Abstract
Snow depth may have a complex influence on carbon cycling in winter. Here we set up a field experiment to investigate how different snow depths (0 cm, 60 cm, 90 cm) influenced carbon dioxide (CO2) in a wetland. The mean ± standard error of CO2 emissions under snow addition treatments (60 cm and 90 cm snow depths) were 0.92 ± 0.16 g·cm−2·s−1 and 0.53 ± 0.16 g·cm−2·s−1, respectively, compared with snow removal treatment (0 cm snow depth), 0.11 ± 0.05 g·cm−2·s−1. In general, snow addition increased CO2 fluxes significantly. As snow depths increased, microbial biomass carbon (MBC) and bacterial diversities increased drastically. More important, the community of bacteria differed under different treatments. Firmicutes, which can resist dehydration and extremely low temperatures, was widely distributed in the snow removal treatment, where it sustained soil biochemical processes. Overall, our study indicates that snow cover counteracts the negative effects on soil microbial activities caused by low temperatures and could play a critical role in winter carbon cycling in wetlands.
Similar content being viewed by others
Introduction
As a result of global warming, precipitation (including snow) has changed significantly during the past several decades, and permafrost in high latitude areas has begun to melt1,2. Soil organic carbon exposed to the air was decomposed by microorganisms and emitted into the atmosphere, which may aggravate global warming3. Therefore, there has been a large amount of research interest on carbon cycling in the context of climate change4,5, especially CO2. Wetlands in particular are generally considered as a large soil organic carbon pool6. Although northern hemisphere wetlands cover only 3% of the earth’s land area, they store 400–600 Gt of C, accounting for 1/4 to 1/3 of global soil organic carbon7,8,9. So it is very important to develop studies of carbon cycling in these regions. However, most of the studies were set up in growing season, but few in winter10. Several studies have shown that carbon emissions still occur in winter in northern wetlands, and these emissions also play an important role in global carbon cycling11,12,13. More than 3–40% of carbon accumulated through photosynthesis in the growing season will release through winter respiration14, accounting for about 4.4–21.6% of annual carbon releases11,12,13,15. However, this type of research has not yet been performed sufficiently, due to the difficulties of field measurements in winter, and still requires further discussion10,16.
Snow is the major variable in northern wetlands in winter, impacting CO2 fluxes in wetlands. For example, Blankinship and Hart (2012) found decreased snow depth could cause a 35% reduction in CO2 fluxes17. Li et al. (2016) also found that increased snow depth could increase the soil-atmosphere CO2 fluxes18. Since snow cover isolates soil from cold air, it usually generates a soil microclimate for carbon emissions19,20. However, there are still few experiments which have mainly studied how different snow depths impact on winter CO2 fluxes20,21,22,23,24,25.
Many researchers found that soil enzymes were catalysts in soil, participating in almost all soil biochemical processes, and they have a major influence on the carbon cycling of terrestrial ecosystems26,27. Among these, soil invertase belongs to hydrolase, which plays an important role in the process of carbon cycling. Under the condition of snow cover, soil temperature usually remains at approximately 0 °C and sometimes even higher, which might be warm enough to maintain soil biotic activities during the winter period28. Thus, higher soil temperatures may enhance soil enzyme activities to accelerate soil decomposition processes during winter time, which might be one of the most important ways that snow depths impact CO2 fluxes in winter29,30,31. However, to our knowledge, few studies have focused on this.
Several studies have reported that soil microbial biomass is even greater in the winter time than that in summer season in some regions32,33. More important, microorganisms are capable of maintaining catabolic (CO2 production) and anabolic (biomass synthesis) processes under sub-zero temperatures30,34,35. For example, Brooks et al. (1996) found that deep and continuous snow cover significantly increased microbial biomass carbon (MBC) and microbial decomposition rates22. All above studies indicated that CO2 emitted by soil microbial respiration in winter is an important part of the annual carbon budget36,37. Thus, there is an urgent need to explore how microbial community composition responds to snow cover, which is important for better understanding the mechanisms of subnivean CO2 emissions38,39. However, traditional methods such as phospholipid fatty acid (PLFA) analysis or polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) provided limited phylogenetic or taxonomic resolution of the influence of snowpack on microbial communities40. By comparison, high-throughput sequencing technology can clearly describe the soil microbial diversities and composition. For example, Liptzin et al. (2015) reported that in the Niwot area of Colorado, winter soil bacteria mainly included Cytophaga and Flexibacter, but the dominant microorganism under snowpack was SMF (snow mold fungi)41. Whether or not this conclusion is suitable for other regions has yet needed to be confirmed. Therefore, the mechanisms of which snow depth influences CO2 fluxes were insufficiently clear, especially regarding the microorganisms involved.
