Abstract
Real-world data comparing the effectiveness of various glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in type 2 diabetes mellitus (T2DM) are limited. We investigated the clinical effectiveness of liraglutide and dulaglutide in Japanese T2DM in a real-world setting. This retrospective study included 179 patients with T2DM who were treated with GLP-1 RA for at least 12 months (liraglutide, n = 97; dulaglutide, n = 82). We used stabilized propensity score-based inverse probability of treatment weighting (IPTW) to reduce selection bias and confounding by observed covariates. Changes in glycated hemoglobin (HbA1c) at the end of the 12-month treatment were evaluated. After adjustment by stabilized propensity score-based IPTW, no significant differences were observed in patient characteristics between the liraglutide and dulaglutide groups. HbA1c was significantly lower at 12 months in both groups (liraglutide, 8.9 to 7.4%; dulaglutide, 8.7 to 7.5%). Multivariate linear regression analysis showed no differences in the extent of changes in HbA1c at 12 months between the two agents. High baseline HbA1c, the addition of GLP-1 RA treatment modality, and in-hospital initiation of GLP-1 RA treatment were identified as significant contributing factors to HbA1c reduction. The effects of liraglutide and dulaglutide on lowering HbA1c levels at 12 months were comparable in a real-world setting.
Similar content being viewed by others
Introduction
Glucagon-like peptide-1 (GLP-1), an incretin hormone secreted by L-cells in the distal ileum and colon1, has various effects, including glucose-dependent enhancement of insulin secretion2, inhibition of glucagon secretion3, appetite suppression4, and suppression of gastric emptying5. Various GLP-1 receptor agonists (GLP-1 RAs) are available in the market at present. GLP-1 RAs can effectively lower blood glucose levels and facilitate weight loss with a low risk of hypoglycemia; hence, they are commonly used in patients with type 2 diabetes mellitus (T2DM). Furthermore, some GLP-1 RAs are reported to reduce the risk of 3-point major adverse cardiovascular events (MACE)6, 7, and a meta-analysis of four large-scale clinical studies that assessed GLP-1 RAs concluded that these agents can reduce the risk of cardiovascular events and all-cause mortality8.
Controlled clinical trials have demonstrated that GLP-1 RAs have distinct effects in terms of lowering glycated hemoglobin (HbA1c) levels and facilitating weight loss, as well as adverse reactions, such as nausea, vomiting, and diarrhea8, 9. In Japan, dulaglutide and liraglutide are the most widely used GLP-1 RAs in routine clinical practice10. In a randomized controlled trial (RCT), dulaglutide (up to 1.5 mg/week) was reported to be non-inferior to liraglutide (up to 1.8 mg/day)11. In a Japanese comparative study of these agents used at different doses, HbA1c-lowering effects after 52 weeks of treatment were greater with dulaglutide (up to 0.75 mg/week) than liraglutide (up to 0.9 mg/day)12. However, the above study evaluated patients who were not taking antidiabetic drugs or had discontinued antidiabetic monotherapy, thereby differing greatly from real-world clinical situations. In a real-world setting, only a few studies have investigated the effectiveness of liraglutide and dulaglutide in patients with T2DM, including those treated concomitantly with more than one oral hypoglycemic agent and insulin. To establish real-world evidence, we retrospectively examined changes in HbA1c after 12 months of GLP-1 RA treatment and compared liraglutide and dulaglutide. In this study, we used stabilized propensity score-based inverse probability of treatment weighting (IPTW) in order to reduce selection bias and confounding by observed covariates.
