Abstract
The serious problems of environmental pollution and energy shortage have pushed the green economy photocatalysis technology to the forefront of research. Therefore, the development of an efficient and environmentally friendly photocatalyst has become a hotpot. In this work, magnetic Fe3O4/C/MnO2/C3N4 composite as photocatalyst was synthesized by combining in situ coating with low-temperature reassembling of CN precursors. Morphology and structure characterization showed that the composite photocatalyst has a hollow core–shell flower-like structure. In the composite, the magnetic Fe3O4 core was convenient for magnetic separation and recovery. The introduction of conductive C layer could avoid recombining photo-generated electrons and holes effectively. Ultra-thin g-C3N4 layer could fully contact with coupled semiconductor. A Z-type heterojunction between g-C3N4 and flower-like MnO2 was constructed to improve photocatalytic performance. Under the simulated visible light, 15 wt% photocatalyst exhibited 94.11% degradation efficiency in 140 min for degrading methyl orange and good recyclability in the cycle experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
In recent years, with the increase of wastewater discharge, a large amount of toxic and harmful organic pollutants are put into the water, which are difficult to completely degrade1,2,3,4,5,6. Advanced photocatalytic oxidation technology with strong oxidation capacity and high efficiency was considered as a very promising wastewater treatment means for the degradation of organic pollutants7,8,9,10,11,12,13,14. Among them, semiconductor-based photocatalysts with mild reaction conditions were most widely used15,16,17,18,19,20,21,22. In addition, various styles of self-assembled photocatalyst nanostructures were synthesized, such as one-dimensional nanotubes23, two-dimensional layered structure24,25, three-dimensional network structure26, three-dimensional flower-like structure27 and etc.
MnO2 has some advantages including low cost, high stability and environmental friendliness. Besieds, its narrow band gap can increase the utilization of visible light, which is very promising as a photocatalyst28,29,30,31,32. The layered structure of δ-MnO2 is formed by the MnO6 octahedral layer with shared edges, and there are some cations and H2O molecules between the layers to maintain the charge balance33. δ-MnO2 has been widely used as a catalyst to purify the environment34,35,36. However, while the narrow band gap increases the usage of visible light, it is also accompanied by the rapid recombination of photo-generated electrons and holes. The low photocatalytic efficiency restricts pure MnO2 for practical applications37,38,39.
Coupling MnO2 with other semiconductors to build a heterojunction is an effective method to prevent the recombination of photo-generated carriers. Wang et al.40 first reported that g-C3N4 could decompose water to generate hydrogen by visible light irradiation. Subsequently, owing to excellent visible light activity and chemical stability, g-C3N4 as photocatalyst has attracted a lot of attention41,42,43. Recently, Zhu et al.44 coated g-C3N4 on the semiconductor surface to form an ultra-thin g-C3N4 layer, avoiding the low contact rate between the bulk g-C3N4 and the coupling semiconductor interface. Therefore, the design of the composite with ultra-thin g-C3N4 layer and MnO2 is expected to achieve significant charge transfer at their interface by facilitating the separation of photo-generated carriers, thereby enhancing photocatalytic activity.
In our previous research, Fe3O4 was introduced as a magnetic core to construct a magnetically recyclable core–shell structure photocatalyst. The existence of magnetic core was beneficial to the recycle of the catalyst45,46. In this paper, hollow Fe3O4 microspheres were also synthesized by using a hydrothermal method as magnetic cores. Subsequently, the carbon layer was obtained by calcining the polymer layer formed on the periphery of Fe3O4 microspheres, which can protect the core and act as an electronic conductor simultaneously. Then, flower-like MnO2 was grown on the periphery of Fe3O4/C microspheres through the hydrothermal method. Finally, the transparent CN precursor produced with the aid of the neutral hydrothermal process was polymerized in situ on the surface of above microspheres at a low temperature. A flower-like Fe3O4/C/MnO2/C3N4 composite photocatalyst with core–shell structure was obtained. The photocatalyst displayed remarkable photocatalytic activity for degrading the organic dye methyl orange (MO). The specific synthetic steps are shown in Fig. 1.
