Abstract
Crop rotation is an important management tactic that farmers use to manage crop production and reduce pests and diseases. Long-term crop rotations may select groups of microbes that form beneficial or pathogenic associations with the following crops, which could explain observed crop yield differences with different crop sequences. To test this hypothesis, we used two locations each with four long-term (12–14-year), replicated, rotation treatments: continuous corn (CCC), corn/corn/soybean (SCC), corn/soybean (CSC), and soybean/corn (SCS). Afterwards, soybean was planted, and yield and soil health indicators, bulk soil microbiome, and soybean root-associated microbiome were assessed. Soybean yields, as well as soil protein, and POXC as soil health indicators were higher following CCC than in the other three treatments at both locations. A bacterial taxon in family JG30-KF-AS9 was enriched in CCC, whereas Microvirga, Rhodomicrobium, and Micromonosporaceae were enriched in SCS. Several ascomycetes explain lowered yield as soybean pathogens in SCS. Surprisingly, Tumularia, Pyrenochaetopsis and Schizothecium were enriched in soybean roots after CCC, suggesting corn pathogens colonizing soybean roots as nonpathogens. Our finding of associations between soil health indicators related to microbiomes and soybean yield has wide-ranging implications, opening the possibility of manipulating microbiomes to improve crop yield potential.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Both the composition and function of microbial communities can be substantially manipulated by management tactics for “smart farming”1. Cumulative management effects can be identified by long-term experiments which help to identify problems that threaten future productivity as an early warning system2, explain the reasons behind existing agricultural production problems3, and assist in formulating solutions. Additionally, it is important to understand the cumulative effects of enduring management strategies in order to sustain optimum soil properties4, specifically the effects on microbial communities and soil health as a result of crop rotation sequences.
Shifts in plant or soil-associated microbial communities are driven by a myriad array of legacy and emerging factors such as plant genetics, soil chemical/physical properties and environmental conditions or soil processes5. Because the microbiome is an integral part of almost all soil processes6, the structure of the microbial communities associated with soil and plants can be directly affected by management strategies such as crop rotation sequences7. In the Midwest U.S., corn (Zea mays) and soybean (Glycine max) cover about 75% of acres used to grow crops8 and are commonly grown in rotation, which generally improves yields of both crops. However, shifts in the resulting microbial communities due to this crop rotation and variations in crop sequencing are unclear, and may explain yield differences as well as provide new knowledge for future yield improvements.
We selected three of the most biologically-oriented, dynamic soil health indicators that have been recently recommended for routine use in agricultural soils by United States Department of Agriculture Natural Resources Conservation Service9. These three measurements described below, represent the top recommendations for indicators of organic C availability, organic N availability, and general organic decomposition activities9. The amount of permanganate-oxidizable carbon (POXC) represents the labile portion of the organic C which is the most reactive and dynamic driver of C mineralization within the pool of soil organic C (SOC)10,11. Labile organic C as measured by POXC has been directly associated with soil C and N mineralization12, and may promote plant productivity due to its positive influence on soil activities and nutrient availability13. Positive correlations have been found between POXC and soil-microbial parameters, comprising microbial biomass and, in particular organic C10,14. Therefore, POXC is the recommended method for C food source of microbes. A second soil health indicator, the autoclaved citrate extractable (ACE) protein content, refers to bioavailable N in the soil organic matter (SOM)15. The largest organic N pool in the soil is represented by proteins16,17,18. The labile organic N pool is used to evaluate soils’ capacity to provide N19 by promoting N mineralization in soils20. Regarding plant growth and development, N mineralization is a critical process in the soil to provide an adequate amount of N for the use of the plant21. Since protein content is an indicator of biological and chemical soil health, especially for SOM quality, it is directly linked to general soil health status22. Soil protein includes an N-linked glycoprotein, glomalin, which is produced by arbuscular mycorrhizal fungi hyphae23,24. Glomalin has been reported to enhance soil structure, drainage, microbial activity, and C sequestration in soil ecosystems25 and is sensitive to crop rotation and tillage22,26,27,28. A third soil health indicator, β-glucosidase, is an enzyme which plays a central role in the C cycle in soil29 and serves as an important indicator of general microbial activity30. In terms of the C cycle, the importance of soil microorganisms in many ecosystems hinges on breaking down cellulose in plant cell walls31; cellulose is one of most common organic compounds in the biosphere32. β-glucosidase, which has a role in the final stage of cellulose degradation in soils, supplies important energy sources, like simple sugar, for microorganisms33. A variety of microorganisms are involved in β-glucosidase activity in soils including filamentous fungi34,35,36,37,38,39,40,41,42, yeast43, and bacteria44,45.
The lack of strong correlations between rotation-induced crop yield differences and soil chemical and physical properties suggest that soil-associated and plant-associated microbiomes could be determinants for these differences4,46. Since soil bacteria and fungi directly mediate the C and N cycles, and regulate the nutrient availability for plants, soil health indicators are expected to be correlated with members of the soil microbiome. We hypothesized that crop yield differences that result from crop rotation would correspond to soil health indicators and soil or root-associated microbiome. To test this hypothesis, we used two sites, each with three replicated, long-term (12–14 year) crop rotations: continuous corn (CCC), corn/corn/soybean (SCC), and corn/soybean (CSC), with each phase of CCS and CSC present each year. In total, there were 4 CCS cycles, 6 SC cycles, and 12 CCC cycles, but corn was in place 2 years prior to the other rotation schemes were fully established. Soil samples were taken right before the soybean was planted the sampling year. In Year 15 (the sampling year) when the continuous corn had been in place for 14 years, soybean was planted in all plots, producing treatments with 14 (CCC), 2 (SCC), and 1 (CSC) year of corn, and of one year (SCS) of soybean as the previous crop. Samples were collected for analyses of soil and root-associated microbiome, soil health indicators, and soybean yield.
Results
Soybean yield and soil health indicators
Data for soybean yield, soil protein, POXC, and β-glucosidase are summarized in Table 1. At both sites there were significant differences in soybean yield following the long-term crop sequences of CCC, SCC, CSC, and SCS. Soybean yields from CCC (5614 and 5256 kg ha−1 at Urbana and Monmouth, respectively) were significantly higher than SCC, CSC, and SCS at both sites. At Urbana, SCC (with two previous years of corn) yielded more than SCS, with the previous crop of soybean. At the Monmouth site, there were no significant differences among the SCC, CSC, and SCS treatments, but yields trended lower as the proportion of corn crops prior to the soybean crop decreased, and were lowest where soybean was the previous crop. Across both sites, the rotations with a higher frequency of corn (CCC, SCC) produced higher yields of soybean than those with corn in alternate years (CSC, SCS).
