Abstract
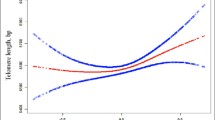
Accelerated telomere attrition is related to various diseases, and multiple factors have been reported to influence telomere length. However, little attention has focused on the relationship between serum phosphate levels and mean telomere length. The purpose of this study was to explore the relationship between serum phosphate levels and mean telomere length in the US general population. A total of 7,817 participants from the 1999–2002 NHANES were included. The association between serum phosphate levels and mean telomere length was investigated using regression models. A remarkably positive relationship between serum phosphate levels and mean telomere length emerged after adjustments were made for covariates. The adjusted β coefficient of serum phosphate levels for mean telomere length was 0.038 (95% confidence intervals (CIs), 0.022 to 0.095, p = 0.002). A longer telomere length was observed in participants with serum phosphate levels in the highest quartiles, and a dose-dependent association was observed. Our study demonstrated that higher quartiles of phosphate had a remarkable correlation with longer telomere length.
Similar content being viewed by others
Introduction
Phosphate is an essential mineral in the body1 and plays a crucial role in many physiological processes, such as energy generation, signal transduction, acid-base balance and bone mineralization2. Phosphate homeostasis is modulated by dietary phosphate intake, intestinal absorption, renal reabsorption and excretion3. There are various regulators of phosphate metabolism, including parathyroid hormone (PTH), calcitriol, vitamin D and fibroblast growth factor 23 (FGF23)2. And imbalance of phosphate homeostasis may induce hypo- and hyperphosphatemia4. Hyperphosphatemia is correlated with a higher risk of cardiovascular diseases5 and all-cause mortality6. In contrast, hypophosphatemia is associated with hypertension7 and reduced insulin resistance8. Kalaitzidis, R. et al. reported that individuals with metabolic syndrome had remarkably lower serum phosphate levels than did healthy participants9. Hence, higher and lower serum phosphate levels have been associated with, respectively, increased cardiovascular risks and more parameters of metabolic syndrome.
Telomeres capping the end of eukaryotic chromosomes protect chromosomes from loss and end-to-end fusion10. Telomeres shorten with repeated cell division and DNA replication11. Telomere length has been positively correlated with healthy life years in elderly individuals12. Furthermore, shorter telomere length is correlated with higher all-cause mortality13. Numerous factors have been found to be associated with telomere length14. Accelerated telomere attrition was correlated with lower socioeconomic status and poor diet15. A review in 2011 demonstrated that various nutrients, such as vitamin A, vitamin D, folate, vitamin B12, magnesium, zinc and iron, may affect telomere length16. In a cross-sectional analysis, Xu, Q., et al. found a positive relationship between multivitamin use and telomere length in women17. Higher dietary intake of magnesium was also associated with longer telomere length17. In contrast, shorter telomere length was found in iron supplement users18.
Despite a large amount of evidence on factors affecting telomere length, little research has clarified the relationship between serum phosphate and telomere length. The aim of our study was to investigate the correlation between serum phosphate and mean telomere length in the US general population.
Results
Characteristics of the study population
The characteristics of the participants divided by serum phosphate quartiles are listed in Table 1. In the study, the mean age of all participants was 49.42 ± 18.82 years, and 48.2% of all participants were men. Mean telomere length, creatinine and total calcium levels were significantly positively associated with serum phosphate levels, whereas age, body mass index (BMI), SBP, serum FG and serum albumin were negatively correlated with serum phosphate levels.
Association between serum phosphate levels and mean telomere length after stratification by sex and ethnicity
Tables 2, 3 presents the results of the correlation between serum phosphate levels and mean telomere length stratified by sex and ethnicity. A significant correlation was found between higher serum phosphate levels and longer mean telomere lengths in the three models (p < 0.05). Additionally, male participants demonstrated an association between higher serum phosphate levels and longer mean telomere lengths in model 2 and in the fully adjusted model (model 2: β = 0.043, p = 0.011; model 3: β = 0.045, p = 0.009). In the non-Hispanic white group and other racial groups, a positive association between serum phosphate levels and mean telomere length was found in 3 adjusted models (p < 0.05).
