.8–100% identical to other isolates from different hosts in other parts of China, and they were located on the same clade (Fig. 2). Anaplasma bovis has experienced genetic stability and the absence of geographical and host isolation in China based on 16S rRNA gene, which is consistent with the earlier report of Yang et al.42. Therefore, more experiments should be performed to explore the geographical and host isolation of A. bovis.
Anaplasma ovis is widely distributed in North America, Asia, Africa, and Europe and has been identified in sheep, goats, Dermacentor abaensis, and Haemaphysalis tibetensis43,44. However, there have been no reports of A. ovis being carried by H. longicornis documented to date. In Shaanxi, Shanxi, Guizhou, and Henan, the overall A. ovis infection percentage of H. longicornis was determined to be 13.9%, which is higher than that of H. qinghaiensis in Qinghai (4.0%)30. In addition, A. ovis was detected at all sampling sites except Shanxi, which may be attributable to the different geographical environment or limited number of samples. In the current study, four msp4 gene sequences of A. ovis were identified in H. longicornis ticks. And these four strains also showed high similarities to those isolates previously obtained from China, Portugal, Serbia, Cyprus and Iran, indicating low diversity of A. ovis in the study ticks. Furthermore, A. ovis isolates of H. longicornis in this investigation were closely related to a human isolate from Cyprus (FJ460443), suggesting this pathogen may post a threat to public health (Fig. 3).
It has been documented that ticks of Rhipicephalus sp. and Haemaphysalis sp. are responsible for the transmission of A. marginale to a variety of vertebrate hosts45. The prevalence of A. marginale in H. longicornis in the present study was 1.35% (5/371), and the detection result is consistent with previous reports to a certain extent. Even though A. marginale populations worldwide typically infect cattle, causing bovine anaplasmosis, and have a significant economic impact on the cattle industry46, the species has also been detected in sheep47 and goats48,49. Because the ticks in this study were collected from goats, it is necessary to explore the presence of A. marginale in goats and other animals at the sampling sites.
Anaplasma capra is an emerging zoonotic pathogen that has been detected in sheep, goats, H. qinghaiensis, I. persulcatus, and humans16,30. In this study, 10.38% of H. longicornis individuals were positive for A. capra, which is a higher percentage than found for A. capra infection of H. longicornis ticks in Qinghai (0.03%), Korea (0.03%), and Shandong (0.84%)30,39,50. These discrepancies may be attributed to the variations in climate, vegetation, hosts, tick activity, time of sampling, and detection methods. The A. capra gltA gene isolated in our experiments exhibited high genetic diversity relative to A. capra isolated (KR261626, KX417324, and KM206274) in earlier surveys. When we phylogenetically analyzed the A. capra isolates based on the gltA gene, the all sequences of A. capra fell into two clades. Additionally, A. capra isolates obtained in this study and other A. capra sequences isolated from ticks and ruminants available in the GenBank fell into the same clade with isolate from human. Therefore, these A. capra isolates obtained in this present study may be zoonotic (Fig. 4).
Our study had a number of limitations that should be acknowledged. While the results highlight the current status of Anaplasma infections in H. longicornis in four provinces of China, investigations in other parts of China have yet to be conducted. Moreover, ticks were not collected from the environment and other hosts, and tick larvae and nymphs were not used in the experiments. Although the present study has revealed the current status of H. longicornis tick infestation with Anaplasma spp. in the investigated areas, the specific biological vector for the individual Anaplasma species need to be further studied by transmission experiments. In addition, the infections of Anaplasma species in different hosts (including animals and humans) and regions should be investigated to understand the true impact of anaplasmosis.
Conclusion
Our investigation revealed a prevalence of A. phagocytophilum-like 1 (Anaplasma sp.-Japan), A. bovis, A. ovis, A. marginale and A. capra in H. longicornis from four provinces in China. These findings add to the existing body of knowledge on the public health risk of H. longicornis carrying Anaplasma pathogens and may provide a basis for strategies to prevent and control anaplasmosis.
References
Yang, B. H. et al. Molecular evidence of coinfection of Anaplasma species in small ruminants from Anhui Province, China. Parasitol. Int. 71, 143–146 (2019).
Rar, V. et al. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 11(8), 1842–1861 (2011).
Yang, J. F. et al. A novel genotype of “Anaplasma capra” in wildlife and its phylogenetic relationship with the human genotypes. Emerg. Microbes Infect. 7(1), 210–213 (2018).
Zhan, L. et al. Anaplasma phagocytophilum from rodents and sheep, China. Emerg. Infect. Dis. 15(6), 764–768 (2010).
Zhang, L. J. et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA 300, 2263–2270 (2008).
Li, H. et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 15(6), 663–670 (2015).