Soils in the CCC treatment had higher protein content compared with other crop sequences at Urbana. The CCC (7360 mg per kg soil) treatment was significantly greater than SCC (5627 mg per kg soil), CSC (5417 mg per kg soil) and SCS (5613 mg per kg soil) treatments (p < 0.05). There were no significant differences among the SCC, CSC and SCS treatments. At the Monmouth location, we found no significant differences in protein content among the CCC (5,842 mg per kg soil), SCC (5426 mg per kg soil), CSC (5453 mg per kg soil) and SCS (5,469 mg per kg soil) crop sequences, although CCC had the highest (numerically) average protein.
Soil β-glucosidase enzyme activity was significantly higher in SCS (Urbana: 1.19 and Monmouth: 1.73 mg p-nitrophenol per g of soil) treatments compared with other rotations (p < 0.05) at both sites. We found no differences among the CCC (0.93 mg p-nitrophenol per g soil), SCC (0.91 mg p-nitrophenol per g of soil) and CSC (0.87 mg p-nitrophenol per g of dried soil) rotations at the Urbana site. At Monmouth, SCS (1.73 mg p-nitrophenol per g of soil) and CSC (1.73 mg p-nitrophenol per g of soil) had more β-glucosidase enzyme activity than CCC (1.51 mg p-nitrophenol per g of soil) and SCC (1.24 mg p-nitrophenol per g of soil). Distribution analysis showed that soil β-glucosidase activity values were normally distributed.
After 12–14 years of the above-mentioned crop rotation regimes, we found that at Monmouth site POXC was significantly greater in the CCC (816 mg C per kg soil) and SCC (734 mg C per kg soil) (P < 0.001) treatments than in the CSC and SCS treatments (Table 1). There was no difference in POXC between CSC (606 mg C per kg soil) and SCS (585 mg C per kg soil) treatments. At the Urbana site, POXC was significantly higher in CCC (647 mg C per kg soil) and SCC (777 mg C per kg soil) compared to the SCS (562 mg C per kg soil) and CSC (490 mg C per kg soil) treatments (P < 0.05). Differences between the CCC and SCC, and among the CCC, CSC, and SCS treatments were not significant.
Analysis across sites with site treated as a random effect, there were no significant differences in soybean yield, soil protein and POXC between the Urbana and Monmouth locations for the same treatments. However, β-glucosidase enzyme analysis was significantly different for the same treatments at the two locations.
Bulk soil microbiome
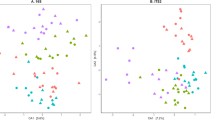
Based on ANCOM results in the bulk soil data from Monmouth, an uncultured bacterium belonging to the order of JG30-KF-AS9 within the Chloroflexi phylum had a descending relative abundance order of CCC > SCC > CSC > SCS (Fig. 1A). In the fungal community in the bulk soil from the same site, the relative abundance of Ascomycota effectively discriminated among the four crop sequences. Specifically, the Macrophomina genus was detected as the most abundant in the SCS crop sequence with a decreasing order of abundance as SCS > SCC > CSC > CCC (Fig. 1B). The relative abundance of the genus of Corynespora was significantly higher in the SCS rotation with a decreasing relative abundance in the order of SCS > SCC > CSC > CCC rotations. The genus Mycoarthris was more abundant in the SCC and CCC rotation groups; and less abundant in the two-year rotation treatments CSC and SCS.
Differential abundance of taxa analyzed by ANCOM approach. Box-and-whisker plots of bacterial (A) and fungal (B) abundance at the Monmouth site, and bacterial (C) and fungal (D) abundance at the Urbana site associated with bulk soil; bacterial (E) and fungal (F) abundance at the Monmouth site, and bacterial (G) and fungal (H) abundance at the Urbana site associated with roots are shown with relative abundance distributions for the taxa that varied significantly among rotation treatments.
In the bulk soil from the Urbana site, the bacterial genus Microvirga, belonging to family Beijerincklaceae under class Alphaproteobacteria, was an informative taxon distinguishing the four treatments, with higher abundance in low-yield rotation groups (CSC and SCS) compared to high-yield (CCC and SCC) rotation groups (Fig. 1C). An uncultured fungus belonging to family Corynesporascaceae in order Pleosporales under class Dothideomycetes in the phylum of Ascomycota was found significantly different in relative abundance separating the four crop sequences, with the order of SCS > CSC > CCC > SCC at the Urbana site (Fig. 1D). From the same order, a taxon under family Pleomassariaceae and genus Tumularia has been found significantly different in relative abundance in decreasing order of CCC > SCC > CSC > SCS. Two additional taxa from genus Clonostachys and Idriella under class Sordariomycetes and phylum Ascomycota were found to be significantly different in terms of relative abundance. Clonostachys was found to be in a decreasing order of SCS > SCC > CCC > CSC, while Idriella was higher in abundance in high-yield groups in the decreasing order of CCC > SCC > CSC > SCS.
Root-associated microbiome
The most informative root-associated bacterial genus at the Monmouth site was Rhodomicrobium under class Alphaproteobacteria which has significantly different in relative abundance among the four crop sequences in the order of SCC > SCS > CSC > CCC (Fig. 1E). For fungal community from the same site, three taxa all belonging to phylum Ascomyota under order Pleosporales (genus Pyrenochaetopsis), Leotiomycetes (genus unknown) and Sordariales (genus Schizothecium) were found to be significantly different in relative abundance with the order of CCC > SCC > CSC > SCS, SCS > CSC > CCC > SCC and CCC > CSC > SCC > SCS, respectively (Fig. 1F). At the Urbana site, a root associated bacterium that was differentially abundant among the rotations was Micromonosporaceae under phylum Actinobacteria with the order of increasing previous crop corn frequency: SCS > CSC > SCC > CCC (Fig. 1G). A root-associated uncultured fungus belonging to family Chaetothyriaceae in order Chaetothyriales under class Eurotiomycetes in the phylum of Ascomycota was found to be the only differential fungal taxa at the Urbana site and had the greatest abundance in the SCS crop sequence and a decreasing abundance order of SCS > SCC > CSC > CCC (Fig. 1H).
Following the ANCOM analysis, differential abundance analysis of taxa between higher-yielding (CCC and SCC) and lower-yielding (CSC and SCS) soybean crops was performed using balances in Gneiss. In the bulk soil from Monmouth, members of the genus Rubrobacter were proportionally higher in the high-yield treatments and Sphingomonas were proportionally higher in the low-yield treatments (Fig. 2A). Fungi from the genus Aspergillus were proportionally higher in the low-yield rotation groups (Fig. 2B). At Urbana, bacterial genera Bradyrhizobium, Gemmatimonas, Cellulomonas, family Micrococcaceae, and several uncultured taxa were proportionally higher in high-yield rotation groups (Fig. 2C), while several fungi from genera Plectosphaerella, Tetracladium, Fusarium, Clonostachys, and Purpureocillium were present in higher proportions in low-yield rotation groups (Fig. 2D).