After serum phosphate levels were stratified into four quartiles, gender-specific associations between serum phosphate levels and mean telomere length were determined and are listed in Table 4. Table 4 shows a significantly positive association between the highest serum phosphate level (Q4) and mean telomere length for all designed models (p < 0.05). The mean telomere length was greater in the higher quartiles than in the lowest quartile of serum phosphate, and a dose-dependent association was observed. Male participants with the highest serum phosphate levels (Q4) had a longer mean telomere length than did those with serum phosphate levels in Q1 in the 3 adjusted models.
Table 5 presents the ethnicity-specific association between serum phosphate levels and mean telomere length after multivariable logistic regression analysis. Only in the non-Hispanic white group, participants with higher quartiles of serum phosphate levels (Q2, Q3 and Q4) had a longer mean telomere length than did participants with serum phosphate levels in Q1 in the 3 regression models (p < 0.05).
Discussion
In the present cohort study of the US general population, a positive relationship between serum phosphate levels and mean telomere length was observed. These findings are not consistent with those of previous studies5,19,20,21,22,23. Higher serum phosphate levels were noted to be linked with increased cardiovascular risk and mortality risk5,19,20,21,23,24. Furthermore, McClelland, R., et al. demonstrated that hyperphosphatemia was associated with accelerated aging, which was evaluated by markers of biological age (i.e., telomere length and DNA methylation content)22. Recently, emerging evidence on klotho expression and aging has been published25,26,27,28. Klotho was associated with phosphate homeostasis via the fibroblast growth factor (FGF) receptor, and decreased klotho expression may induce hyperphosphatemia29. Thus, a plausible association between phosphate toxicity and accelerated aging in klotho-deficient mice was also reported30,31.
Telomere length was viewed as a biomarker of age32. Accelerated telomere attrition was associated with various diseases, such as coronary heart disease33, diabetes mellitus34, hypertension35 and cancer36. Telomere length was influenced by multiple factors, including psychosocial, environmental, and behavioral factors14. Different lifestyles and diets also played prominent roles in telomere length37,38. Mirabello, L., et al. demonstrated that a healthy lifestyle with more exercise, cigarette abstinence and a diet high in fruit and vegetables were significantly correlated with longer telomere length38. Cassidy, A., et al. reported that intake of dietary fiber was positively linked with telomere length, while waist circumference, body mass index (BMI) and the intake of polyunsaturated fatty acids were negatively linked37. The inverse correlation between obesity and telomere length was observed in females39. Increased adiposity and increasing BMI were correlated with shorter telomere length40. A previous study by Song et al. revealed that BMI was significantly positively associated with biomarkers of DNA damage41. Furukawa et al. proposed that elevated chronic oxidative stress was observed in accumulated fat42. Kurz, D.J., et al. demonstrated that oxidative stress may accelerate telomere erosion43. Hence, we speculated that a higher BMI may increase oxidative stress, which could cause DNA damage and might induce telomere attrition.
Few studies have demonstrated a negative association between serum phosphate and BMI44,45. Lower BMI might increase the risk of hyperphosphatemia46. Haglin et al. observed that hypophosphatemia was correlated with higher BMI in females47. Our results were in agreement with these articles that showed an inverse relationship between serum phosphate and BMI. Several possible mechanisms have been proposed for this phenomenon. Haglin et al. speculated that it might be due to a high caloric diet with low nutrient density and low protein intake, which could cause phosphate depletion47. Obeid, O.A. hypothesized that lower serum phosphate may reduce ATP production, which is important for energy expenditure45. Depending on the inverse relationship, we hypothesized that a higher serum phosphate level might be associated with a lower BMI and that a lower BMI may be associated with a greater telomere length. Therefore, these hypothesized relationships might serve as a possible explanation for our results. The hypotheses that participants with higher serum phosphate levels had longer telomere lengths are biologically plausible.
Our study demonstrated that statistically significant relationships between higher serum phosphate levels and longer mean telomere length, particularly in male participants. Previous published studies have shown gender differences in serum phosphate levels48,49. Few studies have examined the effects of gonadal steroids on phosphorus homeostasis. Sex hormone deprivation was found to be correlated with elevated serum phosphate levels in men50. Moreover, testosterone and estrogen were associated with mean telomere length51,52. A possible mechanism may account for this phenomenon53.
