Abstract
In alpine zones, soil microbial biomass and activity are strongly dependent on the seasonal snow cover. Current models assume that microbial biomass reaches an annual peak in winter under the insulating snowpack with a subsequent sharp decline during snowmelt. In this study, we investigated the seasonal dynamics of the soil microbial biomass in the Central Alps, where usually early snowfall buffers winter soil temperatures. We conducted a large-scale survey in three mountains around Davos (Switzerland) along altitudinal gradients from approximately 1900 to 2800 m above sea level. Using a space-for-time approach during snowmelt, soil samples were taken (1) under, (2) at the edge of, and (3) one meter away from remaining snow patches. One additional sample per site was taken in summer to further evaluate the seasonal dynamics. In total, 184 soil samples from 46 different sites were analyzed. We measured microbial biomass C and N, enzymatic activity and dissolved C and N. We observed an increase of microbial biomass and dissolved C during and immediately after snowmelt, as well as an increase from spring to summer. We suggest that the absence of soil freezing in winter and the growing amounts of dissolved C supported a continued growth, without a sudden collapse of the microbial biomass. Our results underline the importance of the insulating effect of the seasonal snow cover for the microbial dynamics. Global warming is modifying the timing and abundance of the seasonal snow cover, and our results will help to refine models for the dynamics of soil microbes in alpine ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mountains cover more than 20% of the earth’s land surface (Barry 1992) and contribute significantly to the worldwide soil C stocks (Ni 2002; Bockheim and Munroe 2014). On a global scale, soils store more C than the atmosphere and vegetation combined (Eswaran et al. 1993; Stockmann et al. 2013), making them of major interest in the context of global C fluxes and climate change. Soil microorganisms are thought to be the main drivers of stable soil organic C (SOC) formation by growing on labile plant-derived C and forming more stable microbial necromass C (Schimel and Schaeffer 2012; Liang et al. 2017). Thus, understanding microbial dynamics in alpine soils becomes essential to predict and model how global warming will affect SOC stocks (Donhauser and Frey 2018).
Alpine regions are characterized by a consistent snow cover throughout the winter, but they are disproportionately affected by global warming (Schröter et al. 2005), with a decrease of the snow cover (Gobiet et al. 2014). The insulating effect of the snow cover, if deeper than ~ 50 cm, decouples soil from air temperature (Zhang 2005), providing a relatively moist, stable and protected environment for soil microbial activity (Brooks et al. 1998). Current models assume that the soil microbial biomass in alpine grassland reaches its annual peak in winter under the snowpack just before snowmelt (Schmidt and Lipson 2004; Ernakovich et al. 2014). Soil microbial biomass was observed to suddenly decline at snowmelt in response to warming and changes in C availability (Schmidt and Lipson 2004; Ernakovich et al. 2014). Concomitantly, an abrupt transition from the cold-adapted winter soil microbial community to a summer community of different composition was observed (Buckeridge et al. 2013; Broadbent et al. 2021). In winter, soil microbes are assumed to obtain C mainly from litter, while root exudates are the main source in summer (Schmidt and Lipson 2004). The microbial biomass C/N ratio (thereafter MB-C:N) was found to decrease at the early stages of snowmelt due to microorganisms taking up N from the melting snow (Brooks et al. 1998; Lipson et al. 1999). Following snowmelt, a pulse of available N, linked to the sudden die-off of the winter microbial community, was observed at sites where the winter snow cover had been shallow and shorter (Brooks et al. 1998; Brooks and Williams 1999); however, the N-pulse was not consistently observed (Buckeridge et al. 2010; Lazzaro et al. 2015). Thus, it appears that in environments with harsh winter conditions where soils freeze before the snow cover is established (Brooks et al. 1998; Lipson and Schmidt 2004; Edwards et al. 2006), such as the intensively studied Niwot Ridge in the Rocky Mountains (Colorado, USA) and the arctic tundra (e.g. Manitoba, Canada), a cryogenic winter microbial community develops (Ley et al. 2004). This microbial community dies when soil temperatures rise above 0 °C in spring (Lipson et al. 2000; Edwards et al. 2006). Observations at these sites were generalized to predict the responses of arctic and alpine ecosystems to altered seasonality under climate change (Ernakovich et al. 2014).