Differential abundance of taxa analyzed by Gneiss approach. Abundances of bacterial (A) and fungal (B) population at the Monmouth site, and bacterial (C) and fungal (D) population at the Urbana site associated with bulk soil; bacterial (E) and fungal (F) population at the Monmouth site, and bacterial (G) and fungal (H) population at the Urbana site associated with roots are shown. The differential abundance analysis was performed based on high- and low-yield groups. The high yield group is CCC (continuous corn) and SCC (two years corn). The low yield group is CSC (one year corn) and SCS (one year soybean). Figure was created using built-in function in85.
Root-associated microbiome communities from Monmouth, however, showed that Actinobacteria from the family Micromonosporacea and genus Streptomyces were proportionally higher in low-yield rotation groups and bacteria from genus Bradyrhizobium were proportionally higher in high-yield rotation groups (Fig. 2E). Fungi from the family Lasiosphaeriaceae were found in higher proportion in low-yielding rotations, including some unidentified fungi in both high- and low-yield rotation groups (Fig. 2F). At Urbana, several bacteria belonging to genus Streptomyces, family Micromonosporaceae, which are both under phylum Actinobacteria, and order Sacharimonadales were in higher proportions in low-yield treatments (Fig. 2G). Root-associated fungi from Urbana, such as genera Tausonia, Solicoccozyma, and Cladophialophora in high-yield groups were found in higher proportion, whereas Leptosphaeria and several uncultured fungal taxa were more abundant in the low-yield groups (Fig. 2H).
Rarefaction curves were graphed to visualize the minimum amount of sequencing reads required for the analysis. In Figure S1, the X-axes represent the number of sequences extracted from each sample and the Y-axes represent the alpha diversity based on the Shannon index. The rarefaction curves for the four rotation groups plateaued for both soil (Figure S1; A-D) and root-associated (Figure S1; E–H) microbial communities, indicating that the sequencing depth (as a function of sampling) was sufficient and any incremental change in the reads of sequencing data would not have further contributed to the species diversity or discovery of any additional species.
With data combined between the two locations for the analysis of alpha diversity, there were no significant differences among the treatments analyzed with either bulk soil or root-associated microbiome data. Data was also analyzed separately for the two locations, and no differences were found in the bulk soil microbiome based on the Shannon index (P > 0.05) among the four rotations (Fig. 3A–D). However, significant differences (P < 0.05) were found among the four treatments in the Shannon index for root-associated bacterial (Fig. 3E) and fungal (Fig. 3F) communities from Monmouth. Also, root-associated bacterial communities were found to be significantly different among treatments at Urbana (Fig. 3G), whereas fungal communities were not significantly different among the four treatments (Figure S2; 3H). Interestingly, CSC rotation had consistently the lowest average diversity in the root-associated microbiome.
Alpha diversity estimated by the Shannon index. Bacterial (A) and fungal (B) diversities at the Monmouth site, and bacterial (C) and fungal (D) diversities at the Urbana site associated with bulk soil; bacterial (E) and fungal (F) diversities at the Monmouth site, and bacterial (G) and fungal (H) diversities at the Urbana site associated with roots are shown.
Beta-diversity analyses of the bacterial communities from bulk soil did not produce separate clusters for the four rotation treatments (Figure S2; A and C) but did for the fungal communities (Figure S2; B and D). On the other hand, beta-diversity of both bacterial and fungal communities associated with roots did produce separate clusters for four rotation treatments (Figure S2; E–H).
To correlate the soil health indicators with bulk soil microbiomes and the crop rotation treatments, the results of canonical correspondence analysis (CCA) were plotted. The rotations that preceded higher-yielding soybean (CCC and SCC) were associated with POX-C and protein, while those preceding lower-yielding regimes (CSC and SCS) were associated with β-glucosidase (Fig. 4A–D). We did not see strong association of significantly abundant microbial taxa with any of the soil health indicators analyzed for Monmouth and Urbana sites (Fig. 4A–D).
Relationships between operational taxonomic units from different crop rotations and the soil health indicators and soybean yield. A multivariate approach using canonical correspondence analysis (CCA) was used. Only significant taxa are shown by colored-coded circles. Rotation regimes are denoted by color-coded triangles (Treatments) and soil health indicators are denoted by hollow shapes (Indicators). Bacterial (A) and fungal (B) taxa with differential abundances at the Monmouth location, and CCA for bacterial (C) and fungal (D) taxa with differential abundances at the Urbana location are shown.
Discussion
Studies have shown higher soybean yields when soybean follows grain crops such as corn or sorghum instead of soybean, with two or more years of corn as the previous crop sequence often producing higher soybean yields than a single corn crop47,48,49,50. Similarly, we found that 12–14 years of continuous corn produced higher soybean yields than fewer years, and two years producing marginally higher soybean yields than one year of corn preceding soybeans. A recent study conducted by Farmaha et al.51 reported higher soybean yield in a corn-corn-soybean rotation than in a soybean-corn-soybean rotation across a large number of irrigated fields. The results from our study expanded the conclusion that two or more previous years of corn crop sequences resulted in increased soybean yield as compared to one previous year of corn crop sequences. These trials were well-managed, with adequate fertilizer provided, and we do not believe that differences in chemical or physical properties caused the observed differences in yield. One study reported that the rotation of corn and soybeans had a neutral effect on above-ground biomass52. Whiting and Crookston53 found that the yield benefit from the rotation of soybean with corn was not due to decreases in the incidence of leaf diseases. It has been speculated that rotation-related increased yields were due to enhanced root function54,55,56, decreased soil pathogenic microorganisms, or parasites affecting root growth57,58,59,60.
In the same plots we compared in this study, Hoss et al.61 concluded that these rotations did not produce significant differences in soil physical and chemical properties, but that effects of crop sequence on soybean yield seemed to be the result of multiple interactive biological components in the soil. Indeed, as we show here, biological properties did have an association with yield, likely because soil microorganisms directly drive the carbon62,63 and nitrogen cycles64. For example, the present study showed that the labile organic carbon pool measured by POXC was higher in CCC and SCC, both sequences with high proportions of corn in the rotation, which corresponded to higher yields of soybean in those plots.
Based on ANCOM analysis of the bulk soil, an uncultured bacterial taxon under family JG30-KF-AS9 (order Ktedonobacterales), was found to be significantly abundant at the Monmouth site with a decreasing order of CCC > SCC > CSC > SCS. Although JG30-KF-AS9 was associated with high-yield rotation groups in our study, their biological significance is not known. Similarly, we identified differentially abundant fungal taxa in bulk soil that are of particular interest. Among those observed as a dominant taxon in SCS plots, included some of the most devastating pathogens of crop plants, such as Macrophomina phaseolina65,66. Pathogens under genera Macrophomina are also known to infect soybean roots causing charcoal rot disease67. Similarly, Corynespora, a genus mostly composed of plant pathogens68,69, was found to be significantly abundant in low-yield SCS plots in decreasing orders of SCS > SCC > CSC > CCC at Monmouth and SCS > CSC > SCC > CCC at Urbana. A fungal pathogen from the genera Corynespora is the causal agent of soybean frogeye leaf spot disease70. Mycoarthris, a genus under order Helotiales, was another fungal taxon that was significantly abundant at Monmouth and was associated with high-yield rotation groups. However, its biological significance is not well-studied.
