Abstract
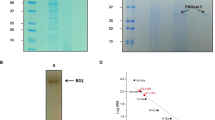
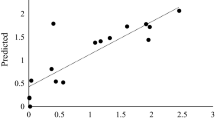
A GH3 β-glucosidase (BGL) from Penicillium brasilianum was purified to homogeneity after cultivation on a cellulose and xylan rich medium. The BGL was identified in a genomic library, and it was successfully expressed in Aspergillus oryzae. The BGL had excellent stability at elevated temperatures with no loss in activity after 24 h of incubation at 60°C at pH 4–6, and the BGL was shown to have significantly higher stability at these conditions in comparison to Novozym 188 and to other fungal GH3 BGLs reported in the literature. The BGL had significant lower affinity for cellobiose compared with the artificial substrate para-nitrophenyl-β-d-glucopyranoside (pNP-Glc) and further, pronounced substrate inhibition using pNP-Glc. Kinetic studies demonstrated the high importance of using cellobiose as substrate and glucose as inhibitor to describe the inhibition kinetics of BGL taking place during cellulose hydrolysis. A novel assay was developed to characterize this glucose inhibition on cellobiose hydrolysis. The assay uses labelled glucose-13C6 as inhibitor and subsequent mass spectrometry analysis to quantify the hydrolysis rates.




Similar content being viewed by others
Reference
Barnett CC, Berka RM, Fowler T (1991) Cloning and amplification of the gene encoding an extracellular β-glucosidase from Trichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates. Biotechnology (N Y) 9:562–567
Bhatia Y, Mishra S, Bisaria VS (2002) Microbial β-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol 22:375–407
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Carlsen M (1994) α-amylase production by Aspergillus oryzae. PhD thesis. Department of Biotechnology, The Technical University of Denmark
Chirico WJ, Brown RD Jr (1987) Purification and characterization of a β-glucosidase from Trichoderma reesei. Eur J Biochem 165:333–341
Christakopoulos P, Goodenough PW, Kekos D, Macris BJ, Claeyssens M, Bhat MK (1994) Purification and characterisation of an extracellular β-glucosidase with transglycosylation and exo-glucosidase activities from Fusarium oxysporum. Eur J Biochem 224:379–385
Christensen T, Woeldike H, Boel S, Mortensen SB, Hjortshoej K, Thim L, Hansen MT (1988) High level expression of recombinant genes in Aspergillus oryzae. Nat Biotechnol 6:1419–1422
Copa-Patino JL, Rodriguez J, Perez-Leblic MI (1990) Purification and properties of a β-glucosidase from Penicillium oxalicum autolysates. FEMS Microbiol Lett 55:191–196
Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies G, Henrissat B, Svensson B (eds) Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, pp 3–12
Dan S, Marton I, Dekel M, Bravdo BA, He S, Withers SG, Shoseyov O (2000) Cloning, expression, characterization, and nucleophile identification of family 3, Aspergillus niger β-glucosidase. J Biol Chem 275:4973–4980
Decker CH, Visser J, Schreier P (2000) β-glucosidases from five black Aspergillus species: study of their physico-chemical and biocatalytic properties. J Agric Food Chem 48:4929–4936
Decker CH, Visser J, Schreier P (2001) β-glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Appl Microbiol Biotechnol 55:157–163
Hidalgo M, Steiner J, Eyzaguirre J (1992) β-glucosidase from Penicillium purpurogenum: purification and properties. Biotechnol Appl Biochem 15:185–191
Hrmova M, Streltsov VA, Smith BJ, Vasella A, Varghese JN, Fincher GB (2005) Structural rationale for low-nanomolar binding of transition state mimics to a family GH3 β-d-glucan glucohydrolase from barley. Biochem 44:16529–16539
Agency IE (2005) Land use and feedstock availability issues. In: Difiglio C (ed) Biofuels for transport. International Energy Agency, Paris, pp 123–145
Kantham L, Vartak HG, Jagannathan V (1984) β-glucosidase of Penicillium funiculosum.II. Properties and mycelial binding. Biotechnol Bioeng 27:786–791
Kawai R, Igarashi K, Kitaoka M, Ishii T, Samejima M (2004) Kinetics of substrate transglycosylation by glycoside hydrolase family 3 glucan (1–>3)-β-glucosidase from the white-rot fungus Phanerochaete chrysosporium. Carbohydr Res 339:2851–2857
Krogh KB, Morkeberg A, Jorgensen H, Frisvad JC, Olsson L (2004) Screening genus Penicillium for producers of cellulolytic and xylanolytic enzymes. Appl Biochem Biotechnol 113–116:389–401
Kubicek CP (1992) The cellulase proteins of Trichoderma reesei: structure, multiplicity, mode of action and regulation of formation. Adv Biochem Eng Biotechnol 45:1–27
Lee YH, Fan LT (1983) Kinetic studies of enzymatic hydrolysis insoluble cellulose. Biotechnol Bioeng 25:939–966
Lin J, Pillay B, Singh S (1999) Purification and biochemical characteristics of β-d-glucosidase from a thermophilic fungus, Thermomyces lanuginosus-SSBP. Biotechnol Appl Biochem 30:81–87