In the Central Alps, however, the snow cover is usually deep and often falls before the soil freezes, thus soil temperatures remain around 0 °C throughout most of the winter (Meteoswiss Federal Office of Meteorology and Climatology 2022). Biogeochemical responses under such conditions can be expected to differ profoundly from observations in harsher environments.
To investigate the soil microbial biomass and nutrient dynamics during snowmelt and in summer, we conducted a large-scale survey in the Central Alps (Davos, Switzerland). In spring, soils were sampled using a space-for-time approach to detect changes during snowmelt at 46 sites in three mountains. One additional sample per site was taken in summer to analyze seasonal changes (184 soil samples in total). We hypothesize that the seasonal changes we will observe would not be as dramatic as those previously reported from regions with a harsher climate and freezing soils in winter. We discuss the implications of our results for modelling the effects of global warming for alpine ecosystems.
Materials and methods
Study area and sampling design
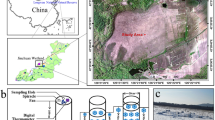
The study was conducted in three mountains, Jakobshorn, Schwarzhorn and Wannengrat, around the city of Davos in the eastern Central Alps in Switzerland (Fig. 1a–c). The climate in this region is characterized by a mean annual temperature of 4.0 °C, 187 days of frost, a total annual precipitation of 1046 mm and snow fall on 68 days per year (data from 1991 to 2020) (Meteoswiss Federal Office of Meteorology and Climatology 2022). The mineral bedrock is composed of acidic rocks, mostly gneiss and mica schists (Koordinationsorgan für Geoinformation des Bundes 2022). The sampling was designed with two sampling campaigns, to capture the biogeochemical changes (1) during snowmelt and (2) from spring to summer in alpine grasslands. For this, we sampled 15 sites along an altitudinal transect on each mountain (16 on Jakobshorn), covering altitudes between 1972 and 2816 m above sea level (Fig. 1c, e). The spring sampling took place during the snowmelt period in late spring 2020 (mid May to beginning of July) when sites with remaining snow patches were selected. At each site, we collected three soil samples, constituting a space-for-time series along a straight line of 2 m (Fig. 1d). These three samples reflected different stages of snowmelt, (i) “under snow”, (ii) “edge”, at the edge of the melting snow patch and (iii) “snow-free”, in an area recently freed from snow, i.e., since one to a few days, depending on the previous snow height and the weather. In the summer sampling campaign, between the 20st and the 25th of August 2020, we collected another sample (“summer”) on the same spot that had been under the snow in spring (Fig. 1d). These two samples (“under snow” and “summer”) were compared to assess the seasonal differences. In total, we collected 184 soil samples (4 samples on 46 sites each).
Maps of the collection sites and sampling scheme. a The Alps and Davos in Europe. b The three mountains where the study took place. c View of the sites at the top of the Jakobshorn during the spring sampling. d A scheme of the sampling design per site. e Sites and dataloggers position along the altitudinal gradient in each mountain
Coordinates and altitudes were recorded with a GPS (Trimble Geo XH 6000, Trimble Inc. Sunnyvale, CA, US), with a precision of a few cm. For each sample, about 200 g of soil were collected just below the grass roots at 5–8 cm depth for the determination of edaphic properties, using a clean metal spoon, and stored at 4 °C in thin polyethylene bags (air-permeable). Samples were shipped in a thermal box to the University of Cologne, where they were sieved at 2 mm before the analysis. All samples were stored at 4 °C and analyzed within 4 weeks upon arrival.