At Urbana, Ascomycota belonging to family Chaetothyriacea (root-associated) were among the most abundant fungal taxa. Although a majority of the Chaetothyriacea genera are saprobes and only a very few are known to be plant pathogens and host specific parasites71,72,73, they were associated with low-yield with a higher abundance in SCS rotation, suggesting it is a pathogen.
Based on both ANCOM and Gneiss analysis, root-associated bacterial family Micromonosporaceae under order Micromonosporales and phylum Actinobacteria was associated with low yield. Micromonosporaceae are known to act as plant saprophytes or symbionts that thrive under anaerobic conditions74, and they have been reported as pathogenic. Gneiss analysis showed several bacterial and fungal taxa present in higher proportions in high and low-yield rotation groups that were not found to be significantly higher by ANCOM analysis. Among those, two bacterial taxa, Streptomyces, which were proportionally higher in low-yield, and Bradyrhizobium, which were proportionally higher in high-yield, are particularly interesting. Although mostly symbionts, some Streptomyces species are known to produce extracellular hydrolytic enzymes that can break down highly stable organic compounds inaccessible to other microbes, infect living plant cells and cause diseases of roots75,76. Bradyrhizobium helps plants with nitrogen fixation and P and K solubilization77,78, thus affecting overall yield. Based on our data, Streptomyces only were associated with roots, whereas Bradyrhizobium were also present in bulk soil.
The multivariate CCA analysis revealed that the yield of high-yield rotation groups (CCC and SCC) was associated with certain soil health indicators, specifically, protein and POX-C; while low-yield rotation groups (CSC and SCS) were associated with β-glucosidase. Bacterial taxa JG30-KF-AS9, enriched in the CCC plots, was negatively correlated with β-glucosidase in Monmouth, whereas Microvirga, enriched in the low-yield treatment, was positively associated with β-glucosidase in Urbana. Both the Tumularia and Idriella, enriched in the high yield CCC plots, were positively associated with soil protein in Urbana. Some of the differentially abundant fungal taxa from the bulk soil samples were associated with the soil health indicators. For example, Mycoarthris correlated positively with POXC, whereas Macrophomina and Corynespora correlated negatively with POXC and soil protein in Monmouth. Notably, Corynespora also negatively correlated with POXC in Urbana.
In this study, the long-term rotational treatments provided the opportunity to determine the impact of crop sequencing on specific microbial taxa and their relationship with yield. Notably, alpha diversity was not significantly different between treatments, which means continuous corn did not result in a reduction of microbiome richness than the other regimes. Although alpha-diversity analysis of microbes did not indicate differences in species richness between high and low-yield rotation groups, we still saw changes in relative species abundances between the rotation groups based on the separation of clusters with a multidimensional scaling analysis, particularly with regards to the root-associated data. Also, some of the bacterial and fungal taxa were found in higher abundance between the rotation groups based on ANCOM and Gneiss analyses.
Soybeans following 14 years of continuous corn (CCC) yielded significantly more than the other three (SCC, CSC, SCS) crop rotations at both locations. The application of custom crop rotation systems in the field could provide many important benefits enhancing soil C concentration and fertility, improving soil physical properties, providing diverse bacterial and fungal communities, and increasing crop yields. This study provided evidence that soil biological properties, including POXC, protein content, specific bacterial 16S rDNA and fungal ITS sequence relative abundances, are significantly correlated with yield shown by the CCA plots. This finding is particularly important given that measuring chemical and physical properties did not provide an adequate explanation for soybean yields differences following these crop sequences61. The results suggest that soybean pathogen populations may be determinants, as well as some uncultured bacterial taxa, which still require efforts in culturing and further characterization. Culturability of bacteria has been greatly improved in recent years, and our finding that adding preparations of bacteria, such as those under the order of JG30-KF-AS9 could perhaps be used as a way to increase soybean yields even in fields where soybeans are grown more frequently than once every three or more years.
Crop rotation and sequencing are management tactics that can increase crop yields. The current study found that differential abundances of bacterial and fungal taxa were related to yield differences in a site-specific manner. The soils at Monmouth (Muscatune silt loam) and Urbana (Flanagan silt loam) both developed under prairie vegetation in similar (loess) parent material, and both are highly productive. Soil organic matter content in the top 30 cm was 3.6% at Monmouth and 3.7% at Urbana. Tillage, pesticide application, crop cultivars, and fertilizer usage were similar between the two sites, but averaged over the twelve years before sampling, yields were higher at Monmouth (13.5 t/ha for corn, 4.6 t/ha for soybean) than at Urbana (11.1 and 3.8 t/ha) (E. Nafziger, unpublished data). We believe that yield differences were partially due to weather differences between the two sites which can be found at https://www.isws.illinois.edu/warm/weather/, so it is likely that accumulative weather differences also contributed to differences in microbial composition over time to some degree. Multivariate analysis results indicated that soil- and root-associated microbiome members contributed towards some of the observed yield differences that correlated well with different indicators of soil health. Pathogens, as expected, are associated with low yield, and correlated negatively with soil protein and POXC, whereas taxa selected by the high yield treatment had a positive correlation with soil protein and POXC.
Methods
Fields descriptions and original source of the soil and soybean root samples
Field experiments were described in a previously published article61.The Urbana site was on Flanagan silt loam soil, and the Monmouth site on Muscatune silt loam; both are highly productive soils. Soil test values (0–15 cm) in fall 2014 were available P 17.6 mg kg−1, available K 218 mg kg−1, pH 6.6, and soil organic matter (SOM) 3.82% at Monmouth; and available P 21.5 mg kg−1, available K 214 mg kg−1, pH 5.9, and SOM 3.72% at Urbana. Soils were sampled in July 2016 at a 0–15 cm depth from rotation plots in place for the previous 12–14 years at the University of Illinois Crop Sciences Research Center at Monmouth IL (40.931–90.722) and at Urbana IL (40.048–88.232). The experiment had 4 prior rotation treatments: T1: Continuous corn (CCCCCCCCCCCCCC-S), T2: 2 year of corn-1 year of soybean (SCCSCCSCCSCC-S), T3: 1 year of corn-1 year of soybean (SCSCSCSCSCSC-S) and T4: 1 year of soybean-1 year of corn (CSCSCSCSCSCS-S) ahead of soybean × 4 rep (block) × 3 subsamples per plot in 2016. The field layout followed a random complete block design at both locations. Soybean cultivars P34T07 and P35T58R, both from Pioneer (DuPont Pioneer, Johnston, Iowa) were planted at seeding rates of 355,000 and 395,000 ha−1 on 9 May and 16 May at Monmouth and Urbana, respectively. Soybean harvest was done using a plot combine, and yields were corrected to 13% moisture.