Here, we conducted a field experiment using snow gradient manipulation in Jinchuan wetland located in the west of Jinchuan Town, Huinan County, Jilin Province, China, which is known as the most concentrated group of crater lake wetlands, during the winter period from 2016 to 2017. Firstly, we tried to clarify how different snow depths influence CO2 emissions; Secondly, we attempted to explore the influence mechanisms of snow depth on CO2 fluxes, and whether soil invertase could make sense of subnivean CO2 emissions; Thirdly, whether MBC and bacterial composition might also influence subnivean CO2 emissions.
Materials and Methods
Site description and experimental design
The experiment was developed in a Jinchuan wetland located in Longwan National Natural Reserve, Jilin Province, China (42°20′56″N, 126°22′51″E) (Fig. 1a), as described by Shi et al.42. It has a north temperate monsoon climate, with a long winter, from October to May. The average annual temperature and precipitation are approximately 3 °C and 1053.9 mm, respectively43. Previous investigations have shown that Jinchuan wetland has a constant peat accumulation rate of approximately 1 mm/a, providing a carbon stock of approximately 4,599,300 m343.44. The wetland has a thick layer of peat, with a maximum thickness of 10 m.
Map of the study area located in Longwan National Natural Reserve in the Jinchuan wetland in China (a)42; Diagram of the experimental device (b: Schematic diagram of chamber; c: Actual layout of device).
Previous studies by our group have included an investigation of the background conditions of the study area, including hydrological conditions, elevation, vegetation and other environmental factors. The average snow depth was approximately 30-70 cm. According to these data, a region of 300 m2 were selected in which most of the biotic and abiotic properties were relatively homogeneous to develop our experiment. The snow gradient manipulation field study was conducted using a completely random design. Three snow depth treatments were included in this study, including 0 cm, 60 cm, and 90 cm, with five replicates of each snow depth randomly distributed in the area. There were 15 plots, each of them measured 1.5 m × 1.5 m and was encircled by four iron rods fixed in the soil. Closed static-chambers were fixed by connecting with the iron rods. The plots were separated by a buffer strip (3 ± 2 m) to control edge effects31 (Fig. 1c).
Electronic temperature recorders (DS1921G-F5, China, Shanghai Wassersen Electronic Technology Co. LTD.) were installed at 30 cm soil depth to record the soil temperature of each snow depth treatment. Also a 3-hour interval was set to measure the soil temperature continuously during the entire experimental period.
CO2 flux measurements
CO2 fluxes were measured by closed static-chamber technique and subsequent gas chromatographic analysis. Chambers were made of polymethyl methacrylate, with a diameter of 20 cm and a height of 100 cm. Every chamber was equipped with a spiracle, and a sampling hole, connected with silicone tubes, which were sealed by water stoppers. In addition, to record the real-time temperature in the chambers, ST-1A digital thermometers (−50 °C– + 80 °C, BT-12M 1.3AT, Digital thermometers) were installed in the chambers. Fans (12 v, 7 cm × 7 cm) were powered by accumulators, and they were necessary to keep gases homogeneous in the chambers (Fig. 1b).
We used shovels to manipulate snow depths. As for 0 cm snow depth treatment, a small amount of snow was left on the ground to keep the albedo homogeneous among the plots45. The treatments with snow depths of 60 cm and 90 cm were controlled by chambers. To keep the snow depths equal in and outside the chambers, nylon mesh and iron wire were used to delineate the plots, which were then filled in with different quantities of snow. Moreover, as soon as snow events occurred, snow depth modulation was performed immediately in order to maintain the snow depth within appropriate limits.
Collection of gasses took place when melting events occurred. Gases were collected into evacuated aluminum plastic composite membrane sampling bags with a sampling pump fortnightly. Collection always occurred after the fans had been running for 5 min. All the gases were collected at the interval of 15 min, namely 0, 15, 30, and 45 min, and then we took them back to the laboratory. Gases samples were stored at room temperature before gas chromatographic analysis. Fluxes were calculated by the change rate of gas concentration as follows:
where F (g/cm2·s) refers to gas flux, ρ (g/ml) is the density of gas under standard conditions, V (cm3) is the volume of air in the chamber, A (cm2) is the covered area of the chamber, P (Pa) is the pressure of the sampling point, P0 (Pa) is the absolute air pressure under standard conditions, T0 (K) is the absolute air temperature under standard conditions, T (K) is the absolute temperature in the chamber when sampling, and dCt/dt is the change rate of gas concentration.