One-year soil temperature records
In September 2019, ten data loggers (ibutton DS1922L, Maxim Integrated Products, San Jose, CA, US) were set up in each of the three mountains along the altitudinal gradients (c. every 50 m of altitudinal difference) to record soil temperature in 2 h intervals with a precision of 0.0625 °C over one year. They were inserted in a rubber balloon for protection, attached to a 10 cm screw and placed at 5–8 cm below the soil surface. Data loggers were recovered during the summer sampling.
Edaphic properties
Gravimetric water content was measured immediately after returning to the lab, by taking a sample of c. 4 g of wet soil and drying it at 60 °C for 48 h before being reweighted. Four grams of sieved dry soil were mixed into 40 ml of distilled water, shaken for 1 h and after a short standstill period the pH was measured using a pH meter (pH/ION 340i with Sentix 4T electrode, WTW GmbH, Germany). SOC and total N (TN) content of dried and milled soil from the spring samples were measured with a C/N element analyzer (Flash 2000, Thermo Fisher Scientific GmbH, Germany). Soil microbial biomass C (MB-C) and N (MB-N) were determined by the chloroform fumigation-extraction method (Vance et al. 1987). Four grams of fresh soil were used for fumigated and non-fumigated samples and C and N was extracted with 16 ml of 0.5 M K2SO4. Soil extracts were subsequently analyzed for dissolved organic C (DOC) and total dissolved N (TDN) with a C/N analyzer (Multi N/C 2100S, Analytik Jena, Jena, Germany). Soil microbial biomass C and N were estimated as the difference of the DOC and TDN content of fumigated and non-fumigated samples, respectively, and corrected by the extraction efficiency factors of 0.45 and 0.54 for C and N, respectively (Joergensen 1996; Joergensen and Mueller 1996). Results from the non-fumigated samples were used as estimates of TDN and DOC in the soils.
Microbial enzyme activities
To estimate the microbial nutrient acquisition we measured the potential activities of four commonly used indicator enzymes (Sinsabaugh et al. 2009), i.e., ß-1,4-glucosidase (GLU), acid phosphatase (PHO); leucine aminopeptidase (LAP), and ß-1,4-N-acetylglucosaminidase (NAG), using the microplate fluorimetric method (Marx et al. 2001). The enzyme substrates (Sigma-Aldrich Chemie GmbH, Munich, Germany) were diluted in 0.05 M sodium acetate buffer (pH 4). Two hundred μl of soil slurry (1 g fresh soil in 50 ml deionised water) and 50 μl of substrate solution (500 µM), with four technical replicates per soil sample, were incubated (2 h, RT) and used to measure fluorescence on a microplate reader (Varioskan™, Thermo Electron Corporation, Germany) at 365 nm wavelength. A quenching coefficient was calculated for each soil. The amount of processed substrate (substrate conversion, SC) per hour was calculated from calibration curves of the fluorescent markers of the substrates (4-methylumbelliferone or 7-amino-4-methyl coumarin). We summarized the four analyzed enzyme activities to total enzyme activity because they showed the same trends (Table S1). For better comparison, biomass-specific enzyme activity was calculated by dividing the enzyme activity by MB-C.
Statistical analyses
All statistical analyses and data visualization were carried out in R (R version 4.1.2, RStudio 2022.02.0 Build 443) (R Core Team 2022; RStudio Team 2022). All graphs were created using the package “ggplot2” (Wickham 2016). There were missing values in only three samples and the sites (J01, W02, S02) to which they belonged were excluded from the corresponding analysis. Statistical tests were performed separately for the snowmelt samples (spring, space-for-time approach) and the spring (“under snow”) to summer seasonal comparison. A one-way-ANOVA (function: aov) followed by post-hoc Tukey tests (function: HSD.test; package: agricolae) (de Mendiburu and Yaseen 2021) was carried out to evaluate differences between spring samples. We evaluated whether altitude (ANCOVA, and as a factor in a 2-way ANOVA) and mountain (2-way ANOVA) had an effect on the observed differences by showing interactions with the samples (function: aov) (Table S2). Seasons (spring and summer) were compared by a repeated-measures t-test (function: t.test; paired = TRUE). For spring samples, under snow samples and relative changes in edge and snow-free compared to under snow samples were compared with altitude (function: lm).