At V6 growth stage, soybean roots were sampled from the corresponding plots (with identical prior crop rotation treatments) in July 2017. All experimental research and field studies on the rotation sequences, including the collection of soil samples and soybean root tissues comply with University of Illinois experimental station guidelines. Samples were kept cool during transportation on ice and stored in a − 80 °C horizontal freezer immediately until further processing. Subsamples were taken for DNA extraction in 2 ml microtubes, and a subsample of about 200 g were transferred to a brown paper bag for drying on a greenhouse bench. The soil samples were broken up by a rolling pin and sieved to pass a 2 mm sieve. During processing, the roots were kept on dry ice to prevent microbiome changes.
Determination of permanganate oxidizable carbon (POXC)
The procedure defined by Weil et al.10 was followed for the measurement of POXC. The colorimetric method was used to measure the absorbance by a microplate reader (BioTek Synergy 2 Multi-Mode Microplate Reader) at the wavelength of 550 nm. Samples exceeding the range of the standard curve were diluted with water.
ACE soil protein index
Soil protein content was measured in triplicate for each soil sample by following a protocol modified from Wright and Upadhyaya and Moebius-Clune et al.15,79 by autoclaving (121 °C @ 15 psi for 30 min) a citric acid (pH 7.0) soil extraction and measuring protein in the extract by a colorimetric method (Pierce BCA Protein Assay Kit, Thermo Scientific). Protein assays were performed in 96 well plates incubated at 60 °C for 30 min. After the incubation, the microplate reader (BioTek Synergy 2 Multi-Mode Microplate Reader) was used to obtain the optical density reading at the wavelength of 562 nm.
Soil β-glucosidase enzyme activity
Soil β-glucosidase enzyme activity was assayed according to the method described by Deng and Tabatabai (1994)80. THAM buffer (pH 10) was used to dilute samples at the rate of 1:4 (note: the controls were not diluted) and samples were pipetted into 96 well microplates. The absorbance was measured using a microplate reader (BioTek Synergy 2 Multi-Mode Microplate Reader) at the wavelength of 405 nm.
Soil and root DNA extraction
The FastDNA Spin Kit (For soil, Cat.No.116560200, MP Biomedicals, Solon, Ohio) was used following the manufacturer’s protocol for DNA extraction from soil with some minor modification. Samples were kept at − 20 °C until further hexadecyltrimethylammonium bromide (CTAB) purification of DNA for post-extraction cleanup81. Root DNA was extracted by pulverizing 0.2 g of root that was cut into small pieces (< 0.5 cm) and dry with speed-vac for 4–6 h in bead beating tubes containing two stainless steel BB gun beads. The tubes were dipped in liquid nitrogen and ground for 10 s and return to the liquid nitrogen and ground again repeated until the roots were ground to fine powder. In the tube, 490 μl of water, 20 μl of SDS, and 20 μl of EDTA were added. The tubes were then vortexed vigorously with the horizontal as well as the vertical vortex adapters. The tubes were incubated in a heat block at 68 °C for 10 min and centrifuged at 13,000 rpm for 8 min to pellet cell debris. The supernatant was transferred to a new tube and 30 μl cold potassium acetate was added to each tube, and incubated on ice for 10 min, and then subjected to centrifugation at 13,000 rpm for 8 min. The supernatant was transferred to a new tube and cleaned up using a genomic DNA clean and concentrator-5 kit (Zymo Research, Irvine, CA).
Bacterial and fungal DNA amplicon sequencing
Bacterial 16S rDNA and fungal nuclear ribosomal internal transcribed spacer (ITS) classifications were amplified and sequenced by University of Minnesota Genomic Center (Minneapolis, Minnesota, USA) using MiSeq-V3 chemistry from a published protocol with a dual-index approach82. The 16S V3-V4 and ITS-1 regions were targeted for the bacterial community and fungal community, respectively. Data was deposited in NCBI BioProject SRA accession: PRJNA521547.
Data analyses
Sample details can be found in Table S1, and soil health indicator data can be found in Table S2. Statistical analyses were performed using R statistical software83. Yield, POXC, protein index, and β-glucosidase activities were analyzed by fitting a mixed-effect model with block as a random effect using ‘lme4’84 followed by the posthoc tests of LSMeans Differences with ‘emmeans’ packages in R. The following assumptions for linear mixed models were also tested: that errors are linear, independent, normally distributed and homogeneity of variance. The threshold was designated for probability at P < 0.05. The classifications of bacteria and fungi were determined using QIIME285, sequences were denoised and filtered using DADA286, and resulting feature tables were then rarefied to perfom core diversity analysis, followed by analyses to detect differential abundance of taxa with the ANCOM87 and Gneiss88 tests. Taxonomic assignment of representative sequences of fungi and bacteria were performed based on the trained ITS and 16S RNA OTUs clustered at 99% similarities within Unite (version 8)89 and Silva13290 databases, respectively, using the Naïve-Bayes classifiers91 built in QIIME2. Kruskal–Wallis92 tests followed by Wilcoxon post hoc tests (where p values were significant [P < 0.05] for omnibus Kruskal–Wallis tests) were performed to determine significant alpha diversity metrics between the rotation types. Rarefaction curves were generated from multiple samplings of the same sample data with QIIME2 adjusting for variation in the sequencing depth. Canonical correspondance analysis (CCA) was performed using the ‘vegan’ package in R. Data was summarized in the supplementary file, named as CCA. High throughput sequencing data has been submitted to NCBI SRA with the accession number available upon revision or the acceptance of the manuscript.
Data availability
Sequence data is publicly available under NCBI BioProject SRA accession: PRJNA521547.
Abbreviations
- ANCOM:
-
Analysis of composition of microbiomes
- CCC:
-
Continuous corn
- SCC:
-
2-Yr of corn in a 3-year rotation
- CSC:
-
1-Yr of corn in a 2-yr rotation
- SCS:
-
1-Yr of soybean in a 2-yr rotation
- POXC:
-
Permanganate-oxidizable carbon
- SOC:
-
Soil organic carbon
- SOM:
-
Soil organic matter
References
Hartman, K. et al. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 6, 14 (2018).
Berzsenyi, Z., Győrffy, B. & Lap, D. Effect of crop rotation and fertilisation on maize and wheat yields and yield stability in a long-term experiment. Eur. J. Agron. 13, 225–244. https://doi.org/10.1016/S1161-0301(00)00076-9 (2000).
Körschens, M. The importance of long-term field experiments for soil science and environmental research: a review. Plant Soil Environ. 52, 1–8 (2006).
Zuber, S. M., Behnke, G., Nafziger, E. & Villamil, M. B. Crop rotation and tillage effects on soil physical and chemical properties in Illinois. Agron. J. 107, 971–978. https://doi.org/10.2134/agronj14.0465 (2015).