Collection of soil samples
We collected 0-30 cm soil samples, and 2 soil cores in each plot, then mixed them together, subsequently put them in marked sealed bags and took them back to the laboratory. After quick removal of gravel, main roots and other debris in soil, some of the soil samples were stored in the refrigerator at 4 °C, and were used for measuring MBC. Some of the soil samples were air-dried for measuring invertase activities. Other soil samples were stored in the Ultra-low temperature freezer at -80°C for high throughput sequencing.
MBC and soil invertase measurements
MBC was measured via the method called “chloroform fumigation extraction”46, “3,5-dinitrosalicylic acid colorimetry” was used to measure invertase activities47,48,49.
Soil high-throughput sequencing technology
The experiment included three snow depth treatments (0, 60 cm, 90 cm), with three repetitions for every treatment, marked 1, 2, 3, respectively. Therefore, the samples were marked as 0-1, 0-2, 0-3, 60-1, 60-2, 60-3, 90-1, 90-2, 90-3. After DNA extraction, genomic DNA was detected by 1% agarose gel electrophoresis. The next step was to conduct PCR amplification, the V3-V4 hypervariable region of bacterial 16S rRNA gene were amplified with the primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT)50. For each soil sample, 10-digit barcode sequence was added to the 5’end of the forward and reverse primers (provided by Allwegene Company, Beijing). The PCR was carried out on a Mastercycler Gradient (Eppendorf, Germany) using 25 μl reaction volumes, containing 12.5 μl 2 × Taq PCR MasterMix, 3 μl BSA (2 ng/μl), 2 Primer (5 uM), 2 μl template DNA, and 5.5 μl dd H2O. Cycling parameters were 95 °C for 5 min, followed by 32 cycles of 95 °C for 45 s, 55 °C for 50 s and 72 °C for 45 s with a final extension at 72 °C for 10 min. The PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN, Germany), quantified using Real Time PCR, and sequenced at Allwegene Company, Beijing. Then PCR products were detected and quantified by the “blue fluorescence quantitative system QuantiFluor™-ST (Promega Company)”. Then, a MiSeq library was constructed and sequenced on the machine. Raw sequencing reads obtained in this study have been deposited into the NCBI Sequence Read Archive (SRA) database with the accession number of SRP250149.
Statistical analyses
All of the statistical analyses were performed using IBM SPSS software (Version 19.0) and graphed by SigmaPlot 12.5. All potential factors of influence (MBC and soil invertase activities) on CO2 fluxes under different snow depths were assessed by one-way anova analysis (with interactions by Duncan test). Daily average soil temperature was computed by taking the mean values measured at an interval of 3 hours.
Operational taxonomic units (OTUs) were formed by cluster analysis, and then bioinformatics and phylogenetic analyses were conducted with a similarity of 97%. The Kruskal-Wallis test was used to evaluate the difference of soil bacteria under different snow depths. Principal coordinates analysis (PCoA) was performed using R to examine microbial community structural variances between different snow depth treatments based on Weighted-UniFrac distance. The P value was calculated by permanova via python. Alpha diversity indices of bacteria (Chao1 richness) were calculated through the number of soil bacteria species (OTUs) and 16S rDNA sequence number (Reads). The formula of Chao1 richness (Bacterial richness index) is:
where Schao1 is the estimated number of OTUs, Sobs is the number of observed OTUs, n1 is the number of OTUs with only one sequence, and n2 is the number of OTUs with only two sequences. The data were analyzed with QIIME51. Based on the unweighted UniFrac distance matrix, UPGMA (Unweighted Pair Group Method with Arithmetic Mean) was used for cluster and tree construction, and the results of clustering were integrated with the relative abundance of species at the phylum level.
Results
CO2 fluxes
Soil CO2 fluxes varied under different snow depth treatments. The mean CO2 flux under snow removal treatment (0 cm) was 0.11 ± 0.05 g·cm−2·s−1, while CO2 fluxes under 60 cm and 90 cm snow depth treatments were 0.92 ± 0.16 g·cm−2·s−1 and 0.53 ± 0.16 g·cm−2·s−1, respectively. It indicated that 60 cm and 90 cm snow depth treatments showed significantly increased CO2 flux compared with 0 cm snow depth (Fig. 2, P = 0.004), but there was no significant difference between snow cover treatments.