Results
Soil temperature
In all sites, the snow cover was established before the soil temperature dropped below 0 °C (Fig. 2). Soil temperatures constantly remained above 0 °C under the insulating snowpack in all sites except for the highest elevations, where they dropped slightly below 0 °C, yet stayed above − 1 °C (Fig. 2). After snowmelt, no freeze–thaw cycles occurred in the soils, as temperatures quickly increased.
Dynamics during snowmelt
We observed MB-C to increase by 27% during snowmelt from under snow to snow-free, with intermediate values at the snow edge (Fig. 3a). MB-N also increased during snowmelt, although not significantly (Fig. 3b). MB-C:N showed high variation but did not show any significant pattern among the snowmelt gradient or seasonally. Both MB-C and MB-N decreased with increasing altitude (Fig. 3c, d). This did not affect the trend of increasing MB-C (and MB-N) during snowmelt, which was consistent along the altitudinal gradient (Fig. 3e, f) and among the three mountains (Table S2). DOC and TDN showed very similar patterns to MB-C and MB-N during snowmelt (Fig. 4). DOC increased by 21% from under snow to snow-free (Fig. 4a) while TDN remained nearly stable (Fig. 4b). DOC/TDN ratio did not show significant changes during snowmelt (Table S2). There was also no significant effect of snowmelt on biomass specific enzyme activity (Fig. 4c). Soil pH, SOC and TN showed no significant differences among the spring samples over the snowmelt gradient (Table S2). Instead, large variations were observed between sites (Table 1).
Variation of MB-C and MB-N during snowmelt. a MB-C during snowmelt in spring, from samples under the snow, at the snow edge and in the snow-free soil (n = 44). b MB-N during snowmelt (n = 44). c The relationship between MB-C in under snow samples and altitude. d The relationship between MB-N in under snow samples and altitude. e Relative change (%) of MB-C of edge and snow-free samples compared to the under snow samples. f Relative change (%) of MB-N of edge and snow-free samples compared to the under snow samples. Mean values are displayed as bar charts with standard error visualized as error bars. Different letters above bar charts indicate significant differences at the p < 0.05 level. The only significant differences were found between under snow and snow-free soils for MB-C. MB-C and MB-N in under snow samples negatively correlated with altitude. Relative change of MB-C and MB-N in edge and snow free samples did not show a correlation with altitude
Variation of DOC, TDN and enzyme activity during snowmelt. a DOC during snowmelt (n = 44). b TDN during snowmelt (n = 44). c Biomass specific enzyme activity during snowmelt (n = 45). Mean values are displayed as bar charts with standard error visualized as error bars. Different letters above bar charts indicate significant differences at the p < 0.05 level. DOC significantly increased from under snow to snow-free while TDN and enzyme activity showed no significant changes
Spring to summer dynamics
From spring to summer, MB-C and MB-N more than doubled (Fig. 5a, b) resulting in relatively stable MB-C:N ratios (Table S2) within sites, while variation between sites was high. DOC increased by 81% and TDN by 68% (Fig. 5c, d) and DOC/TDN ratio did not change significantly (Table S2). Biomass-specific enzyme activity was the only measured parameter to decrease (−63%) (Fig. 5e).