Stott, D. E. Recommended Soil Health Indicators and Associated Laboratory Procedures. National Soil Health Specialist, Soil Health Division, U.S. Department of Agriculture (USDA), Natural Resources Conservation Service (NRCS), Washington, D.C. (2019).
Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 64, 269–285. https://doi.org/10.1016/j.ecolecon.2007.03.004 (2007).
Garbeva, P., van Veen, J. A. & van Elsas, J. D. Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 42, 243–270. https://doi.org/10.1146/annurev.phyto.42.012604.135455 (2004).
Hatfield, J., Prueger, J. & Kustas, W. Spatial and temporal variation of energy and carbon fluxes in central Iowa. Agron. J. 99, 285–296. https://doi.org/10.2134/agronj2005.0116S (2007).
Lehman, R. M. et al. Understanding and enhancing soil biological health: the solution for reversing soil degradation. Sustainability 7, 988–1027. https://doi.org/10.3390/su7010988 (2015).
Weil, R. R., Islam, K. R., Stine, M. A., Gruver, J. B. & Samson-Liebig, S. E. Estimating active carbon for soil quality assessment: a simplified method for laboratory and field use. Am. J. Alter. Agric. 18, 3–17. https://doi.org/10.1079/AJAA200228 (2003).
Idowu, O. et al. Farmer-oriented assessment of soil quality using field, laboratory, and VNIR spectroscopy methods. Int. J. Plant-Soil Relatsh. 307, 243–253. https://doi.org/10.1007/s11104-007-9521-0 (2008).
Gunapala, N. & Scow, K. M. Dynamics of soil microbial biomass and activity in conventional and organic farming systems. Soil Biol. Biochem. 30, 805–816. https://doi.org/10.1016/S0038-0717(97)00162-4 (1998).
Plaza-Bonilla, D., Álvaro-Fuentes, J. & Cantero-Martínez, C. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Tillage Res. 139, 19–22. https://doi.org/10.1016/j.still.2014.01.006 (2014).
Mirsky, S., Lanyon, L. & Needelman, B. Evaluating soil management using particulate and chemically labile soil organic matter fractions. Soil Sci. Soc. Am. J. 72, 180–185. https://doi.org/10.2136/sssaj2005.0279 (2008).
Wright, S. F. & Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 161, 575–586 (1996).
Jan, M. T., Roberts, P., Tonheim, S. K. & Jones, D. L. Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils. Soil Biol. Biochem. 41, 2272–2282. https://doi.org/10.1016/j.soilbio.2009.08.013 (2009).
Nannipieri, P. & Eldor, P. The chemical and functional characterization of soil N and its biotic components. Soil Biol. Biochem. 41, 2357–2369. https://doi.org/10.1016/j.soilbio.2009.07.013 (2009).
Weintraub, M. N. & Schimel, J. P. Seasonal protein dynamics in Alaskan arctic tundra soils. Soil Biol. Biochem. 37, 1469–1475. https://doi.org/10.1016/j.soilbio.2005.01.005 (2005).
Ros, G. H., Temminghoff, E. J. M. & Hoffland, E. Nitrogen mineralization: a review and meta-analysis of the predictive value of soil tests. Eur. J. Soil Sci. 62, 162–173. https://doi.org/10.1111/j.1365-2389.2010.01318.x (2011).
Ros, G. H., Hanegraaf, M. C., Hoffland, E. & van Riemsdijk, W. H. Predicting soil N mineralization: relevance of organic matter fractions and soil properties. Soil Biol. Biochem. 43, 1714–1722. https://doi.org/10.1016/j.soilbio.2011.04.017 (2011).
Chang, E.-H., Chung, R.-S. & Tsai, Y.-H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 53, 132–140. https://doi.org/10.1111/j.1747-0765.2007.00122.x (2007).
Liebig, M., Carpenter-Boggs, L., Johnson, J. M. F., Wright, S. & Barbour, N. Cropping system effects on soil biological characteristics in the Great Plains. Renew. Agric. Food Syst. 21, 36–48. https://doi.org/10.1079/RAF2005124 (2006).
Wright, S. & Upadhyaya, A. Comparison of N-linked oligosaccharides of glomalin from arbuscular mycorrhizal fungi and soils by capillary electrophoresis. Soil Biol. Biochem. 30, 1853–1857 (1998).
Wright, S. F. & Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198, 97–107 (1998).
Lovelock, C. E., Wright, S. F., Clark, D. A. & Ruess, R. W. Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J. Ecol. 92, 278–287. https://doi.org/10.1111/j.0022-0477.2004.00855.x (2004).
Emran, M., Gispert, M. & Pardini, G. Patterns of soil organic carbon, glomalin and structural stability in abandoned Mediterranean terraced lands. Eur. J. Soil Sci. 63, 637–649. https://doi.org/10.1111/j.1365-2389.2012.01493.x (2012).
Nichols, K. A. & Millar, J. Glomalin and soil aggregation under six management systems in the Northern Great Plains, USA. Open J. Soil Sci. 03(08), 5. https://doi.org/10.4236/ojss.2013.38043 (2013).
Rillig, M., Ramsey, P., Morris, S. & Paul, E. Glomalin, an arbuscular-mycorrhizal fungal soil protein, responds to land-use change. Int. J. Plant-Soil Relatsh. 253, 293–299. https://doi.org/10.1023/A:1024807820579 (2003).
Klose, S. & Tabatabai, M. A. Response of phosphomonoesterases in soils to chloroform fumigation. J. Plant Nutr. Soil Sci. 165, 429–434. https://doi.org/10.1002/1522-2624(200208)165:4%3c429::AID-JPLN429%3e3.0.CO;2-S (2002).
Wang, X.-C. & Lu, Q. Beta-glucosidase activity in paddy soils of the Taihu Lake Region, China. Pedosphere 16, 118–124. https://doi.org/10.1016/S1002-0160(06)60033-7 (2006).
Wilson, D. B. Microbial diversity of cellulose hydrolysis. Curr. Opin. Microbiol. 14, 259–263. https://doi.org/10.1016/j.mib.2011.04.004 (2011).
Shewale, J. G. β-Glucosidase: Its role in cellulase synthesis and hydrolysis of cellulose. Int. J. Biochem. 14, 435–443. https://doi.org/10.1016/0020-711X(82)90109-4 (1982).
Acosta-Martínez, V., Reicher, Z., Bischoff, M. & Turco, R. F. The role of tree leaf mulch and nitrogen fertilizer on turfgrass soil quality. Biol. Fertil. Soils 29, 55–61. https://doi.org/10.1007/s003740050524 (1999).
Krogh, K. et al. Characterization and kinetic analysis of a thermostable GH3 β-glucosidase from Penicillium brasilianum. Appl. Microbiol. Biotechnol. 86, 143–154. https://doi.org/10.1007/s00253-009-2181-7 (2010).
Chen, M. et al. Isolation and characterization of a β-glucosidase from Penicillium decumbens and improving hydrolysis of corncob residue by using it as cellulase supplementation. Enzyme Microb. Technol. 46, 444–449. https://doi.org/10.1016/j.enzmictec.2010.01.008 (2010).