Results
Patient demographics after adjustment by stabilized propensity score-based IPTW
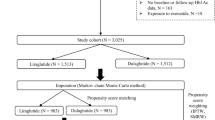
The selection of GLP-1 RA for treatment of these patients, and specifically with liraglutide and dulaglutide was based on the clinical assessment and judgment by the attending physicians. Table 1 (left-side) summarizes the clinical characteristics of the study patients before adjustment. Ninety-seven patients treated with liraglutide and 82 patients treated with dulaglutide at least 12 months. The average liraglutide dose were administered at 0.89 mg/day (SD: 0.26) and dulaglutide dose were 0.75 mg/week at 12 months, respectively. Compared with patients of the dulaglutide group, those of the liraglutide group were significantly younger, had shorter duration of T2DM, higher BMI, and used fewer classes of oral glucose-lowering agents. Higher prevalence of dementia was noted in the dulaglutide group. Next, we calculated the IPTW using the stabilized propensity scores to reduce selection bias and confounding by observed covariates and adjusted the patient characteristics. The adjusted patient characteristics are shown in Table 1 (right side). Ninety-seven and 72 patients were treated with liraglutide and dulaglutide, respectively. The distribution of the patients’ characteristics between the groups was balanced based on the small standardized mean difference and the insignificant P values.
Efficacy
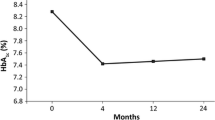
Figure 1 summarizes the effects of each treatment on HbA1c in the two groups after adjustment by stabilized propensity score-based IPTW. HbA1c was significantly lower at 12 months in both groups, relative to the respective value at baseline (before treatment), from 8.9 (SD: 1.5) to 7.4% (SD: 1.6) with liraglutide treatment and from 8.7 (SD: 1.7) to 7.5% (SD: 1.3) with dulaglutide therapy (Fig. 1). The change in HbA1c level was significantly different at 6 months of treatment (liraglutide vs. dulaglutide: − 1.4% [SD: 1.8] vs. − 1.2% [SD: 1.9], P = 0.016), but not at 12 months (− 1.4% [SD: 1.8] vs. − 1.2% [SD: 1.9], P = 0.100). This result was similar to the data before adjustment (Fig. S1). The percentage of patients who achieved HbA1c < 7% at 12 months was significant, compared to that at baseline, in both the liraglutide and dulaglutide groups (liraglutide: 10.3% to 46.4%; dulaglutide: 21.9% to 39.7%, Fig. 2). Similarly, the percentage of patients with HbA1c ≥ 8% significantly decreased in both groups, with no difference observed in the distribution of HbA1c at 12 months between the two groups.
HbAlc levels at baseline and 6 and 12 months of treatment after adjustment by stabilized propensity score-based inverse probability of treatment weighting. Data are mean (95% CI). **P < 0.01 vs. baseline, by Wilcoxon signed-rank test. †P < 0.05 for between the two groups, by Wilcoxon rank-sum test. HbA1c glycated hemoglobin.
Table 2 shows the effect of 12-month treatment with liraglutide and dulaglutide on various parameters, relative to the baseline. Body weight decreased significantly in both groups, though the reduction over the 12-month period differed significantly between the two groups (liraglutide vs. dulaglutide: − 2.7 kg [SD: 4.5] vs. − 1.2 kg [SD: 3.2], P = 0.005). The total and bolus insulin dose also decreased significantly in both groups, but no significant differences were observed between the two groups. eGFR was significantly lower in the liraglutide group, but no significant differences were observed between the two groups.
We employed univariate and multivariate linear regression analysis to determine the factors that contributed to the observed changes in HbA1c after 12 months of treatment (Table 3). Multivariate linear regression analysis showed that the change in HbA1c after 12 months of treatment correlated positively with reducing or switching to GLP-1 RA treatment, and negatively with baseline HbA1c and inpatient initiation of GLP-1 RA treatment, but no difference was detected between the two types of GLP-1 RAs.