Experimental section
Preparation of Fe3O4/C core–shell composite
First, hollow Fe3O4 microspheres were obtained by the hydrothermal method47. Then the polymer layer was prepared through a distillation precipitation process. 0.10 g Fe3O4 microspheres were ultrasonically dispersed in 80 mL acetonitrile. 1.0 mL divinylbenzene (DVB), 1.0 mL methyl methacrylate (MMA), and 0.040 g 2,2-azobisisobutyronitrile (AIBN) were also added into the above solution. The system was heated in 90 °C water bath for 2 h. Afterwards, Fe3O4/P(MMA-DVB) microspheres were obtained by using an external magnet, and washed three times. Then, the Fe3O4/P(MMA-DVB) sample was calcined at 600 °C for 2.0 h to obtain Fe3O4/C microspheres.
Preparation of Fe3O4/C/MnO2 flower-like composite
0.30 g Fe3O4/C microspheres were fully dispersed to 80 mL 0.055 M KMnO4 solution. Next, 1.0 mL HCl was added to the mixture dropwise. Thereafter, the mixture was transferred to an autoclave and heated to 100 °C for 6.0 h. At last, flower-like Fe3O4/C/MnO2 microspheres with core–shell structure were obtained by using an external magnet, and washed three times and lyophilized.
Preparation of Fe3O4/C/MnO2/C3N4 composite
6.0 g dicyandiamide was calcined at 550 °C for 4.0 h to produce g-C3N4. Thereafter, 2.0 g g-C3N4 powder was dispersed in 80 mL deionized water, and then heated at 210 °C for 6 h to form a CN transparent precursor. Fe3O4/C/MnO2 microspheres were added to the precursor (5.0, 10, 15, 20, 30 wt%). The solvent was slowly removed through a lyophilized process. Finally, Fe3O4/C/MnO2/C3N4 flower-like photocatalyst was obtained via annealing at 200 °C for 4.0 h in a tube furnace under N2 protection.
Characterization
Scanning electron microscope (SEM, JSM-6700F, JEOL Ltd., Japan) was employed to obtain a surface topography image of the samples. Transmission images were gotten by using a high-resolution transmission electron microscope (TEM, JEM-3010, Hitachi Co., Japan). X-ray diffraction patterns of samples were obtained by the use of an X-ray diffractometer (XRD, Shimadzu XRD-7000, Shimadzu Co., Japan). X-ray photoelectron spectrometer (XPS, JPS-9010 MC, JEOL Ltd., Japan) was utilized to obtain the samples’ surface elemental composition of the samples. Brunauer–Emmett–Teller (BET, ASAP 2020, Quantachrome, US) means was used to test the pore size and specific surface area of the catalyst. The saturation magnetization of the samples was obtained by employing a vibrating sample magnetometer (VSM, Lake Shore 7307, Lake Shore Ltd., USA). A photochemical reactor (BL-GHX-V, Shanghai Bilang Instruments Co., Ltd., China) was used to simulate the illumination. The ultraviolet–visible absorption spectra were measured on an ultraviolet–visible spectrophotometer (UV–vis, UV-5200PC, YuanXi, China).
Photocatalytic experiment
Firstly, 20 mg Fe3O4/C/MnO2/C3N4 photocatalyst were added to 65 mL, 10 mg/L MO solution. Under dark environment, the mixture was agitated to reach adsorbed-desorbed equilibrium. Secondly, photocatalytic reaction was carried out with simulate light stemming from a 400 W metal halide lamp. The absorbance of the solution at intervals was monitored with the help of UV–visible spectrophotometer. Ultimately, the degradation curves of the MO solution were recorded, followed by the calculation of photocatalytic degradation rate.