Soil temperature and soil invertase activities
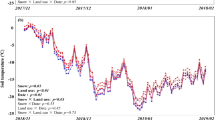
As is known to all, soil enzyme activities are closely related to soil temperature. In this study soil temperature increased with the increased snow depth. It tended to be more stable and was barely affected by air temperature with thicker snow cover (Fig. 3a). However, this snow manipulation experiment did not significantly affect the soil invertase activities (Fig. 3b).
Soil microorganisms
MBC showed the same tendency as CO2 fluxes which also increased markedly under snow cover (Fig. 4, p < 0.001). The concentrations of MBC were 185.52 ± 53.83 g·m−3, 332.95 ± 64.50 g·m−3 and 325.36 ± 82.30 g·m−3 under the treatments of 0 cm, 60 cm and 90 cm snow depths, respectively. High throughput sequencing results are as follows, valid data is shown in Table 1 (raw-tags gained after splicing of the filtered low-quality fastq data, and clean-tags are the results of further removing chimera and short sequence from splicing results). The distribution of OTUs under different snow depths is indicated by the Venn diagram (Fig. 5a). In total, this study generated 2448 OTUs, including 1631 OTUs that were present in all snow depths. The results of PCoA indicated that microbial community varied significantly (P = 0.005) between different treatments (Fig. 5b).
Soil bacterial community structure and diversities under different snow depth treatments: (a) shows a Venn diagram in which the overlapping parts are OTUs shared by different treatments; (b) shows PCoA based on Weighted-UniFrac distance; (c) is a box diagram of alpha diversity index (Chao1 index) for different snow depth groups. (d) is a cluster histogram of samples. On the left is the hierarchical cluster analysis, and the community structure histogram is on the right.
The alpha diversity index was used to compare microbial community diversity among different snow cover groups; snow cover significantly increased the alpha diversity indices of bacteria (Fig. 5c, Chao1 richness, P = 0.003). The dominant bacteria in Jinchuan wetland were Proteobacteria, Acidobacteria, Chloroflexi, Actinobacteria, and Bacteroidetes (Fig. 5d). The distribution of bacteria showed totally different in different snow cover groups. Proteobacteria increased significantly with increasing snow depth (P = 0.039). Chloroflexi (P = 0.027), Firmicutes, and Nitrospirae were relatively abundant in 0 cm snow depth (Table 2). At the genus level, Pseudolabrys, Candidatus_Solibacter, Bradyrhizobium, Candidatus_Koribacter, Bryobacter, Candidatus Competibacter, and Geobacter took an important place in winter soil bacteria; Pseudolabrys, Bradyrhizobium, Bryobacter, and Mucilaginibacter increased significantly with increasing snow depth (P = 0.039, P = 0.027, P = 0.027, P = 0.027) (Table 2).
Discussion
Many researches have studied the influences of snow depth on CO2 fluxes during the winter period20,41. They found that snow removal would cause a reduction of CO2 fluxes, whereas snow addition would increase CO2 fluxes (Table 3). In this study, we also found similar outcomes and also determined that snow addition will cause higher CO2 emissions during the winter period. However, the relationship between snow depth and CO2 fluxes did not show a totally positive correlation, as value under the treatment of 60 cm was slightly higher than that in 90 cm (Fig. 2). Based on our results, CO2 flux may be related to the following three points: Firstly, it may be related to the soil temperature of the optimum active layer in the wetland, which is usually at 30–40 cm. Secondly, the fluctuation of temperature may have a “stimulating” effect, the results of Fig. 3a show that soil temperatures under 60 cm snow depth fluctuated more than those under 90 cm. Neilson et al. (2001) studied forest soil and confirmed our speculations, indicating that the greater the fluctuation in soil temperature, the greater the CO2 fluxes52. Thirdly, the pulse intensities are different under different snow depths, suggesting some of the gases may be trapped in snow and ice. Therefore, further research is needed to investigate the other causes.
Invertase activity is related to an important nutrient for soil microbes (glucose)53, which is also vital for carbon emissions. However, invertase activities showed homogenous among different snow depths, which might prove the effects of snow cover on CO2 fluxes may not result from the shortage of nutrients (eg. sucrose) in soil. Additionally, soil enzyme activities are also driven by soil temperature, which was also effected by snow cover. Through our observation of the whole winter, soil temperature maintained at a constant temperature of 0°C-2°C. Previous studies have shown that snow cover can isolate soil from cold air and temper the atmospheric temperature changes’ influence on soil54. It could also increase soil moisture around the soil particles. As a result, it is beneficial to soil microbial activities18,41, thus ensuring the activity of soil enzymes55,56. In summary, this answered the second question, clarifying that invertase activities can ensure subnivean CO2 emissions in winter, but it might not be the major reason of the different subnivean CO2 emissions.