Finally, we determined the factors that contributed to the observed changes in HbA1c after 12 months of treatment using univariate and multivariate analyses in non-adjusted data (Supplemental Tables S1, S2). Multivariate analysis identified baseline HbA1c, baseline plasma glucose, and inpatient initiation of GLP-1 RA treatment as significant independent factors that contributed to the change in HbA1c over the 12-month liraglutide treatment. Similarly, baseline HbA1c and GLP-1 RA treatment modality were identified as significant factors that contributed to the observed changes in HbA1c over the 12-month dulaglutide treatment.
Safety
Adverse reactions were observed in 27 patients (27.8%) of the liraglutide group and 20 patients (24.4%) of the dulaglutide group (P = 0.602) in non-adjusted data. Only mild adverse reactions were observed in both groups. The reported adverse reactions in the liraglutide group were nausea (n = 20), constipation (n = 8), and diarrhea (n = 1). Liraglutide dose reduction was required in one patient due to the adverse reactions. Hypoglycemia was observed in one patient. In the dulaglutide group, nausea (n = 7), diarrhea (n = 6), hepatic dysfunction (n = 3), and hypoglycemia (n = 2) were reported. Gastrointestinal symptoms were reported early after the initiation of treatment but resolved spontaneously.
Discussion
The present study compared the efficacy and safety of GLP-1 RAs, liraglutide and dulaglutide, in Japanese patients with T2DM in a real-world setting. We used stabilized propensity score-based IPTW in order to reduce selection bias and confounding by observed covariates. In a previously published RCT in Japanese patients, dulaglutide had greater HbA1c-lowering effects than liraglutide12. However, the use of other drugs and patient characteristics in that RCT differed from those in real-world clinical practice. The present study included several patients who used more than one oral glucose-lowering agents, unlike previously reported RCT in Japanese patients. In this study using stabilized propensity score-based IPTW, the change in HbA1c level was lower in the liraglutide treatment group at 6 months, but there was no difference in the HbA1c level at 12 months, indicating similar HbA1c-lowering effect by the two agents.
Our study identified three characteristics that were associated with changes in HbA1c over the 12-month treatment period (Table 3). First, baseline HbA1c; patients with high baseline HbA1c levels were more likely to show improved post-treatment HbA1c levels. Next, GLP-1 RA treatment modality; compared with ‘add-on’, the rate of improvement in HbA1c at 12 months was modest in ‘switch’ and ‘reduce’. Similar findings have been reported following liraglutide treatment13. In clinical practice, treatment is often switched from dipeptidyl peptidase-4 inhibitors to GLP-1 RAs. In such cases, it is necessary to consider that any improvement in HbA1c after 12-month treatment would be smaller than that after the addition of GLP-1 RAs. Third, initiation of GLP-1 RA treatment during hospitalization was more effective in lowering HbA1c levels than applying the same treatment at the outpatient setting. Although patients who started GLP-1 RA treatment during admission to the hospital had higher baseline HbA1c levels than those who underwent outpatient initiation (9.7% [SD: 1.5] vs. 8.3% [SD: 1.4], P < 0.001), their HbA1c levels at 12 months were lower (7.1% [SD: 1.4] vs. 7.6% [SD: 1.5], P = 0.032). This result is quite interesting, but it is likely that unmeasured confounding factors and/or differences in follow-up care patterns contributed to this effect, and therefore, further investigation is warranted in the future.
Several studies reported the weight-reducing effects of liraglutide and dulaglutide, with weight loss of 2.3 kg (95% confidence interval [CI] 2.0–2.5) in one study6 and 1.46 kg (95% CI 1.25–1.67) in another14. However, in the AWARD-6 study using the same two agents, liraglutide (up to 1.8 mg/day) was significantly more effective in reducing body weight than dulaglutide (up to 1.5 mg/week)11. Furthermore, in a Japanese phase III clinical study, body weight reduction by dulaglutide was negligible after 26 weeks of treatment15. Our results also showed that liraglutide was more effective in reducing body weight than dulaglutide. These results may partly explain why liraglutide is commonly used in obese diabetics in real-world clinical practice.