Results and discussion
SEM and TEM images of samples are shown in Fig. 2. In Fig. 2a, Fe3O4 microspheres prepared by the hydrothermal method have good dispersibility and uniform size of about 200 nm. Figure 2b shows Fe3O4/P(MMA-DVB) microspheres prepared by distillation precipitation process. Compared with the former, the surface of the latter becomes much smoother, which proves the successful formation of polymer coating. And these polymer core–shell microspheres have a diameter of 225 nm. To obtain the conductive carbon layer, the polymer microspheres were calcined and carbonized. The SEM image of Fe3O4/C microspheres is displayed in Fig. 2c. One can see that the original core–shell structure of the material is not destroyed after the calcination treatment. And the agglomeration that originally occurred in Fe3O4/P(MMA-DVB) polymer microspheres has been slightly weakened due to the carbonization treatment. From Fig. 2d,e, it can be found out that the flower-like morphology of the composite microspheres produced by the hydrothermal method is composed of MnO2 intersecting sheets. And the overall particle size is about 480 nm. As shown in Fig. 2f, the overall flower-like morphology has not changed, but the thickness of the MnO2 flower sheets has increased significantly. This case indicates that the ultra-thin C3N4 layer is successfully formed on the surface of MnO2 to form a flower-like Fe3O4/C/MnO2/C3N4 composite photocatalyst. It can be seen from Fig. 2g that the synthesized magnetic microspheres have a clear hollow structure with a particle size of about 200 nm. Figure 2h shows the TEM image of the Fe3O4/C microspheres, which have a core–shell structure with 13 nm thickness of C shell. Figure 2i is the TEM image of the flower-like Fe3O4/C/MnO2/C3N4 microspheres. It can be found out that the composite photocatalyst with a complete magnetic core and flower-like shell exhibits the diameter of around 480 nm. According to these results, the composite photocatalyst with a magnetic core and flower-like shell was successfully prepared.
SEM images of hollow Fe3O4 microspheres (a), Fe3O4/P(MMA-DVB) microspheres (b), Fe3O4/C microspheres (c), Fe3O4/C/MnO2 flower-like microspheres (d,e), and Fe3O4/C/MnO2/C3N4 flower-like microspheres (f), TEM images of hollow Fe3O4 microspheres (g), Fe3O4/C core–shell microspheres (h) and flower-like Fe3O4/C/MnO2/C3N4 microspheres (i).
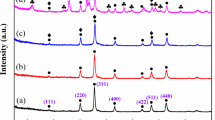
The crystal phase composition of the composite was demonstrated by XRD characterization, as shown in Fig. 3I. Figure 3I-a is the diffraction curve of the bulk g-C3N4 obtained by pyrolysis of dicyandiamide. The strong peak near 27.4° belongs to the (002) plane, corresponding to the crystal plane stack of the CN aromatic system48. The broad peak at 13.0° belongs to the (100) plane ascribed to the triazine repeat unit44. Figure 3I-b is the diffraction curve of Fe3O4 microspheres showing the diffraction peaks at 30.12°, 35.41°, 43.10°, 53.43°, 57.11°, and 62.52°, which are attributed to (220), (311), (400), (422), (511) and (440) crystal planes of Fe3O4 (JCDPS 85-1436)49. The sharp peaks indicate that the synthesized Fe3O4 are well crystallized. In Fig. 3I-c, in addition to the diffraction peaks of Fe3O4 component, the diffraction peaks at 12.20°, 36.70°, and 65.70° correspond to (002), (006), and (119) crystal planes, in consistence with the crystal planes of MnO2 (JCDPS 18-0802)50. This case indicates that the MnO2 is successfully synthesized. The diffraction curve of the Fe3O4/C/MnO2/C3N4 photocatalyst in Fig. 3I-d reveals that except for the diffraction peaks of Fe3O4 and MnO2, the diffraction peaks appear at 13.00° and 27.40° are separately assigned to the (100) and (002) crystal planes of g-C3N4. This situation indicates that CN precursor successfully becomes g-C3N4 after low temperature polymerization and high temperature calcination. XRD results show that the Fe3O4/C/MnO2/C3N4 composite photocatalyst were successfully synthesized.
The XRD patterns cannot verify the existence of the C layer. For further confirming the formation of the C layer, the Raman test was used to characterize the Fe3O4/C sample. The spectrum in Fig. 3II indicates two different peaks at 1344 cm−1 and 1596 cm−1, corresponding to D-band and G-band of carbon material, respectively. These results confirm the carbonization of Fe3O4/P(MMA-DVB) material, and Fe3O4/C microspheres are successfully obtained. These two bands are related to the A1g phonon of sp3 carbon atoms in disordered graphite and the in-plane vibration of sp2 carbon atoms in the crystalline graphite, respectively51. The peak intensity ratio (ID/IG) can evaluate the carbon material’s crystallinity. The smaller the value is, the higher the degree of atomic order is52. Herein, the value is 0.79, meaning that the carbon material is graphitized partially. Therefore, the presence of the carbon matrix can improve the electronic conductivity and help avoid the recombination of photo-generated electron holes.