Changes of microbial parameters and dissolved nutrients from spring (under snow) to summer. a MB-C (n = 44). b MB-N (n = 44). c DOC (n = 44). d TDN (n = 44). e Biomass specific enzyme activity (n = 45). Mean values are displayed as bar charts with standard error visualized as error bars. Different letters above bar charts indicate significant differences at the p < 0.05 level. All parameters showed significant changes between seasons. Enzyme activity decreased (63%). MB-C and MB-N more than doubled. DOC (81%) and TDN (68%) both increased
Discussion
C and N dynamics during snowmelt and in summer
The steady increase of MB-C during snowmelt and from spring to summer indicated a smooth transition between seasons without any sudden die-off of microbes. With raising temperature and thus more plant activity, the increase in microbial biomass was likely supported by more dissolved C from root exudates, which were shown to be the major C sources for microbes in alpine grassland in spring and summer, in contrast to litter-C in winter (Schmidt and Lipson 2004). The theory that microorganisms were supplied with sufficient C and N in summer was supported by a decrease of potential extracellular enzymes activity. It is usually considered that microbes increase enzyme production when the nutrients are scarce (Rosinger et al. 2022b), so that the observed decrease of enzyme activity can be explained by the increase of dissolved C and N (Fig. 5c–e). However, potential enzyme activities have to be interpreted with caution because all measurements were performed at room temperature and might not represent the actual enzyme activity in situ (Nannipieri et al. 2018). Even though atmospheric N from the melting snowpack has been shown to contribute substantially to ecosystem N input in spring in the Alps (Hiltbrunner et al. 2005), we could not observe a pulse of available N during or shortly after snowmelt in our study, consistent with the absence of a microbial die-off. In studies in the arctic tundra, a strong stability in dissolved N pool sizes was observed just after snowmelt, similar to our observations (Buckeridge et al. 2010).
Although pH, SOC and TN showed variation within some sites, there was no significant change along the snowmelt gradient in spring (Table 1 and Table S2). This justified our space-for-time approach, allowing us to attribute the observed changes to the dynamics occurring at snowmelt.
Major impact of snow cover timing for seasonal biochemical dynamics in alpine ecosystems
Our study highlights the importance of early snow cover in autumn and constant snow cover throughout the winter for the microbial dynamics and thus nutrient cycles in alpine soil ecosystems. The early establishment of the snowpack, before soil freezes, ensures constant soil temperatures above 0 °C during the winter, which was observed at most of our sites (Fig. 2). Consequently, we assume that no cryogenic microbial community developed at our sites during winter, contrary to what was described for other seasonally snow-covered ecosystems (Schmidt and Lipson 2004; Edwards et al. 2006). At the intensively studied sites in the Niwot Ridge in the Rocky Mountains, a sudden collapse in microbial biomass was consistently observed after snowmelt (Brooks et al. 1998; Schmidt and Lipson 2004). However, at these sites the snowpack usually formed after the soils had already frozen, so they remained frozen throughout the winter (Brooks et al. 1998). Under these conditions, only a cryogenic microbial community can develop, which will then quickly die off with increasing soil temperatures during snowmelt (Schmidt and Lipson 2004). This was also reported for arctic grasslands (Edwards et al. 2006). Starvation of the winter community after consumption of all litter-derived C in winter has also been suggested to partly explain this sudden collapse (Lipson et al. 2000; Edwards et al. 2006). In the aforementioned studies, microbial biomass reached its annual peak in late winter/early spring before snowmelt (Schmidt and Lipson 2004), supported by high litter-derived C availability due to a vegetation die-off in frozen soil. This is in contrast to our study where microbial biomass peaked in summer, as the early snow cover might have mitigated such strong effects on the plant community, thus reducing the input of labile C in winter. At our sites, the peak of microbial biomass in summer is supported by high availability of C and N (Fig. 4) linked to increased ecosystem activity, while in the Rocky Mountains, the microbial biomass in summer remains low due to strong C limitation and competition with plants for available N (Brooks and Williams 1999; Schmidt and Lipson 2004). To summarize, higher winter soil temperatures due to early snow cover and the greater amounts of available C and N in the studied ecosystems in the Alps explain our observations in contrast to that in the Rocky Mountains. Generally, mountain ecosystems show great heterogeneity in climate and available nutrients depending on region, altitude and topography (Gruber and Haeberli 2009), complicating the establishment of a general model for annual microbial dynamics.