GLP-1 RAs are reported to help reduce HbA1c, body weight, and insulin dose among insulin users16,17,18. Although there are no studies that directly compared changes in insulin dose after GLP-1 RA initiation (liraglutide vs. dulaglutide), our results showed total and bolus insulin dose significantly decreased in liraglutide and dulaglutide, no significant differences were observed between the two agents.
Several reports compared the real-world use of GLP-1 RAs, but most comparisons were restricted to treatment maintenance19, 20 and cost-effectiveness21. In contrast, our study examined changes in HbA1c levels. A real-world study from Taiwan22 showed there was a statistically significant change in HbA1c at 12 months from baseline in each treatment group (dulaglutide: − 1.06% [SD: 1.70] and liraglutide: − 0.83% [SD: 1.61]), with a significant between-group difference in HbA1c reduction of − 0.23% (95% CI − 0.38 to − 0.08%). A study from United States also showed that treatment with dulaglutide significantly reduced HbA1c compared with liraglutide23. These results are different from our study, probably due to differences in drug dosage; the dose of dulaglutide in about 40% of the patients of the above study was 1.5 mg/week.
Few studies have investigated the real-world use of GLP-1 RAs in Japanese patients. Although more than 900 patients were enrolled in the JDDM-57 study10, HbA1c was analyzed after only 6 months of treatment with GLP-1 RAs. Despite the small sample size, the present study examined the contributing factors to changes in HbA1c induced by GLP-1 RAs in Japanese patients. We anticipate our data to help establish real-world evidence for the role of these factors in GLP-1 RA treatment.
Our study has several limitations. First, the sample size was relatively small, as described earlier, and the study was conducted in patients at a single institution treated by diabetologists; therefore, the results may not be generalizable to the entire population. However, since diabetologists often initiate the administration of GLP-1 RAs in Japan, our findings are considered plausible as real-world data at least in Japan. Second, the plasma glucose data were somewhat unreliable because not all blood samples were collected in a fasting state. However, evaluation of HbA1c, which was the primary endpoint, should be sufficient for the purpose of this study. Third, since liraglutide was the first to be launched in the Japanese market, there was a selection bias that dulaglutide could not be used in patients enrolled early in this study. In addition, the approved maximum dose of liraglutide in Japan under the public health system is 1.8 mg/day, and thus a higher dose of liraglutide was prescribed only in a few patients in the present study. Therefore, it is important to update this study in future investigations to establish more-up-date real-world evidence. Finally, propensity score is only appropriate when the strongly ignorable treatment assignment assumption is satisfied24. However, there is no reliable way to test this assumption. Conventionally, consideration is given to C-statistics and propensity score-adjusted estimates of variables, but these also have no standard criteria. In this study, the estimates of the propensity score-adjusted estimates of variables are very close in each group compared to the pre-adjustment, although some variables remained significant, but it is uncertain whether these sufficiently satisfied the strongly ignorable treatment assignment assumption.
In conclusion, we have demonstrated in the present retrospective study the presence of significant differences in the characteristics of patients treated with liraglutide or dulaglutide in a real-world setting, implying that GLP-1 RAs were selected according to individual patient characteristics. Even after reducing the selection bias and confounding using stabilized propensity score-based IPTW between liraglutide and dulaglutide treatments, the effects of these two agents on HbA1c levels after 12 months of treatment were comparable. Our results also suggest that baseline HbA1c level, GLP-1 RA treatment modality, and inpatient initiation of GLP-1 RA treatment may be associated with reduction in HbA1c levels in a real-world setting.