The surface chemical composition and the chemical state of the products were demonstrated by XPS characterization. Figure 4a is the full-scan spectrum of the photocatalyst, presenting the peaks of Mn, O, N, and C elements. From Fig. 4b, as for the Mn 2p spectrum, two peaks at 653.9 eV and 642.3 eV correspond to Mn 2p1/2 and Mn 2p3/2. With respect to the O1s, as illustrated in Fig. 4c, three peaks at 529.7 eV, 531.3 eV, 533.2 eV are fitted, which are separately attributed to the Mn–O–Mn lattice oxygen, surface hydroxyl and surface adsorbed oxygen. The C1s spectrum in Fig. 4d shows the sub-bands centered at 284.8 eV and 288.5 eV, which are ascribed to the C–C coordination of the surface-unstable carbon and N=C–N2 of g-C3N4. In addition, there is another peak centering at 286.3 eV, which is assigned to the C-O bond formed between the C of C3N4 and the O of MnO2. This result indicates that MnO2 and g-C3N4 are closely connected and form a solid MnO2/g-C3N4 interface, promoting the transfer and separation of photo-generated carriers. In the case of the N1s spectrum (Fig. 4e), the peaks at 399.4 eV, 400.5 eV, 401.8 eV, and 405.3 eV are separately designated as carbon-bonded sp2 hybrid aromatic C=N–C, a tertiary nitrogen bonded to a carbon atom in the form N–(C)3, NH and the charge effect or positive charge localization in the heterocyclic ring. The XPS spectra powerfully verify the surface chemical composition of the Fe3O4/C/MnO2/C3N4 photocatalyst.
The specific surface area and the pore structure of Fe3O4, Fe3O4/C, Fe3O4/C/MnO2 and Fe3O4/C/MnO2/C3N4 products are listed in Table 1. The former of the Fe3O4/C/MnO2 and Fe3O4/C/MnO2/C3N4 products are 119.56 m2/g and 120.25 m2/g, and the latter of them are 0.35 cm3/g and 0.31 cm3/g. Since C3N4 does not significantly affect the morphology of the composite structure, these parameters of the two samples are almost similar. The higher values are owing to the flower-like structure of the composite photocatalyst. The increase in specific surface area is conducive to exposing more active sites and increasing more surface adsorption, followed by improving catalytic performance.
To evaluate the saturation magnetization value of Fe3O4, Fe3O4/C, Fe3O4/C/MnO2 and Fe3O4/C/MnO2/C3N4, VSM measurement is conducted. It can be seen from Fig. 5a that the magnetization value of the Fe3O4 microspheres is 70.58 emu/g. After the carbon layer is recombined, the value of Fe3O4/C microspheres decreases to 56.97 emu/g (Fig. 5b). After the flower-like MnO2 was fabricated, the content of Fe3O4 component is decreasing, which leads to the value of Fe3O4/C/MnO2 microspheres decreases obviously to 37.62 emu/g (Fig. 5c). With the further formation of g-C3N4, the value is 30.02 emu/g (Fig. 5d). This value still meets the needs of magnetic separation. As shown in the illustration, when the magnet is placed next to the Fe3O4/C/MnO2/C3N4 photocatalyst suspension, the photocatalyst can be quickly attracted to the side of the cuvette in a short time. The results show that the photocatalyst has a good magnetic response to the magnetic field, favoring the magnetic separation from the mixed solution.
Determining the adsorption capacity of the photocatalyst in dark reaction, then degrading MO under simulated light is used to investigate the photocatalytic activity of the prepared photocatalyst, and the results are shown in Fig. 6. Figure 6a reveals the mixture reached adsorption–desorption equilibrium within 60 min. And Fe3O4/C/MnO2/C3N4 can adsorb about 22% of MO within 60 min, which is related to its higher specific surface area (120.25 m2/g). Figure 6b displays that UV–Vis is employed to monitor the change in the absorbance of the solution during the photocatalytic reaction. In Fig. 6b, one can clearly view that MO was almost completely degraded with adding Fe3O4/C/MnO2/C3N4 composite photocatalyst after 140 min. The photocatalytic degradation MO over Fe3O4/C/MnO2/C3N4 could be described by the following reactions:
Adsorption of MO on sample in dark reaction (a), UV–vis spectra of MO solution at different times in the presence of Fe3O4/C/MnO2/C3N4 photocatalyst (b), Ct/C0 change with time (c), first-order kinetic model linear fit (d). Reaction conditions: [MO] = 65 mL, 10 mg/L, [photocatalyst] = 20 mg, [light] = 400 W metal halide lamp, T = 25 °C, t = 140 min.