Studies indicated that MBC is also pivotal for CO2 emissions. Brooks and Williams (2010) found deeper and continuous snow cover could accelerate decomposition rates and lead to a higher MBC57. Similarly, our study showed that snow addition significantly increased MBC. Moreover, Sylvia et al. (2005) indicated that higher snow depths increased litter biomass, which increases labile carbon input58. This may explain the increase of MBC under snow addition. More important, MBC is one of the most active component of soil organic carbon and can directly participate in soil biochemical processes59. As a result, increased MBC might increase carbon output (CO2), thus affirmatively answering our third question as to whether MBC also impacts subnivean CO2 emissions.
Soil bacterial composition and richness also differs among different snow depths in our study. At the phylum level, major taxa in our study area were similar with those found in other studies60. At the genus level, Liptzin et al. (2015) reported that the major soil bacteria in Niwot Ridge of the Colorado Rocky Mountains were Cytophaga and Flexibacter in winter41. By contrast, our research has drawn different conclusions. Flexibacter were not observed in our study, and Cytophaga was also not a major taxon. In this study, Pseudolabrys, Candidatus_Solibacter, Bradyrhizobium, Candidatus_Koribacter, Bryobacter, Candidatus_Competibacter and Geobacter occupied an important position. It was considered that this dissimilarity was caused by differences in soil background or regional environment. Also, Dong et al. (2017) reported that higher snow depth caused a significant increase of bacterial richness and diversity, and played an important role in shaping microbial communities61. Additionally, Gao et al. (2020) found 16S rRNA gene abundance was positively correlated with soil CO2 emissions62. Microbial abundances and elevated temperature may increase the metabolic activity of microbes63 and decomposition of organics64. Similarly, this study reported that higher richness of bacterium appeared in subnivean soil, suggesting that this condition may provide more niches for microbial taxon. Nevertheless, how these dominant bacteria impact subnivean CO2 emissions still needs to be further investigated. Different bacteria also responded to snow depth with different sensitivities even in the same area. Several studies have reported most of the taxa found in cold winter conditions can correspond to either microorganisms with specific adaptive strategies like spore formation, or to opportunists65,66. Through our study, Proteobacteria increased significantly with increasing snow depth, whereas Firmicutes decreased. Ma (2013) conducted a study on Antarctic tundra and reported that soil carbon showed a negative correlation with the relative abundance of Proteobacteria67. It may correlate with the enhancement of other modalities of carbon, such as CO2. Moreover, many kinds of Firmicutes can resist dehydration and extreme conditions through producing spores at low temperature to maintain the soil biochemical processes. Additonally, previous study proved that the distinct presence of the phylum Chloroflexi at the temperature-reducing condition68, possibly due to the symbiosis with other bacteria by utilising cellular compounds and metabolites derived from dead biomass69.
The impact of snow depth on carbon dioxide emissions in winter is complex and worthy of further study in the context of global climate change. The insulating function of snow cover ensures liquid water around soil particles by increasing soil temperature. Also the elevated subnivean temperatures enhance the metabolic and decomposition activity of microorganisms, thus enhancing the carbon emissions.
Conclusion
The effects of altered snow depth on wetland greenhouse gases were complex. Thicker snow depth could accelerate the process of carbon cycling. Our research also revealed the impacts of snow depth on CO2 fluxes were mainly related to soil microbial activities (soil invertase activities, MBC, bacterial composition and bacterial richness). Higher snow depth increased microbial diversities, which had played a positive effect on carbon cycling. This field experiment could indicate the flux of carbon emissions under various snow depth and climate change scenarios. Additionally, the balance of the carbon budget in wetland ecosystem were associated with many vital factors, especially microbiological changes in freeze-thaw cycle stage. Therefore, more long-term studies should be developed to explore the impacts of soil microorganisms during this period.
Change history
15 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Romanovsky, V. E. et al. Thermal state of permafrost in Russia. Permafrost & Periglacial Processes 21, 136–155 (2010).
Zhao, F. B. et al. Predicting the climate change impacts on water-carbon coupling cycles for a loess hilly-gully watershed. Journal of Hydrology 518, 514388 (2020).
Schuur, E. A. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015).