There are several limitations in the present study. First, because our study was a cross-sectional observational analysis of a database, causal inferences were not clear. Further longitudinal studies are warranted. Second, adjustments were not made in regression models for unmeasured confounding variables, such as lifestyle factors and dietary patterns that might interfere with both mean telomere length and serum phosphate levels. Third, there might be a possible selection bias in our study. Our participants were mostly Caucasian; therefore, the generalizability of the results might be limited to specific racial populations.
In conclusion, a significant positive correlation between serum phosphate levels and mean telomere length was observed in the study. The findings of the paucity of currently published studies were consistent with our results, and our study provided epidemiologic evidence for further studies on the relationship between mean telomere length and serum phosphate levels.
Materials and methods
Study populations
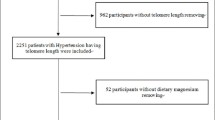
All data were obtained from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2002. NHANES was a cross-sectional study of noninstitutionalized US citizens and was conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). NHANES database included personal data including demographic information, past medical history and laboratory data. The participants’ information was collected by a household interview and a subsequent physical examination. All of the study protocol, consent documents and relevant information were detailed on the NHANES website. The NHANES study protocol was conducted according to the NCHS Institutional Review Board (IRB). Before data collection and the health examinations, all informed consents had been obtained. All the experimental protocols were approved by NCHS IRB. Initially, a total of 7,817 participants who aged 20 years old or older were included in our study.
Covariates
For causal relationship from cross-sectional study, it was essential to control confounding, but it was hard to recognize a potential confounder. A confounding variable was associated with the exposure and with the outcome or the occurrence of a disease. A confounder obscured the real causal path between the exposure and outcome. Based on the variables correlation and previous studies, we drew our Directed Acyclic Graph (DAG) analysis of the study, showed in Fig. 1. Demographic confounders were collected with a computer-assisted personal interviewing system, including age, gender, race, personal history, and past medical status. Other medical histories were recorded, including congestive heart failure, coronary artery disease, angina, stroke and cancer/malignancy, which were diagnosed or revealed by a doctor.
The level of serum C-reactive protein (CRP) was measured by the Dade Behring Nephelometer II Analyzer System using latex-enhance nephelometry (Dade Behring Diagnostics Inc., Somerville, NJ). The level of serum fasting glucose (FG) was measured by the Instrumentation Cobas Mira Chemistry System (Roche Diagnostic Systems, Inc., Montclair, New Jersey). The resting biochemical profiles, including creatinine, alanine aminotransferase (ALT), total calcium, serum total cholesterol (TC) and serum albumin, were measured with the Beckman Synchron LX20. All protocols followed the standardized guidelines and record accuracy based on CDC reference methods.
Serum phosphate measurement
The level of serum phosphate was measured by a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Inorganic phosphorus reacted with ammonium molybdate in an acidic solution to make a colored phosphomolybdate complex. The quantification of serum phosphate was performed using the change in absorbance at 365 nm of phosphomolybdate.
Mean telomere length measurement
The telomere length assay for the measurement of telomere length relative to standard reference DNA (T/S ratio) was performed using the quantitative polymerase chain reaction (PCR) method in the laboratory of Dr. Elizabeth Blackburn at the University of California, San Francisco. More detailed information can be found on the NHANES web site in the laboratory section.
Statistical analysis
We used SPSS version 18 (SPSS Inc., Chicago, IL, USA) to perform all statistical analyses. Continuous variables are indicated as the means and standard deviations (SDs); categorical variables are indicated as numbers and percentages. The chi-square test and one-way ANOVA were used for categorical data and continuous data, respectively. To examine the variables of interest, we divided 7,817 participants into quartiles based on serum phosphate levels. Two-sided p-values <0.05 were considered statistically significant.
We investigated the relationship between serum phosphate levels and mean telomere length using multivariable logistic regression analysis. Covariate adjustments were conducted using 3 extended-model methods: model 1 was adjusted for age, gender and race; model 2 was further adjusted for associated clinical laboratory data; and model 3 was further adjusted for smoking history and past medical history. We tested for effect modification by serum phosphate levels and race and sex by including interaction terms in the models for the mean telomere length. Based on the statistically significant findings of the interaction effect, we used stratified test to perform further analyses.