Figure 6c indicates the change of the MO concentration ratio Ct/C0 with varying the light time, in which C0 and Ct are the initial concentration of MO and the concentration of MO during the reaction, respectively. The degradation rate of MO solution with Fe3O4/C/MnO2/C3N4 photocatalyst reaches 94.11%. From Fig. 6d, this reaction is attributed to a pseudo first-order reaction, which belongs to the Langmuir–Hinshelwood model with ln (Ct/C0) = −kt. In the formula, k is the apparent first-order rate constant. The calculated rate constant k of Fe3O4/C/MnO2/C3N4 photocatalyst is 0.022 min−1. The excellent photocatalytic performance of Fe3O4/C/MnO2/C3N4 composite material benefits from the synergistic effect between the various components.
In order to find the optimal ratio, the effect of amount of g-C3N4 on the photocatalytic efficiency was investigated. Meanwhile, determining the minimum optimal amount of photocatalyst in practical applications is important to reduce the costs. The composite photocatalyst containing different amounts of g-C3N4 (5%, 10%, 15%, 20%, 30%) were used to degrade MO dyes under the same conditions. From Fig. 7a,b, when the amount of g-C3N4 is 15%, the Fe3O4/C/MnO2/C3N4 composite photocatalyst has the highest value. In Fig. 7c, the effect of the amount of photocatalyst on the degradation efficiency is examined. The results show that the photocatalytic efficiency gradually increases when the amount of photocatalyst increases in the range of 0–20 mg, due to the effective reaction area and the reactive site increase. When the amount of photocatalyst continues to increase, the photocatalytic efficiency does not change significantly, which may be caused by the particle agglomeration affecting the increase of active sites. Therefore, the optimal dosage of Fe3O4/C/MnO2/C3N4 photocatalyst is 20 mg. Considering the industrial application of Fe3O4/C/MnO2/C3N4 nanoparticles, it is essential to investigate the recyclability and stability of the photocatalyst. The Fe3O4/C/MnO2/C3N4 was reused four times to examine their performances. And Fig. 7d reveals the results that the degradation rates for the four cycles are 94.11%, 90.42%, 88.37% and 79.69%, respectively. There is no doubt that after the photocatalyst is recycled, the conversion rate will decrease, which might result from the loss of sample during the cycle. However, even after four cycles, the value still has 79.69% that might be related with the structure stability of the used photocatalysts, strongly demonstrating that the designed photocatalyst has excellent recyclability.
Effect of the amount of C3N4 on the degradation of MO (a,b), effect of the amount of Fe3O4/C/MnO2/C3N4 photocatalyst on the degradation of MO (c), cyclic test of MO degradation of Fe3O4/C/MnO2/C3N4 photocatalyst (d). Reaction conditions: [MO] = 65 mL, 10 mg/L, [light] = 400 W metal halide lamp, T = 25 °C, t = 140 min. For (a,b,d), [photocatalyst] = 20 mg.
In this study, Fe3O4/C/MnO2/C3N4 photocatalyst was synthesized by compounding g-C3N4 on the surface of MnO2. In terms of enhanced photocatalytic activity, it is assumed that the charge transfer in the photocatalyst uses the Z-type mechanism, as shown in Fig. 8. For the individual g-C3N4 or MnO2 component, due to thermodynamic effects, photo-generated holes in g-C3N4 cannot oxidize OH- to form •OH radicals, while photo-generated electrons in MnO2 cannot generate ·O2− radicals effectively. Therefore, individual g-C3N4 or MnO2 material cannot possess good photocatalytic performances. However, after a heterojunction was fabricated between these two components, the photo-generated electrons in the conduction band of MnO2 can be transferred to the valence band of g-C3N4 and combined with the photo-generated holes there. This configuration of the Z-type scheme makes the utilization of holes from MnO2 and electrons from g-C3N4 remarkably enhanced. In addition, the conductive C layer can also increase the photo-generated electron–hole pairs’ separation in MnO2, which effectively prevents the recombination of photo-generated carriers. In the meantime, the higher specific surface area supplies much more active sites for photocatalytic activities. The prepared flower-like Fe3O4/C/MnO2/C3N4 photocatalyst forms a Z-type photocatalytic system, which effectively enhances the separation of carrier, so that the composite material has excellent photocatalytic degradation efficiency.