Dugat, T. et al. Opening the black box of Anaplasma phagocytophilum diversity: Current situation and future perspectives. Front. Cell Infect. Microbiol. 5, 61. https://doi.org/10.3389/fcimb.2015.00061 (2015).
Said, M. B. et al. Molecular typing and diagnosis of Anaplasma spp. closely related to Anaplasma phagocytophilum in ruminants from Tunisia. Ticks Tick Borne Dis. 8(3), 412–422 (2017).
Kang, Y. J. et al. Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol. Biol. 14, 167. https://doi.org/10.1186/s12862-014-0167-2 (2014).
Said, M. B. et al. Molecular survey of anaplasma species in small ruminants reveals the presence of novel strains closely related to A. phagocytophilum in Tunisia. Vector Borne Zoonotic Dis. 15(10), 580–590 (2015).
Chilton, N. B. et al. Prevalence of Anaplasma bovis in Canadian populations of the Rocky Mountain wood tick, Dermacentor andersoni. Ticks Tick Borne Dis. 9(6), 1528–1531 (2018).
Cabezas-Cruz, A. et al. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasit. Vectors. 12(1), 3. https://doi.org/10.1186/s13071-018-3269-7 (2019).
Al-Hosary, A. et al. Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasites Vectors. 13(1), 495. https://doi.org/10.1186/s13071-020-04372-z (2020).
Aubry, P. & Geale, D. A review of Bovine anaplasmosis. Transbound Emerg. Dis. 58(1), 1–30 (2011).
Ashraf, S. et al. A report on molecular detection and phylogenetic evaluation of Anaplasma marginale in ticks and blood samples collected from cattle in District Layyah in Punjab (Pakistan). Curr. Microbiol. 78, 1–8 (2020).
Seo, M. et al. Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma capra, an emerging tick-borne zoonotic pathogen. Vet. Microbiol. 226, 15–22 (2018).
Niu, Q. et al. Genetic diversity and molecular characterization of Babesia motasi-like in small ruminants and ixodid ticks from China. Infect. Genet. Evol. 41, 8–15 (2016).
Li, Z. et al. Molecular characterization of hard tick Haemaphysalis longicornis from China by sequences of the internal transcribed spacers of ribosomal DNA. Exp. Appl. Acarol. 74(2), 171–176 (2018).
Li, Z. B. et al. Mitochondrial gene heterogeneity and population genetics of Haemaphysalis longicornis (Acari Ixodidae) in China. Acta Parasitol. 64, 360–366 (2019).
Deng, G. F. Economic Insect Fauna of China, Acari: Ixodidate. M. 39 (Science Press, 1992).
Chen, Z. et al. Illustrated keys to families and genera of the superfamily Ixodoidea under new taxonomic system. Chin. J. Jarasitol. Parasit. Dis. 29, 302–304 (2011).
Krakowetz, C. et al. Genetic diversity in Ixodes scapularis (Acari Ixodidae) from six established populations in Canada. Ticks Tick Borne Dis. 2, 143–150 (2011).
Mwamuye, M. M. et al. Novel Rickettsia and emergent tick-borne pathogens A molecular survey of ticks and tick-borne pathogens in Shimba Hills National Reserve, Kenya. Ticks Tick Borne Dis. 8(2), 208–218 (2017).
Barlough, J. et al. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus). Vet. Parasitol. 63, 319–329 (1996).
Kawahara, M. et al. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 72(2), 1102–1109 (2006).
Wang, G. Q. Detection and Quantification of Anaplasma DNA by Nested-PCR and Real-Time PCR. (Huazhong Agricultural University, 2007).
Fuente, J. D. L. et al. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector Borne Zoonotic Dis. 5, 390–401 (2005).
Yang, J. F. et al. A novel zoonotic Anaplasma species is prevalent in small ruminants: Potential public health implications. Parasit. Vectors. 10, 264. https://doi.org/10.1186/s13071-017-2182-9 (2017).
Tenquist, J. et al. A revision of the annotated checklist of ectoparasites of terrestrial mammals in New Zealand. J. R. Soc. N. Zeal. 31(3), 481–542 (2001).
Han, R. et al. Molecular detection of Anaplasma infections in ixodid ticks from the Qinghai-Tibet Plateau. Infect. Dis. Poverty. 8(1), 83–90 (2019).
Ybanez, A. et al. Molecular analyses of a potentially novel Anaplasma species closely related to Anaplasma phagocytophilum detected in sika deer (Cervus nippon yesoensis) in Japan. Vet. Microbiol. 157(1–2), 232–236 (2012).
Yan, Y. Q. et al. Molecular detection of Anaplasma spp. in dairy cattle in southern Xinjiang, China. Vet. Parasitol. Reg. Stud. Rep. 20, 100406. https://doi.org/10.1016/j.vprsr.2020.100406 (2020).