Current models and the effects of global warming on microbial dynamics in alpine regions
Due to the high vulnerability of alpine ecosystems to climate change (Schröter et al. 2005), it is crucial to establish reliable models of microbial dynamics and how their contribution to C cycling may be affected by global warming. Our study showed that current models, which assume a die-off of the microbial community at snowmelt, cannot be generalized to all alpine ecosystems (Ernakovich et al. 2014). Our results suggest that thaw biogeochemistry is controlled by winter snow cover which is drastically decreasing in the Alps (Beniston 2012; Gobiet et al. 2014; Matiu et al. 2021). The absence of sufficient snow cover or a later snowfall will lead to soil freezing and more freeze–thaw cycles (Edwards et al. 2007) and these “colder soils in a warmer world” (Groffman et al. 2001) will challenge the stability of the microbial communities and ecosystem nutrient cycles (Buckeridge and Grogan 2008; Broadbent et al. 2021; Rosinger et al. 2022a). They could lead to the switch between winter and summer microbial communities that was observed in ecosystems with harsher climate (Schmidt and Lipson 2004; Edwards et al. 2006). If this scenario will apply to alpine grasslands, it will have consequences for soil C stocks (Sofi et al. 2016) but the exact effects are unpredictable with the current state of knowledge. Whether the soils of these ecosystems will turn into a C-sink or a C-source is highly depending on microorganisms and their respiration, growth and transition to necromass C (Hagedorn et al. 2010; Nikrad et al. 2016). Our study showed, that only large-scale monitoring of seasonal microbial dynamics and biogeochemical cycles in different alpine ecosystems can improve the modelling of the expected changes due to global warming.
Data availability
The dataset generated and analyzed during the current study is available as supplementary information (Table S1).
References
Barry RG (1992) Mountains and their climatological study. Mountain weather and climate, 3rd edn. Cambridge University Press, Cambridge, pp 1–23
Beniston M (2012) Is snow in the Alps receding or disappearing? Wiley Interdiscip Rev Clim Change 3:349–358. https://doi.org/10.1002/wcc.179
Bockheim JG, Munroe JS (2014) Organic carbon pools and genesis of alpine soils with permafrost: a review. Arctic Antarct Alp Res 46:987–1006. https://doi.org/10.1657/1938-4246-46.4.987
Broadbent AAD, Snell HSK, Michas A et al (2021) Climate change alters temporal dynamics of alpine soil microbial functioning and biogeochemical cycling via earlier snowmelt. ISME J 15:2264–2275. https://doi.org/10.1038/s41396-021-00922-0
Brooks PD, Williams MW (1999) Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrol Process 13:2177–2190. https://doi.org/10.1002/(SICI)1099-1085(199910)13:14/15%3c2177::AID-HYP850%3e3.0.CO;2-V
Brooks PD, Williams MW, Schmidt SK (1998) Inorganic nitrogen and microbial biomass dynamics before and during spring snowmelt. Biogeochemistry 43:1–15. https://doi.org/10.1023/A:1005947511910
Buckeridge KM, Grogan P (2008) Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl Soil Ecol 39:210–222. https://doi.org/10.1016/j.apsoil.2007.12.010
Buckeridge KM, Cen YP, Layzell DB, Grogan P (2010) Soil biogeochemistry during the early spring in low arctic mesic tundra and the impacts of deepened snow and enhanced nitrogen availability. Biogeochemistry 99:127–141. https://doi.org/10.1007/s10533-009-9396-7
Buckeridge KM, Banerjee S, Siciliano SD, Grogan P (2013) The seasonal pattern of soil microbial community structure in mesic low arctic tundra. Soil Biol Biochem 65:338–347. https://doi.org/10.1016/j.soilbio.2013.06.012