Conclusions
In summary, a magnetic recyclable flower-like Fe3O4/C/MnO2/C3N4 heterojunction photocatalyst was prepared for degrading organic dyes. The Fe3O4 core was used to facilitate magnetic separation and recovery. The C layer could conduct photo-generated electrons in MnO2 and protect the core. The thin g-C3N4 layer was compounded on the surface of MnO2, which greatly improved the specific surface area and the reactive sites of the material. The obtained Fe3O4/C/MnO2/C3N4 composites exhibited enhanced photocatalytic performance for the degradation of MO solution (65 mL, 10 mg/L) under simulated light irradiation. The maximum photocatalytic degradation efficiency was 94.11% within 140 min. It was assumed that a Z-type heterojunction was fabricated between MnO2 and g-C3N4, which stimulated the electron transfer from the valence band of MnO2 to the conduction band of g-C3N4. This structure promoted the photo-generated electron–hole pairs’ separation, inhibited the free charges’ recombination, and improved effective use of visible light. In here, an effective method to construct heterostructure nanomaterials was provided for efficient photocatalytic degradation.
References
Masuda, Y. Bio-inspired mineralization of nanostructured TiO2 on PET and FTO films with high surface area and high photocatalytic activity. Sci. Rep. 10, 13499 (2020).
Lee, M. Y. et al. Comparison of UV/H2O2 and UV/PS processes for the treatment of reverse osmosis concentrate from municipal wastewater reclamation. Chem. Eng. J. 388, 124260 (2020).
Liu, N. et al. Magnetic Fe3O4@MIL-53(Fe) nanocomposites derived from MIL-53(Fe) for the photocatalytic degradation of ibuprofen under visible light irradiation. Mater. Res. Bull. 132, 111000 (2020).
Takanabe, K. Photocatalyticwater splitting: quantitative approaches toward photocatalyst by design. ACS Catal. 7, 8006–8022 (2017).
Kumar, M., Basera, P., Saini, S. & Bhattacharya, S. Theoretical insights of codoping to modulate electronic structure of TiO2 and SrTiO3 for enhanced photocatalytic efficiency. Sci. Rep. 10, 15372 (2020).
Fu, J., Xu, Q., Low, J., Jiang, C. & Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2 production photocatalyst. Appl. Catal. B Environ. 243, 556–565 (2019).
Trojanowicz, M., Bojanowska-Czajka, A., Bartosiewicz, I. & Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS): a review of recent advances. Chem. Eng. J. 336, 170–199 (2018).
Hodges, B. C., Cates, E. L. & Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018).
Xu, J., Olvera-Vargas, H., Loh, B. J. H. & Lefebvre, O. FTO-TiO2 photoelectrocatalytic degradation of triphenyltin chloride coupled to photoelectro-Fenton: a mechanistic study. Appl. Catal. B Environ. 271, 118923 (2020).
Zhang, G. et al. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: a review. Chem. Eng. J. 392, 123684 (2020).
Sakhare, P. A. et al. Magnetically recoverable BiVO4/NiFe2O4 nanocomposite photocatalyst for efficient detoxification of polluted water under collected sunlight. Mater. Res. Bull. 129, 110908 (2020).
Zhang, Z. et al. Synthesis of 1D Bi12O17ClxBr2–x nanotube solid solutions with rich oxygen vacancies for highly efficient removal of organic pollutants under visible light. Appl. Catal. B Environ. 269, 118774 (2020).
Ma, Y. et al. Tuning polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. Polymer 156, 128–135 (2018).
Ma, Y. et al. Morphology-dependent electrochemical supercapacitors in multi-dimensional polyaniline nanostructures. J. Mater. Chem. A 5, 14041–14052 (2017).
Ran, J., Jaroniec, M. & Qiao, S. Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv. Mater. 30, 1704649 (2018).
Liu, D. et al. In-situ fabrication of atomic charge transferring path for constructing heterojunction photocatalysts with hierarchical structure. Appl. Catal. B Environ. 248, 459–465 (2019).
Jiang, D. et al. MoS2/SnNb2O6 2D/2D nanosheet heterojunctions with enhanced interfacial charge separation for boosting photocatalytic hydrogen evolution. J. Colloid Interface Sci. 536, 1–8 (2019).