Zhao, F. B. et al. Climatic and hydrologic controls on net primary production in a semiarid loess watershed. Journal of Hydrology 568, 803–815 (2019).
Sun, P. C. et al. Remote sensing and modeling fusion for investigating the ecosystem water-carbon coupling processes. Science of the Total Environment 697, 134064 (2019).
Zhao, F. B. et al. Spatiotemporal features of the hydro-biogeochemical cycles in a typical loess gully watershed. Ecological Indicators 91, 542–554 (2018).
Gorham, E. Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications 1, 182–195 (1991).
Turunen, J., Tomppo, E., Tolonen, K. & Reinikainen, A. Estimating carbon accumulation rates of undrained mires in Finland – application to boreal and subarctic regions. The Holocene 12, 69–80 (2002).
Charman, D. J. et al. Climate-related changes in peatland carbon accumulation during the last millennium. Biogeosciences 10, 929–944 (2013).
Liu, B. Q. et al. Annual soil CO2 efflux in a cold temperate forest in northeastern China: effects of winter snowpack and artificial nitrogen deposition. Scientific Reports 6, 18957 (2016).
Bubier, J., Crill, P. & Mosedale, A. Net ecosystem CO2 exchange measured by autochambers during the snow-covered season at a temperate peatland. Hydrological Processes 16, 3667–3682 (2002).
Olsson, P. Q., Sturm, M., Racine, C. H., Romanovsky, V. & Liston, G. E. Five Stages of the Alaskan Arctic Cold Season with Ecosystem Implications. Arctic Antarctic & Alpine Research 35, 74–81 (2003).
Wang, T. et al. Controls on winter ecosystem respiration in temperate and boreal ecosystems. Biogeosciences & Discussions 8, 2009–2025 (2011).
Edwards, K. A., McCulloch, J., Kershaw, G. P. & Jefferies, R. L. Soil microbial and nutrient dynamics in a wet arctic sedge meadow in late winter and early spring. Soil Biology and Biochemistry 38, 2843–2851 (2006).
Lafleur, P. M., Roulet, N. T. & Admiral, S. W. Annual cycle of CO2 exchange at a bog peatland. Journal of Geophysical Research Atmospheres 106, 3071–3081 (2001).
Campbell, J. L., Mitchell, M. J., Groffman, P. M., Christenson, L. M. & Hardy, J. P. Winter in northeastern North America: a critical period for ecological processes. Frontiers in Ecology and the Environment 3, 314–322 (2005).
Blankinship, J. C. & Hart, S. C. Consequences of manipulated snow cover on soil gaseous emission and N retention in the growing season: a meta-analysis. Ecosphere 3, Article 1 (2012).
Li, W. B. et al. Response of terrestrial carbon dynamics to snow cover change: A meta-analysis of experimental manipulation (II). Soil Biology & Biochemistry 103, 383–393 (2016).
Hirota, T. et al. Decreasing soil-frost depth and its relation to climate change in Tokachi, Hokkaido, Japan. Journal of the Meteorological Society of Japan 84, 821–833 (2006).
Ohkubo, S., Iwata, Y. & Hirota, T. Influence of snow-cover and soil-frost variations on continuously monitored CO2 flux from agricultural land. Agricultural & Forest Meteorology 165, 25–34 (2012).
Clein, J. S. & Schimel, J. P. Microbial activity of tundra and taiga soils at sub-zero temperatures. Soil Biology and Biochemistry 27, 1231–1234 (1995).
Brooks, P. D., Williams, M. W. & Schmidt, S. K. Microbial activity under alpine snowpacks, Niwot Ridge, Colorado. Biogeochemistry 32, 93–113 (1996).
Groffman, P. M., Driscoll, C. T., Fahey, T. J., Hardy, J. P. & Fitzhugh, R. D. Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 56, 135–150 (2001).
Russell, K. M. et al. Winter forest soil respiration controlled by climate and microbial community composition. Nature 439, 711–714 (2006).
IPCC, 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.
Dick, R. P., Pankhurst, C., Doube, B. M., Gupta, V. V. S. R. Soil enzyme activities as integrative indicators of soil health. Biological Indicators of Soil Health, 121–156(1997).
Yao, X. H., Min, H., Lv, Z. H. & Yuan, H. P. Influence of acetamiprid on soil enzymatic activities and respiration. European Journal of Soil Biology 42, 120–126 (2006).
Romanovsky, V. E. & Osterkamp, T. E. Effects of unfrozen water on heat and mass transport processes in the active layer and permafrost. Permafrost & Periglacial Processes 11, 219–239 (2015).