Patients and methods
Patients
This retrospective study included patients with T2DM who received outpatient or inpatient care at the Hospital of the University of Occupational and Environmental Health, Japan between September 2010 and August 2019, who underwent GLP-1 RA treatment for the first time, and who continued treatment with liraglutide or dulaglutide for at least 12 months. The following exclusion criteria were applied: patients with type 1 diabetes mellitus, severe infection or serious trauma, and hepatic dysfunction (transaminase level at least threefold higher than the normal upper limit). In addition, we also excluded patients who had used GLP-1 RA previously. This study was approved by the Institutional Ethics Review Committee of the University of Occupational and Environmental Health (approval #H27-186). Informed consent was obtained from the participants, and the study was performed in accordance with the Declaration of Helsinki.
Biochemical and clinical measurements
We collected patient data, including age, sex, disease duration, body mass index (BMI), arterial blood pressure, presence of diabetic microangiopathy or macroangiopathy, presence of hypertension, dyslipidemia, as well as use of glucose-lowering agents, antihypertensive agents, and antilipidemic medications. Blood and urine samples were collected either in a fasting or non-fasting state. In addition, the levels of plasma glucose, HbA1c, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), and estimated glomerular filtration rate (eGFR) were measured. HbA1c levels (%) were measured using a high-performance liquid chromatography method with a Tosoh HLC-723 G8 analyzer (Tosoh Co., Kyoto, Japan) and expressed in National Glycohemoglobin Standardization Program (NGSP) equivalent values, calculated based on the following equation: HbA1c (NGSP) = HbA1c (Japan Diabetes Society [JDS]) (%) + 0.4%25.
Baseline drug adjustments at liraglutide or dulaglutide initiation were categorized as follows: ‘add-on’, when the number of classes of glucose-lowering agents increased; ‘switch’, when the number of classes of glucose-lowering agents remained unchanged; ‘reduce’, when the number of classes of glucose-lowering agents decreased. In addition, if the number of the glucose-lowering agents increased during the 12-month period of GLP-1 RA treatment, the patient was recorded as ‘augmentation’.
Stabilized propensity score-based IPTW
To adjust for baseline patient characteristics between the two groups, the calculated stabilized propensity scores were weighted using the ‘proportion of patients receiving dulaglutide to all patients/propensity score’ in the dulaglutide group and the ‘proportion of patients receiving liraglutide to all patients/1 − propensity score’ in patients treated with liraglutide as the weighting coefficient on stability26. To calculate the stabilized propensity scores, multivariable logistic regression analysis was performed with the concomitant use of dulaglutide as the dependent variable and the following independent variables: age, sex, disease duration, BMI, HbA1c, AST, ALT, eGFR, dyslipidemia, dementia, GLP-1RA treatment modality (add-on, switch or reduce), initiation of GLP-1 RA treatment (outpatient or inpatient), number of oral antidiabetic agent classes, glucose-lowering agents (none, DPP-4 inhibitors, biguanides, SGLT-2 inhibitors, insulin), insulin dose. The area under the curve of the propensity scores model was 0.869 (95% CI 0.818–0.919).
Statistical analysis
Variables are expressed as mean (standard deviation) or number (%) of patients. Categorical variables were evaluated using the χ2 test. Student’s t-test or Wilcoxon rank-sum test was employed to compare the two groups, depending on the data distribution pattern. We applied the paired t-test or Wilcoxon signed-rank test depending on data distribution to assess changes within the group. Differences between liraglutide and dulaglutide were tested using Student's t-test or Wilcoxon rank-sum test. HbA1c target achievement at baseline and 12 months were compared using McNemar’s test. Univariate and multivariate logistic regression analyses were performed to assess the effects of GLP-1 RAs on changes in HbA1c at 12 months. Factors with P < 0.05 on univariate linear regression analysis and the type of GLP-1 RA treatment were entered into multivariate linear regression analysis. Missing data were imputed using the last observation carried forward method, and the results did not differ with or without imputation. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Armonk, NY).
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information file.
References
Drucker, D. J. Glucagon-like peptides. Diabetes 47, 159–169 (1998).