de Mendiburu F, Yaseen M (2021) agricolae: statistical procedures for agricultural research. R package version 1.4.0. https://CRAN.R-project.org/package=agricolae. Accessed Sep 2022
Donhauser J, Frey B (2018) Alpine soil microbial ecology in a changing world. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiy099
Edwards KA, McCulloch J, Kershaw GP, Jefferies RL (2006) Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biol Biochem 38:2843–2851. https://doi.org/10.1016/j.soilbio.2006.04.042
Edwards AC, Scalenghe R, Freppaz M (2007) Changes in the seasonal snow cover of alpine regions and its effect on soil processes: a review. Quat Int 162–163:172–181
Ernakovich JG, Hopping KA, Berdanier AB et al (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol 20:3256–3269. https://doi.org/10.1111/gcb.12568
Eswaran H, Van Den Berg E, Reich P (1993) Organic carbon in soils of the world. Soil Sci Soc Am J 57:192–194. https://doi.org/10.2136/sssaj1993.03615995005700010034x
Gobiet A, Kotlarski S, Beniston M et al (2014) 21st century climate change in the European Alps—a review. Sci Total Environ 493:1138–1151. https://doi.org/10.1016/j.scitotenv.2013.07.050
Groffman PM, Driscoll CT, Fahey TJ, et al (2001) Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem
Gruber S, Haeberli W (2009) Mountain permafrost. In: Margesin R (ed) Permafrost soils. Springer, Berlin, Heidelberg, pp 33–44
Hagedorn F, Martin M, Rixen C et al (2010) Short-term responses of ecosystem carbon fluxes to experimental soil warming at the Swiss alpine treeline. Biogeochemistry 97:7–19. https://doi.org/10.1007/s10533-009-9297-9
Hiltbrunner E, Schwikowski M, Körner C (2005) Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmos Environ 39:2249–2259. https://doi.org/10.1016/j.atmosenv.2004.12.037
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Joergensen RG, Mueller T (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol Biochem 28:33–37. https://doi.org/10.1016/0038-0717(95)00101-8
Koordinationsorgan für Geoinformation des Bundes (2022) Geotechnische Karte 200. https://map.geo.admin.ch/. Accessed Sept 2022
Lazzaro A, Hilfiker D, Zeyer J (2015) structures of microbial communities in alpine soils: seasonal and elevational effects. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01330
Ley RE, Williams MW, Schmidt SK (2004) Microbial population dynamics in an extreme environment: controlling factors in talus soils at 3750 m in the Colorado Rocky Mountains. Biogeochemistry 68:297–311. https://doi.org/10.1023/B:BIOG.0000031032.58611.d0
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105. https://doi.org/10.1038/nmicrobiol.2017.105
Lipson DA, Schmidt SK (2004) Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl Environ Microbiol 70:2867–2879. https://doi.org/10.1128/AEM.70.5.2867-2879.2004
Lipson DA, Schmidt SK, Monson RK (1999) Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80:1623. https://doi.org/10.2307/176551
Lipson DA, Schmidt SK, Monson RK (2000) Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem 32:441–448. https://doi.org/10.1016/S0038-0717(99)00068-1
Marx M-C, Wood M, Jarvis S (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640. https://doi.org/10.1016/S0038-0717(01)00079-7
Matiu M, Crespi A, Bertoldi G et al (2021) Observed snow depth trends in the European Alps: 1971 to 2019. Cryosph 15:1343–1382. https://doi.org/10.5194/tc-15-1343-2021
Meteoswiss Federal Office of Meteorology and Climatology (2022) Climate normals Davos Reference period 1991−2020. https://www.meteoswiss.admin.ch/product/output/climate-data/climate-diagrams-normal-values-station-processing/DAV/climsheet_DAV_np9120_e.pdf. Accessed Sept 2022
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19. https://doi.org/10.1007/s00374-017-1245-6
Ni J (2002) Carbon storage in grasslands of China. J Arid Environ 50:205–218. https://doi.org/10.1006/jare.2001.0902