Zhong, J. et al. Opposite photocatalytic oxidation behaviors of BiOCl and TiO2: direct hole transfer vs. indirect (OH)–O-center dot oxidation. Appl. Catal. B Environ. 241, 514–520 (2019).
Weng, B., Qi, M.-Y., Han, C., Tang, Z.-R. & Xu, Y.-J. Photocorrosion inhibition of semiconductor-based photocatalysts: basic principle, current development, and future perspective. Acs Catal. 9, 4642–4687 (2019).
Wang, F., Li, Q. & Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical energy conversion. Adv. Energy Mater. 7, 1700529 (2017).
Ma, Y. et al. Solid polyaniline dendrites consisting of high aspect ratio branches self-assembled using sodium lauryl sulfonate as soft templates: synthesis and electrochemical performance. Polymer 182, 121808 (2019).
Ma, Y. et al. Three-dimensional core-shell Fe3O4/polyaniline coaxial heterogeneous nanonets: preparation and high performance supercapacitor electrodes. Electrochim. Acta 315, 114–123 (2019).
Geng, R. et al. In situ construction of Ag/TiO2/g-C3N4 heterojunction nanocomposite based on hierarchical co-assembly with sustainable hydrogen evolution. Nanomaterials 10, 1 (2020).
Li, K. K. et al. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 61, 728–736 (2018).
Yin, J. J. et al. Facile preparation of self-assembled MXene@Au@CdS nanocomposite with enhanced photocatalytic hydrogen production activity. Sci. China Mater. 63, 2228–2238 (2020).
Jiao, T. F. et al. Self-assembly reduced graphene oxide nanosheet hydrogel fabrication by anchorage of chitosan/silver and its potential efficient application toward dye degradation for wastewater treatments. ACS Sustain. Chem. Eng. 3, 3130–3139 (2015).
Gao, S. et al. A novel 3D hollow magnetic Fe3O4/BiOI heterojunction with enhanced photocatalytic performance for bisphenol A degradation. Chem. Eng. J. 307, 1055–1065 (2017).
Das, S., Sarnanta, A. & Jana, S. Light-assisted synthesis of hierarchical flower-like MnO2 nanocomposites with solar light induced enhanced photocatalyticactivity. ACS Sustain. Chem. Eng. 5, 9086–9094 (2017).
Wang, H., Chen, S., Wang, Z., Zhou, Y. & Wu, Z. A novel hybrid Bi2MoO6 MnO2 catalysts with the superior plasma induced pseudo photocatalytic-catalytic performance for ethyl acetate degradation. Appl. Catal. B Environ. 254, 339–350 (2019).
Liu, Y. et al. Promoted oxygen activation of layered micro-mesoporous structured titanium phosphate nanoplates by coupling nano-sized delta-MnO2 with surface pits for efficient photocatalytic oxidation of CO. Appl. Catal. B Environ. 254, 260–269 (2019).
Ding, Y. et al. Rational design of Z-scheme PtS-ZnIn2S4/WO3-MnO2 for overall photo catalytic water splitting under visible light. Appl. Catal. B Environ. 258, 117948 (2019).
Yang, Y. et al. UV–vis-infrared light-driven photothermocatalytic abatement of CO on Cu doped ramsdellite MnO2 nanosheets enhanced by a photoactivation effect. Appl. Catal. B Environ. 224, 751–760 (2018).
Liang, X. et al. Coupled morphological and structural evolution of delta-MnO2 to alpha-MnO2 through multistage oriented assembly processes: the role of Mn(iii). Environ. Sci. Nano 7, 238–249 (2020).
Zhang, Y., Li, H., Zhang, L., Gao, R. & Dai, W.-L. Construction of highly efficient 3D/2D MnO2/g-C3N4 nanocomposite in the epoxidation of styrene with TBHP. ACS Sustain. Chem. Eng. 7, 17008–17019 (2019).
Huang, J. et al. Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem. Eng. J. 370, 906–915 (2019).
Xu, Y. et al. Selective conversion of syngas to aromatics over Fe3O4@MnO2 and hollow HZSM-5 bifunctional catalysts. ACS Catal. 9, 5147–5156 (2019).
Zhao, J. et al. Energy-efficient fabrication of a novel multivalence Mn3O4–MnO2 heterojunction for dye degradation under visible light irradiation. Appl. Catal. B Environ. 202, 509–517 (2017).