Wang, K. L. et al. Seasonal dynamics of Anaplasma spp. in goats in warm-temperate zone of China. Ticks Tick Borne Dis. 12(3), 101673. https://doi.org/10.1016/j.ttbdis.2021.101673 (2021).
Worthington, R. et al. A review of the infectious diseases of African wild ruminants. Onderstepoort. J. Vet. Res. 68(4), 291–323 (2001).
Sreekumar, C. et al. Morphology and staining characteristics of Ehrlichia bovis. Comp. Immunol. Microbiol. Infect. Dis. 19(1), 79–83 (1996).
Qin, X. R. et al. Anaplasma species detected in Haemaphysalis longicornis tick from China. Ticks Tick Borne Dis. 9(4), 840–843 (2018).
Shpynov, S. et al. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the asiatic part of Russia. Ann. N. Y. Acad. Sci. 1078, 378–383 (2006).
Dong, X. et al. Co-circulation of multiple species of Rickettsiales bacteria in one single species of hard ticks in Shenyang, China. Ticks Tick Borne Dis. 5(6), 727–733 (2014).
Oh, J. et al. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. J. Bacteriol. Virol. 39(4), 257–267 (2009).
Wei, F. et al. Molecular detection and characterization of zoonotic and veterinary pathogens in ticks from Northeastern China. Front. Microbiol. 7, 1913 (2016).
Liu, Z. et al. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 78(2), 464–470 (2012).
Yang, J. et al. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, Northwest China. Parasit. Vectors. 8, 108. https://doi.org/10.1186/s13071-015-0727-3 (2015).
Han, R. et al. Characterization of Anaplasma ovis strains using the major surface protein 1a repeat sequences. Parasit. Vectors. 10(1), 447. https://doi.org/10.1186/s13071-017-2363-6 (2017).
Yin, H. & Luo, J. Ticks of small ruminants in China. Parasitol. Res. 101(Suppl 2), S187-189 (2007).
Léger, E. et al. Changing distributions of ticks: Causes and consequences. Exp. Appl. Acarol. 59(1–2), 219–244 (2013).
Kocan, K. et al. The natural history of Anaplasma marginale. Vet. Parasitol. 167(2–4), 95–107 (2010).
Yousefi, A. et al. Molecular detection of Anaplasma marginale and Anaplasma ovis in sheep and goat in west highland pasture of Iran. Asian Pac. J. Trop. Biomed. 7(5), 455–459 (2017).
Da Silva, N. et al. First report of Anaplasma marginale infection in goats, Brazil. PLoS ONE 13(8), e0202140. https://doi.org/10.1371/journal.pone.0202140 (2018).
Barbosa, I. et al. Anaplasma marginale in goats from a multispecies grazing system in northeastern Brazil. Ticks Tick Borne Dis. 12(1), 101592. https://doi.org/10.1016/j.ttbdis.2020.101592 (2021).
Zhang, H. et al. Prevalence of severe febrile and thrombocytopenic syndrome virus, Anaplasma spp. and Babesia microti in Hard Ticks (Acari: Ixodidae) from Jiaodong Peninsula, Shandong Province. Can. J. Infect. Dis. Med. Microbiol. 17(2), 134–140 (2017).
Acknowledgements
We thank Suzanne Leech, Ph.D., from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the Earmarked Fund for China Modern Agro-industry Technology Research System (nycytx-38) and the Key Scientific and Technological Project of Henan Province (202102110104).
Author information
Authors and Affiliations
Contributions
Conception and design: C.S.N and L.X.Z. Acquisition of data: Y.Q.Y., K.L.W., Y.C.Z., S.S.Z and Y.J.Z. Analysis and Interpretation of data: Y.Q.Y., Y.Y.C., F.C.J., and R.J.W. Drafting the article: Y.Q.Y., K.L.W. and C.S.N. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, Y., Wang, K., Cui, Y. et al. Molecular detection and phylogenetic analyses of Anaplasma spp. in Haemaphysalis longicornis from goats in four provinces of China. Sci Rep 11, 14155 (2021). https://doi.org/10.1038/s41598-021-93629-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93629-3
- Springer Nature Limited
This article is cited by
-
Prevalence and genetic diversity of Anaplasma and Ehrlichia in ticks and domesticated animals in Suizhou County, Hubei Province, China
Scientific Reports (2024)
-
Exploring the relationship between flumethrin resistance and Anaplasma marginale infection in Rhipicephalus (Boophilus) microplus ticks of cattle
Tropical Animal Health and Production (2024)
-
Anaplasma capra: a new emerging tick-borne zoonotic pathogen
Veterinary Research Communications (2024)