Nikrad MP, Kerkhof LJ, Häggblom MM (2016) The subzero microbiome: microbial activity in frozen and thawing soils. FEMS Microbiol Ecol 92:fiw081. https://doi.org/10.1093/femsec/fiw081
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed May 2022
Rosinger C, Clayton J, Baron K, Bonkowski M (2022a) Soil freezing-thawing induces immediate shifts in microbial and resource stoichiometry in Luvisol soils along a postmining agricultural chronosequence in Western Germany. Geoderma 408:115596. https://doi.org/10.1016/j.geoderma.2021.115596
Rosinger C, Keiblinger KM, Rousk J, Sandén H (2022b) Shifts in microbial stoichiometry upon nutrient addition do not capture growth-limiting nutrients for soil microorganisms in two subtropical soils. Biogeochemistry 159:33–43. https://doi.org/10.1007/s10533-022-00911-1
RStudio Team (2022) RStudio: integrated development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/. Accessed May 2022
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00348
Schmidt SK, Lipson DA (2004) Microbial growth under the snow: implications for nutrient and allelochemical availability in temperate soils. Plant Soil 259:1–7. https://doi.org/10.1023/B:PLSO.0000020933.32473.7e
Schröter D, Cramer W, Leemans R et al (2005) Ecosystem service supply and vulnerability to global change in Europe. Science 310:1333–1337. https://doi.org/10.1126/science.1115233
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. https://doi.org/10.1038/nature08632
Sofi JA, Lone AH, Ganie MA et al (2016) Soil microbiological activity and carbon dynamics in the current climate change scenarios: a review. Pedosphere 26:577–591. https://doi.org/10.1016/S1002-0160(15)60068-6
Stockmann U, Adams MA, Crawford JW et al (2013) The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric Ecosyst Environ 164:80–99. https://doi.org/10.1016/j.agee.2012.10.001
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Zhang T (2005) Influence of the seasonal snow cover on the ground thermal regime: an overview. Rev Geophys. https://doi.org/10.1029/2004RG000157
Acknowledgements
This work was funded by the German Research Foundation (DFG), Grant Number FI 1686/2-1. For help in collecting samples in Davos, we thank Sven Buchmann, Fiona Schwaller and Francesca Jaroszynska. At the University of Cologne, we are grateful to Irene Brockhaus, Anna Herzog and Andrea Meys for helping with the measurement of edaphic properties.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the German Research Foundation (DFG), Grant Number FI 1686/2-1.
Author information
Authors and Affiliations
Contributions
Conceptualization: AMF-D; Collection of samples: AMF-D and CR; Edaphic measurements and statistical anaylses: OR and CR; Interpreting the results: all co-authors; Writing—original draft preparation: OR and AMF-D; Writing—review and editing: CR, CR, MB, TU and NB; Funding acquisition: AMF-D and CR; Resources: MB and CR.
Corresponding author
Ethics declarations
Competing interests
The authors do not have any conflict of interest.
Ethical approval
Not applicable.
Additional information
Responsible Editor: Lettice Hicks.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10533_2022_1005_MOESM2_ESM.xlsx
Supplementary file2 (XLSX 9 KB) Table S2 Statistical analyzes (Anova, Ancova and t-test) to test for the effects of altitude (as factor or continous), mountain, snow coverage and season on selected edaphic parameters
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rindt, O., Rosinger, C., Bonkowski, M. et al. Biogeochemical dynamics during snowmelt and in summer in the Alps. Biogeochemistry 162, 257–266 (2023). https://doi.org/10.1007/s10533-022-01005-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-01005-8