Chen, M. et al. FeOOH-loaded MnO2 nano-composite: an efficient emergency material for thallium pollution incident. J. Environ. Manag. 192, 31–38 (2017).
Zhang, Z. et al. Conversion of CaTi1−xMnxO3 delta based photocatalyst for photocatalytic reduction of NO via structure-reforming of Ti-bearing blast furnace slag. ACS Sustain. Chem. Eng. 7, 10299–10309 (2019).
Wang, X. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009).
Lin, L. et al. Crystalline carbon nitride semiconductors prepared at different temperatures for photocatalytic hydrogen production. Appl. Catal. B Environ. 231, 234–241 (2018).
Fu, J., Yu, J., Jiang, C. & Cheng, B. g-C3N4 based heterostructured photocatalysts. Adv. Energy Mater. 8, 1701503 (2018).
Yang, C., Wang, B., Zhang, L., Yin, L. & Wang, X. Synthesis of layered carbonitrides from biotic molecules for photoredox transformations. Angew. Chem. Int. Ed. Engl. 56, 6627–6631 (2017).
Wang, Y., Yang, W., Chen, X., Wang, J. & Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 220, 337–347 (2018).
Boruah, P. K. & Das, M. R. Dual responsive magnetic Fe3O4–TiO2/graphene nanocomposite as an artificial nanozyme for the colorimetric detection and photodegradation of pesticide in an aqueous medium. J. Hazard Mater. 385, 121516 (2020).
Yuan, D. et al. Superparamagnetic polymer composite microspheres supported Schiff base palladium complex: an efficient and reusable catalyst for the Suzuki coupling reactions. Chem. Eng. J. 287, 241–251 (2016).
Liu, Y. et al. One-pot hydrothermal synthesis of highly monodisperse water-dispersible hollow magnetic microspheres and construction of photonic crystals. Chem. Eng. J. 259, 779–786 (2015).
Dong, Z., Wu, Y., Thirugnanam, N. & Li, G. Double Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 430, 293–300 (2018).
Wang, D., Duan, H., Lü, J. & Lü, C. Fabrication of thermo-responsive polymer functionalized reduced graphene oxide@Fe3O4@Au magnetic nanocomposites for enhanced catalytic applications. J. Mater. Chem. A 5, 5088–5097 (2017).
Zhang, H., Cao, D. & Bai, X. High rate performance of aqueous magnesium-ion batteries based on the δ-MnO2@carbon molecular sieves composite as the cathode and nanowire VO2 as the anode. J. Power Sources 444, 227299 (2019).
Schuepfer, D. B. et al. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 161, 359–372 (2020).
Tang, J. & Yamauchi, Y. MOF morphologies in control. Nat. Chem. 8, 638–639 (2016).
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (Grant No. 51503116), the Shandong Provincial Natural Science Foundation (Grant No. ZR2019BB063) and the Applied Basic Research Foundation of Qingdao City (Grant No. 19-6-2-13-cg).
Author information
Authors and Affiliations
Contributions
M.M., Y.Y. and Y.C. wrote the main manuscript text. Y.Y. drew the flow chart and mechanism diagram. Y.M. ananlyzed the data. J.J.,Y.M., Z.W., W.H., S.W., M.L., D.M. and X.Y. collected data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, M., Yang, Y., Chen, Y. et al. Fabrication of hollow flower-like magnetic Fe3O4/C/MnO2/C3N4 composite with enhanced photocatalytic activity. Sci Rep 11, 2597 (2021). https://doi.org/10.1038/s41598-021-81974-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81974-2
- Springer Nature Limited
This article is cited by
-
Enhancing photocatalytic activity through 2D heterostructured P/MnO2/r-GO nanocomposites: a study on synthesis, structure, and optical properties
Ionics (2023)
-
Research progress of novel magnetic two-dimensional carbon composites in photocatalytic degradation of pollutants: a review
Environmental Science and Pollution Research (2023)
-
Constructing 3D magnetic flower-like Fe3O4@SiO2@Co3O4@BiOCl heterojunction photocatalyst for degrading rhodamine B
Environmental Science and Pollution Research (2022)
-
One-dimensional recoverable ZnFe2O4/C/MnO2/BiOI magnetic composite with enhanced photocatalytic performance for organic dyes degradation
Environmental Science and Pollution Research (2022)