Günata, Z. & Vallier, M.-J. Production of a highly glucose-tolerant extracellular β-glucosidase by three Aspergillus strains. Biotechnol. Lett. 21, 219–223. https://doi.org/10.1023/A:1005407710806 (1999).
Riou, C., Salmon, J.-M., Vallier, M.-J., Gunata, Z. & Barre, P. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta -glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 64, 3607 (1998).
Tsukada, T., Igarashi, K., Yoshida, M. & Samejima, M. Molecular cloning and characterization of two intracellular β-glucosidases belonging to glycoside hydrolase family 1 from the basidiomycete Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 73, 807–814. https://doi.org/10.1007/s00253-006-0526-z (2006).
Yang, S., Wang, L., Yan, Q., Jiang, Z. & Li, L. Hydrolysis of soybean isoflavone glycosides by a thermostable β-glucosidase from Paecilomyces thermophila. Food Chem. 115, 1247–1252. https://doi.org/10.1016/j.foodchem.2009.01.038 (2009).
Arévalo Villena, M., Úbeda Iranzo, J. F., Gundllapalli, S. B., Cordero Otero, R. R. & Briones Pérez, A. I. Characterization of an exocellular β-glucosidase from Debaryomyces pseudopolymorphus. Enzyme Microb. Technol. 39, 229–234. https://doi.org/10.1016/j.enzmictec.2005.10.018 (2006).
Amouri, B. & Gargouri, A. Characterization of a novel β-glucosidase from a Stachybotrys strain. Biochem. Eng. J. 32, 191–197. https://doi.org/10.1016/j.bej.2006.09.022 (2006).
Okamoto, K., Sugita, Y., Nishikori, N., Nitta, Y. & Yanase, H. Characterization of two acidic β-glucosidases and ethanol fermentation in the brown rot fungus Fomitopsis palustris. Enzyme Microb. Technol. 48, 359–364. https://doi.org/10.1016/j.enzmictec.2010.12.012 (2011).
Singhania, R. R., Patel, A. K., Sukumaran, R. K., Larroche, C. & Pandey, A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Biores. Technol. 127, 500–507. https://doi.org/10.1016/j.biortech.2012.09.012 (2013).
Okamoto, K., Nakano, H., Yatake, T., Kiso, T. & Kitahata, S. Purification and some properties of a β-glucosidase from Flavobacterium johnsonae. Biosci. Biotechnol. Biochem. 64, 333–340. https://doi.org/10.1271/bbb.64.333 (2000).
Spano, G. et al. A β-glucosidase gene isolated from wine Lactobacillus plantarum is regulated by abiotic stresses. J. Appl. Microbiol. 98, 855–861. https://doi.org/10.1111/j.1365-2672.2004.02521.x (2005).
Mendes, R. et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100. https://doi.org/10.1126/science.1203980 (2011).
Crookston, R., Kurle, J., Copeland, P. J., Ford, J. H. & Lueschen, W. E. Rotational cropping sequence affects yield of corn and soybean. Agron. J. 83, 108–113 (1991).
Meese, B. G., Carter, P. R., Oplinger, E. S. & Pendleton, J. W. Corn/soybean rotation effect as influenced by tillage, nitrogen, and hybrid/cultivar. J. Prod. Agric. 4, 74–80 (1991).
Kelley, K. W., Long, J. H. & Todd, T. C. Long-term crop rotations affect soybean yield, seed weight, and soil chemical properties. Field Crop Res. 83, 41–50. https://doi.org/10.1016/S0378-4290(03)00055-8 (2003).
Mourtzinis, S. et al. Corn and soybean yield response to tillage, rotation, and nematicide seed treatment. Crop Sci. 57, 1704–1712. https://doi.org/10.2135/cropsci2016.09.0792 (2017).
Farmaha, B. S. et al. Rotation impact on on-farm yield and input-use efficiency in high-yield irrigated maize-soybean systems. Agron. J. 108, 2313–2321. https://doi.org/10.2134/agronj2016.01.0046 (2016).
Crookston, R. K. & Kurle, J. E. Corn residue effect on the yield of corn and soybean grown in rotation. Agron. J. 81, 229–232. https://doi.org/10.2134/agronj1989.00021962008100020018x (1989).
Whiting, K. R. & Crookston, R. K. Host-specific pathogens do not account for the corn-soybean rotation effect. Crop Sci. 33, 539–543. https://doi.org/10.2135/cropsci1993.0011183X003300030024x (1993).
Copeland, P. J., Allmaras, R. R., Crookston, R. K. & Nelson, W. W. Corn-soybean rotation effects on soil water depletion. Agron. J. 85, 203–210. https://doi.org/10.2134/agronj1993.00021962008500020008x (1993).
Li, J. et al. Soil-plant indices help explain legume response to crop rotation in a semiarid environment. Front. Plant Sci. https://doi.org/10.3389/fpls.2018.01488 (2018).
Nickel, S. E., Crookston, R. K. & Russelle, M. P. Root growth and distribution are affected by corn-soybean cropping sequence. Agron. J. 87, 895–902. https://doi.org/10.2134/agronj1995.00021962008700050020x (1995).
Bennett, A. J., Bending, G. D., Chandler, D., Hilton, S. & Mills, P. Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotations. Biol. Rev. 87, 52–71. https://doi.org/10.1111/j.1469-185X.2011.00184.x (2012).
Johnson, N., Copeland, P. J., Crookston, R. & Pfleger, F. L. Mycorrhizae: possible explanation for yield decline with continuous corn and soybean. Agron. J. 84, 387–390 (1992).
Chen, S., Porter, P. M., Reese, C. D. & Stienstra, W. C. Crop sequence effects on soybean cyst nematode and soybean and corn yields this research was supported by Minnesota soybean producers check-off funding through Minnesota research and promotion council and Minnesota agric. exp. stn.. Crop Sci. 41, 1843–1849. https://doi.org/10.2135/cropsci2001.1843 (2001).
Grabau, Z. J. & Chen, S. Determining the role of plant-parasitic nematodes in the corn-soybean crop rotation yield effect using nematicide application: II. Soybean. Agron. J. 108, 1168–1179. https://doi.org/10.2134/agronj2015.0432 (2016).
Hoss, M., Behnke, G., Davis, A., Nafziger, E. & Villamil, M. B. Short corn rotations do not improve soil quality. Compared with corn monocultures. Agron. J. 110, 1274–1288. https://doi.org/10.2134/agronj2017.11.0633 (2018).
Plaza, C., Courtier-Murias, D., Fernández, J. M., Polo, A. & Simpson, A. J. Physical, chemical, and biochemical mechanisms of soil organic matter stabilization under conservation tillage systems: a central role for microbes and microbial by-products in C sequestration. Soil Biol. Biochem. 57, 124–134. https://doi.org/10.1016/j.soilbio.2012.07.026 (2013).