Brooks, P. D., Schmidt, S. K. & Williams, M. W. Winter production of CO2 and N2O from alpine tundra: environmental controls and relationship to inter-system C and N fluxes. Oecologia 110, 403–413 (1997).
McMahon, S. K., Wallenstein, M. D. & Schimel, J. P. A cross-seasonal comparison of active and total bacterial community composition in Arctic tundra soil using bromodeoxyuridine labeling. Soil Biology and Biochemistry 43, 287–295 (2011).
Aanderud, Z. T., Jones, S. E. & Schoolmaster, D. R. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biology & Biochemistry 57, 217–227 (2013).
Lipson, D. A., Schmidt, S. K. & Monson, R. K. Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biology & Biochemistry 32, 441–448 (2000).
Schadt, C. W., Martin, A. P., Lipson, D. A. & Schmidt, S. K. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 301, 1359–1361 (2003).
Panikov, N. S., Flanagan, P. W., Oechel, W. C., Mastepanov, M. A. & Christensen, T. R. Microbial activity in soils frozen to below −39 °C. Soil Biology & Biochemistry 38, 785e794 (2006).
Drotz, S. H., Sparrman, T., Nilsson, M. B., Schleucherc, J. & Öquista, M. G. Both catabolic and anabolic heterotrophic microbial activity proceed in frozen soils. Proceedings of the National Academy of Sciences of the United States of America 107, 21046e21051 (2010).
Price, P. B. & Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proceedings of the National Academy of Sciences of the United States of America 101, 4631–4636 (2004).
Price, P. B. Microbial life in glacial ice and implications for a cold origin of life. Fems Microbiology Ecology 59, 217–231 (2010).
Monson, R. K. et al. Winter forest soil respiration controlled by climate and microbial community composition. Nature 439, 711–714 (2006).
Ernakovich, J. G. & Wallenstein, M. D. Permafrost microbial community traits and functional diversity indicate low activity at in situ, thaw temperatures. Soil Biology & Biochemistry 87, 78–89 (2015).
Grayston, S. J. et al. Assessing shifts in microbial community structure across a range of grasslands of differing management intensity using CLPP, PLFA and community DNA techniques. Applied Soil Ecology 25, 63–84 (2004).
Liptzin, D., Helmig, D., Schmidt, S. K., Seok, B. & Williams, M. W. Winter gas exchange between the atmosphere and snow-covered soils on Niwot Ridge, Colorado, USA. Plant Ecology & Diversity 8, 1–12 (2015).
Shi, et al. Using 13C isotopes to explore denitrification-dependent anaerobic methane oxidation in a paddy-peatland. Scientific Reports 7, 40848 (2017).
Yu. The study on ecological value of Jinchuan Mire in Jilin Longwan National Nature Reserve. Northeast Normal University (2010).
Mao, X. M., Hong, Y. T., Zhu, Y. X. & Wang, H. Discovery of volcanic explosion-derived materials in Jinchuan peatland its significance. Acta Mineralogica Sinica 22, 9–14 (2002).
Comerford, D. P. et al. Influence of experimental snow removal on root and canopy physiology of sugar maple trees in a northern hardwood forest. Oecologia 171, 261 (2013).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biology & Biochemistry 19, 703–707 (1987).
Guan, S. Y. Soil enzyme and its research method. Beijing: China Agricultural Publishing House 120–276 (1986).
Xu, G. H. Manual of soil microorganism analysis method. Beijing: China Agricultural Publishing House 245–285 (1986).
Zhou, L. K. Soil enzymology. Beijing: Science Press 116–190 (1987).
Munyaka, P. M., Eissa, N., Bernstein, C. N., Khafipour, E., Ghia, J. E. Antepartum Antibiotic Treatment Increases Offspring Susceptibility to Experimental Colitis: A Role of the Gut Microbiota. Plos One 10, e0142536. doi:10.1371 (2015).
Schloss, P. D., Gevers, D. & Westcott, S. L. Reducing the Effects of PCR Amplification and Sequencing Artifacts on 16S rRNA-Based Studies. Plos One 6, e27310 (2013).
Nielsen, C. B. et al. Freezing Effects on Carbon and Nitrogen Cycling in Northern Hardwood Forest Soils. Soil Science Society of America Journal 65, 1723–1730 (2001).
Tu, C. M. Effect of four experimental insecticides on enzyme activities and levels of adenosine triphosphate in mineral and organic soils. Journal of Environmental Science & Health Part B 25, 787–800 (1990).