Mandels M, Weber J (1969) The production of cellulases. Adv Chem Ser 95:394–414
McCarter JD, Withers SG (1994) Mechanisms of enzymatic glycoside hydrolysis. Curr Opin Struct Biol 4:885–892
Montero MA, Romeu A (1992) Kinetic study on the β-glucosidase-catalysed reaction of Trichoderma viride cellulase. Appl Microbiol Biotechnol 38:350–353
Murray P, Aro N, Collins C, Grassick A, Penttila M, Saloheimo M, Tuohy M (2004) Expression in Trichoderma reesei and characterisation of a thermostable family 3 β-glucosidase from the moderately thermophilic fungus Talaromyces emersonii. Protein Expr Purif 38:248–257
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Parr SR (1983) Some kinetic properties of the β-d-glucosidase (cellobiase) in a commercial cellulase product from Penicillium funiculosum and its relevance in the hydrolysis of cellulose. Enzyme Microb Technol 5:457–462
Parry NJ, Beever DE, Owen E, Vandenberghe I, Van BJ, Bhat MK (2001) Biochemical characterization and mechanism of action of a thermostable β-glucosidase purified from Thermoascus aurantiacus. Biochem J 353:117–127
Reese ET, Levinson HS, Downing MH (1950) Quartermaster culture collection. Farlowia 4:45–86
Riou C, Salmon J-M, Vallier M-J, Günata Z, Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64:3607–3614
Rose TM, Henikoff JG, Henikoff S (2003) CODEHOP (COnsensus-DEgenerate Hybrid Oligonucleotide Primer) PCR primer design. Nucleic Acids Res 31:3763–3766
Saha BC, Bothast RJ (1996) Production, purification, and characterization of a highly glucose-tolerant novel β-glucosidase from Candida peltata. Appl Environ Microbiol 62:3165–3170
Saloheimo M, Kuja-Panula J, Ylosmaki E, Ward M, Penttilä M (2002) Enzymatic properties and intracellular localization of the novel Trichoderma reesei β-glucosidase BGLII (Cel1A). Appl Environ Microbiol 68:4546–4553
Sambrook J, Fritsch EF, Maniatis T (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY
Seidle HF, Huber RE (2005) Transglucosidic reactions of the Aspergillus niger family 3 β-glucosidase: qualitative and quantitative analyses and evidence that the transglucosidic rate is independent of pH. Arch Biochem Biophys 436:254–264
Seidle HF, Marten I, Shoseyov O, Huber RE (2004) Physical and kinetic properties of the family 3 β-glucosidase from Aspergillus niger which is important for cellulose breakdown. Protein J 23:11–23
Shewale JG (1982) β-Glucosidase: its role in cellulase synthesis and hydrolysis of cellulose. Int J Biochem 14:435–443
Sinnott ML (1990) Catalytic mechanisms of enzymic glycosyl transfer. Chem Rev 90:1171–1202
Skoog B, Wichman A (1986) Calculation of the isoelectric points of polypeptides from the amino acid composition. Trends Anal Chem 3:82–83
Sternberg D, Vijayakumar P, Reese ET (1977) β-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Can J Microbiol 23:139–147
Stokvis E, Rosing H, Beijnen JH (2005) Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? Rapid Commun Mass Spectrom 19:401–407
Tengborg C, Galbe M, Zacchi G (2001) Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam-pretreated softwood. Biotechnol Prog 17:110–117
Tu M, Zhang X, Kurabi A, Gilkes N, Mabee W, Saddler J (2006) Immobilization of β-glucosidase on Eupergit C for lignocellulose hydrolysis. Biotechnol Lett 28:151–156
Varghese JN, Hrmova M, Fincher GB (1999) Three-dimensional structure of a barley β-d-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 7:179–190
Wood TM (1985) Properties of cellulolytic enzyme systems. Biochem Soc Trans 13:407–410
Xie Y, Gao Y, Chen Z (2004) Purification and characterization of an extracellular β-glucosidase with high transglucosylation activity and stability from Aspergillus niger No. 5.1. Appl Biochem Biotechnol 119:229–240
Zorov IN, Gusakov AV, Baraznenok VA, Bekkarevich AO, Okunev ON, Sinitsyn AP, Kondrat'eva EG (2001) Isolation and properties of cellobiase from Penicillium verruculosum. Appl Biochem Micro 37:587–592
Acknowledgements
Johnny Christensen (Novozymes A/S) is acknowledged for the heterologous production of the BGL from Penicillium brasilianum in Aspergillus oryzae. Caslav Savic (Novozymes A/S) is gratefully thanked for purification of the recombinant BGL and for supplying a BGL from the commercial product Novozym 188. M.Sc. student Roberto Archila is thanked for his technical assistance during the enzyme characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krogh, K.B.R.M., Harris, P.V., Olsen, C.L. et al. Characterization and kinetic analysis of a thermostable GH3 β-glucosidase from Penicillium brasilianum . Appl Microbiol Biotechnol 86, 143–154 (2010). https://doi.org/10.1007/s00253-009-2181-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2181-7