Tardy, V. et al. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 90, 204–213. https://doi.org/10.1016/j.soilbio.2015.08.010 (2015).
Tiedje, J. M., Asuming-Brempong, S., Nüsslein, K., Marsh, T. L. & Flynn, S. J. Opening the black box of soil microbial diversity. Appl. Soil. Ecol. 13, 109–122. https://doi.org/10.1016/S0929-1393(99)00026-8 (1999).
Hussain, S., Ghaffar, A. & Aslam, M. Biological-control of macrophomina-phaseolina charcoal rot of sunflower and mung bean. J. Phytopathol. 130, 157–160. https://doi.org/10.1111/j.1439-0434.1990.tb01163.x (1990).
Khan, A. N. et al. Molecular identification and genetic characterization of Macrophomina phaseolina strains causing pathogenicity on sunflower and chickpea. Front. Microbiol. 8, 1309. https://doi.org/10.3389/fmicb.2017.01309 (2017).
Ramezani, M. et al. Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (-)-botryodiplodin but no detectable phaseolinone. J. Nat. Prod. 70, 128–129. https://doi.org/10.1021/np060480t (2007).
Smith, L.J., Datnoff, L.E., Pernezny, K. & Schlub, R.L. Phylogenetic and pathogenic characterization of Corynespora cassiicola isolates. In II International Symposium on Tomato Diseases 808, 51–56 (2007)
Deon, M. et al. Characterization of a cassiicolin-encoding gene from Corynespora cassiicola, pathogen of rubber tree (Hevea brasiliensis). Plant Sci. 185–186, 227–237. https://doi.org/10.1016/j.plantsci.2011.10.017 (2012).
Videira, S. I. R. et al. Mycosphaerellaceae: chaos or clarity?. Stud. Mycol. 87, 257–421 (2017).
Wijayawardene, N. N. et al. Outline of ascomycota: 2017. Fungal Divers. 88, 167–263. https://doi.org/10.1007/s13225-018-0394-8 (2018).
Olofsson, J., Ericson, L., Torp, M., Stark, S. & Baxter, R. Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nat. Clim. Change 1, 220–223. https://doi.org/10.1038/Nclimate1142 (2011).
Wells, L. D. & McManus, P. S. A photographic diagnostic guide for identification of the principal cranberry fruit rot pathogens. Plant Health Prog. https://doi.org/10.1094/php-2013-0729-01-dg (2013).
Yeager, C. M. et al. Polysaccharide degradation capability of actinomycetales soil isolates from a semiarid grassland of the Colorado Plateau. Appl. Environ. Microbiol. https://doi.org/10.1128/aem.03020-16 (2017).
Loria, R., Bukhalid, R. A., Fry, B. A. & King, R. R. Plant pathogenicity in the genus Streptomyces. Plant Dis. 81, 836–846. https://doi.org/10.1094/Pdis.1997.81.8.836 (1997).
Li, Y., Liu, J., Diaz-Cruz, G., Cheng, Z. & Bignell, D. R. D. Virulence mechanisms of plant-pathogenic Streptomyces species: an updated review. Microbiology 165, 1025–1040. https://doi.org/10.1099/mic.0.000818 (2019).
Abdalla, M. H. Solubilization of rock phosphates by rhizobium and bradyrhizobium. Folia Microbiol. 39, 53–56. https://doi.org/10.1007/Bf02814530 (1994).
Bargaz, A., Lyamlouli, K., Chtouki, M., Zeroual, Y. & Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01606 (2018).
Moebius-Clune, B. N. Comprehensive Assessment of Soil Health: The Cornell Framework Manual (Cornell University, 2016).
Deng, S. P. & Tabatabai, M. A. Cellulase activity of soils. Soil Biol. Biochem. 26, 1347–1354. https://doi.org/10.1016/0038-0717(94)90216-X (1994).
Riesenfeld, C. S., Goodman, R. M. & Handelsman, J. Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ. Microbiol. 6, 981–989 (2004).
Gohl, D. et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 34, 942–949. https://doi.org/10.1038/nbt.3601 (2016).
R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (Vienna, Austria, 2020).
Bates, D., Machler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Bolyen, E. et al. Qiime 2: reproducible, interactive, scalable, and extensible microbiome data science. Report No. 2167–9843, (PeerJ Preprints, 2018).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Mandal, S. et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. https://doi.org/10.3402/mehd.v26.27663 (2015).
Morton, J. T. et al. Balance trees reveal microbial niche differentiation. mSystems https://doi.org/10.1128/mSystems.00162-16 (2017).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264. https://doi.org/10.1093/nar/gky1022 (2019).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590-596. https://doi.org/10.1093/nar/gks1219 (2013).
Pedregosa, F. et al. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Kruskal, W. H. & Wallis, W. A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621. https://doi.org/10.1080/01621459.1952.10483441 (1952).
Acknowledgements
We sincerely thank the efforts in assisting soil and soybean sampling by Mr. Jason Niekamp and Mr. Joshua Vonk at University of Illinois, Urbana-Champaign. We thank Connor Pedersen for English editing.
Funding
This study was supported in part by South Dakota Nutrient Research and Education Council and SDSU startup from USDA Hatch fundSD00H606-16 project number with Accession Number 1009451 and National Sclerotinia Initiative Grant to Shin-Yi Lee Marzano. Scholarship from the Ministry of Agriculture and Forestry and Ministry of National Education of Republic of Turkey (to Izzet Bulbul). The funding bodies did not play a role in the design of the study and collection, analysis, and interpretation of the data, nor in writing the manuscript, but only provided the financial support.
Author information
Authors and Affiliations
Contributions
A.N., S.L.M., and Z.W. analyzed the sequencing data, I.B., S.L.M., Z.W. and R.M.L. measured the soil health indicators, and I.B. analyzed the soil health indicators data statistically. E.N. and S.L.M. conceived the study. I.B. and A.N. helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neupane, A., Bulbul, I., Wang, Z. et al. Long term crop rotation effect on subsequent soybean yield explained by soil and root-associated microbiomes and soil health indicators. Sci Rep 11, 9200 (2021). https://doi.org/10.1038/s41598-021-88784-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88784-6
- Springer Nature Limited
This article is cited by
-
Combined metagenomics and metabolomic analysis of microbial community structure and metabolic function in continuous soybean cropping soils of Songnen Plain, China
Chemical and Biological Technologies in Agriculture (2024)
-
Rotation with other crops slow down the fungal process in tobacco-growing soil
Scientific Reports (2024)
-
The impact of different preceding crops on soil nitrogen structure and nitrogen cycling in tobacco-planting soil
Scientific Reports (2024)
-
Legacy effects of preceding crops improve flue-cured tobacco productivity in southwest China by optimizing soil structure, nutrients, and microbial interactions
Plant and Soil (2024)
-
Continuous Replanting Could Degrade Soil Health in Short-Rotation Plantation Forestry
Current Forestry Reports (2023)