Drucker, D. J. & Nauck, M. A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Gutniak, M., Orskov, C., Holst, J. J., Ahrén, B. & Efendic, S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36) amide in normal subjects and patients with diabetes mellitus. N. Engl. J. Med. 326, 1316–1322 (1992).
Gutzwiller, J. P. et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am. J. Physiol. 276, R1541-1544 (1999).
Nauck, M. A., Kemmeries, G., Holst, J. J. & Meier, J. J. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60, 1561–1565 (2011).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Bethel, M. A. et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 6, 105–113 (2018).
Trujillo, J. M., Nuffer, W. & Ellis, S. L. GLP-1 receptor agonists: A review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 6, 19–28 (2015).
Ishigaki, Y. et al. Glucagon-like peptide-1 receptor agonist utilization in type 2 diabetes in Japan: A retrospective database analysis (JDDM 57). Diabetes Ther. 12, 345–361 (2021).
Dungan, K. M. et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet 384, 1349–1357 (2014).
Odawara, M. et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes. Metab. 18, 249–257 (2016).
Simioni, N. et al. Predictors of treatment response to liraglutide in type 2 diabetes in a real-world setting. Acta Diabetol. 55, 557–568 (2018).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Onishi, Y. et al. Subgroup analysis of phase 3 studies of dulaglutide in Japanese patients with type 2 diabetes. Endocr. J. 63, 263–273 (2016).
Lind, M., Jendle, J., Torffvit, O. & Lager, I. Glucagon-like peptide 1 (GLP-1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim. Care Diabetes 6, 41–46 (2012).
Lind, M. et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: Randomised clinical trial (MDI Liraglutide trial). BMJ 351, h5364 (2015).
Lee, J. et al. Dulaglutide as an add-on to insulin in type 2 diabetes; clinical efficacy and parameters affecting the response in real-world practice. Diabetes Metab. Syndr. Obes. 12, 2745–2753 (2019).
Norrbacka, K. et al. Glucagon-like peptide 1 receptor agonists in type 2 diabetes mellitus: Data from a real-world study in Spain. Diabetes Ther. 12, 1535–1551 (2021).
Zimner Rapuch, S. et al. Treatment patterns and persistence with GLP-1 RA treatments among patients with type 2 diabetes in France: A retrospective cohort analysis. Diabetes Ther. 12, 1553–1567 (2021).
Dilla, T. et al. The cost-effectiveness of dulaglutide versus liraglutide for the treatment of type 2 diabetes mellitus in Spain in patients with BMI 30 kg/m2. J. Med. Econ. 20, 443–452 (2017).
Chang, K. C. et al. Comparative effectiveness of dulaglutide versus liraglutide in Asian type 2 diabetes patients: A multi-institutional cohort study and meta-analysis. Cardiovasc. Diabetol. 19, 172 (2020).
Mody, R. et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States. Diabetes Obes. Metab. 21, 920–929 (2019).
Rosenbaum, P. R. & Rubin, D. B. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41–55 (1983).
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 1, 212–228 (2010).
Xu, S. et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 13, 273–277 (2010).
Acknowledgements
The authors thank Ms. N. Sakaguchi for the excellent technical assistance. We also thank Yoshihisa Fujino, MD, PhD, MPH (Institute of Industrial Ecological Sciences, University of Occupational and Environmental Health, Japan) for valuable comments to this work.
Author information
Authors and Affiliations
Contributions
K.T. and Y.O. formulated the concept and design of the study. K.T., T.A. and Y.O. acquired, analyzed and interpreted the data. K.T. drafted the text of the main manuscript and prepared tables and figures. K.T., Y.O. and Y.T. revised the manuscript. Y.T. supervised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, K., Okada, Y., Tokutsu, A. et al. Real-world effectiveness of liraglutide versus dulaglutide in Japanese patients with type 2 diabetes: a retrospective study. Sci Rep 12, 154 (2022). https://doi.org/10.1038/s41598-021-04149-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04149-z
- Springer Nature Limited