Roehm, C. L. & Roulet, N. T. Seasonal contribution of CO2 fluxes in the annual C budget of a northern bog. Global Biogeochemical Cycles 17, 291–239 (2003).
Sinsabaugh., R. L. et al. Stoichiometry of soil enzyme activity at global scale. Ecology Letters 11, 1252–1264, https://doi.org/10.1111/j.1461-0248.2008.01245.x (2008).
Wan, Z. & Wu, J. Study progress on factors affecting soil enzyme activity. Journal of Northwest Sci-Tech University of Agriculture and Forestry 33, 87–91 (2005).
Brooks, P. D. & Williams, M. W. Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrological Processes 13, 2177–2190 (2010).
Sylvia, D. M., Fuhrrmann, J. J., Hartel, P. & Zuberer, D. A. Principles and Applications of Soil Microbiology. Pearson Prentice Hall, Upper Saddle River (2005).
Dilly, O., Blume, H. P., Sehy, U., Jimenez, M. & Munch, J. C. Variation of stabilized, microbial and biologically active carbon and nitrogen in soil under contrasting land use and agricultural management practices. Chemosphere 52, 557–569 (2003).
Tornblom, E. & Sondergaard, M. Seasonal dynamics of bacterial biomass and production on eelgrass Zostera marina leaves. Marine Ecology Progress 179, 231–240 (1999).
Dong, W. et al. Influence of film mulching on soil microbial community in a rain fed region of northeastern China. Scientific Reports 7, 8468 (2017).
Gao, G. F. Examining Soil Carbon Gas (CO2, CH4) Emissions and the Effect on Functional Microbial Abundances in the Zhangjiang Estuary Mangrove Reserve. Journal of Coastal Research 36, 54–62 (2020).
Leopold, A., Marchand, C., Deborde, J. & Allenbach, M. Temporal variability of CO2 fluxes at the sediment-air interface in mangroves (New Caledonia). Science of the Total Environment 502, 617–626 (2015).
Xu, X. et al. Seasonal and spatial dynamics of greenhouse gas emissions under various vegetation covers in a coastal saline wetland in southeast China. Ecological Engineering 73, 469–477 (2014).
Amato, P. et al. Bacterial characterization of the snow cover at Spitzberg, Svalbard. Fems Microbiology Ecology 59, 255–264 (2007).
Møller, A. K., Søborg, D. A., Al-Soud, W. A., Sørensen, S. J. & Kroer, N. Bacterial community structure in high-Arctic snow and freshwater as revealed by pyrosequencing of 16S rRNA genes and cultivation. Polar Research 32, 130–137 (2013).
Ma, D. W. Distribution characteristics of bacterial community and enzyme activity and their affecting factors in Antarctic tundra soils. University of Science and Technology of China (2013).
Zhong, X. Z. et al. A comparative study of composting the solid fraction of dairy manure with or without bulking material: performance and microbial community dynamics. Bioresource Technology 247, 443–452 (2018).
Kindaichi, T., Yuri, S., Ozaki, N. & Ohashi, A. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Science and Technology 66, 2556–2561 (2012).
Groffman, P. M., Hardy, J. P ., Driscoll, C. T. & Fahey, T. J. Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Global Change Biology 12, 1748–1760 (2006).
Tucker, C. L., Tamang, S., Pendall, E., Ogle & K. Shallow snowpack inhibits soil respiration in sagebrush steppe through multiple biotic and abiotic mechanisms. Ecosphere 7, Article e 01297 (2016).
Acknowledgements
We appreciated Kyle D Boutin at Everglades Wetland Research Park, Florida Gulf Coast University (EWRP, FGCU) for his revision of the language. This work was supported by the National Natural Science Foundation of China [grant numbers 41901116]; the Fundamental Research Funds for the Central Universities [grant numbers 2412017QD023]; and the Special Funds of the State Environmental Protection Public Welfare Industry [grant numbers 201509040].
Author information
Authors and Affiliations
Contributions
Xue Wang wrote the main manuscript. Wenbo Luo and Lianxi Sheng revised the article critically for important intellectual content. Xueyuan Bai, Liang Ma, Chunguang He and Haibo Jiang reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Bai, X., Ma, L. et al. Snow depths’ impact on soil microbial activities and carbon dioxide fluxes from a temperate wetland in Northeast China. Sci Rep 10, 8709 (2020). https://doi.org/10.1038/s41598-020-65569-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65569-x
- Springer Nature Limited