References
Berndt, T. J., Schiavi, S. & Kumar, R. “Phosphatonins” and the regulation of phosphorus homeostasis. Am. J. Physiol. Renal Physiol. 289, F1170–1182 (2005).
Penido, M. & Alon, U. S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 27, 2039–2048 (2012).
Marks, J., Debnam, E. S. & Unwin, R. J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Renal. Physiol. 299, F285–296 (2010).
Lee, R. & Weber, T. J. Disorders of phosphorus homeostasis. Curr. Opin. Endocrinol. Diabetes Obes. 17, 561–567 (2010).
McGovern, A. P. et al. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community based cohort study. PLos one 8, e74996 (2013).
Chang, A. R., Lazo, M., Appel, L. J., Gutierrez, O. M. & Grams, M. E. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am. J. Clin. Nutr. 99, 320–327 (2014).
Gudmundsdottir, H., Strand, A. H., Kjeldsen, S. E., Hoieggen, A. & Os, I. Serum phosphate, blood pressure, and the metabolic syndrome–20-year follow-up of middle-aged men. J. Clin. Hypertens. (Greenwich) 10, 814–821 (2008).
Haap, M. et al. Association of serum phosphate levels with glucose tolerance, insulin sensitivity and insulin secretion in non-diabetic subjects. Eur. J. Clin. Nutr. 60, 734–739 (2006).
Kalaitzidis, R., Tsimihodimos, V., Bairaktari, E., Siamopoulos, K. C. & Elisaf, M. Disturbances of Phosphate Metabolism: Another Feature of Metabolic Syndrome. Am. J. Kidney Dis. 45, 851–858 (2005).
Blackburn, E. H. Telomere states and cell fates. Nature 408, 53–56 (2000).
Jiang, H., Ju, Z. & Rudolph, K. L. Telomere shortening and ageing. Z. Gerontol. Geriatr. 40, 314–324 (2007).
Njajou, O. T. et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J. Gerontol. A. Biol. Sci. Med. Sci. 64, 860–864 (2009).
Rode, L., Nordestgaard, B. G. & Bojesen, S. E. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J. Natl. Cancer Inst. 107, djv074 (2015).
Starkweather, A. R. et al. An Integrative Review of Factors Associated with Telomere Length and Implications for Biobehavioral Research. Nurs. Res. 63, 36–50 (2014).
Shiels, P. G. et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLos one 6, e22521 (2011).
Paul, L. Diet, nutrition and telomere length. J. Nutr. Biochem. 22, 895–901 (2011).
Xu, Q. et al. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 89, 1857–1863 (2009).
Aviv, A. Leukocyte telomere length: the telomere tale continues. Am. J. Clin. Nutr. 89, 1721–1722 (2009).
Foley, R. N., Collins, A. J., Herzog, C. A., Ishani, A. & Kalra, P. A. Serum Phosphorus Levels Associate with Coronary Atherosclerosis in Young Adults. J. Am. Soc. Nephrol. 20, 397–404 (2009).
Ix, J. H. et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin. J. Am. Soc. Nephrol. 4, 609–615 (2009).
Kestenbaum, B. et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 16, 520–528 (2005).
McClelland, R. et al. Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging (Albany NY) 8, 1135–1149 (2016).
Tonelli, M., Sacks, F., Pfeffer, M., Gao, Z. & Curhan, G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112, 2627–2633 (2005).
Palmer, S. C. et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305, 1119–1127 (2011).
Izquierdo, M. C. et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transplant. 27(Suppl 4), iv6–10 (2012).
Kooman, J. P. et al. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Renal Physiol. 313, F938–f950 (2017).
Kuro-o, M. Klotho and aging. Biochim Biophys Acta 1790, 1049–1058 (2009).
Stenvinkel, P. & Larsson, T. E. Chronic kidney disease: a clinical model of premature aging. Am. J. Kidney. Dis. 62, 339–351 (2013).
Kuro, O. M. Phosphate and Klotho. Kidney Int. Suppl., S20–23 (2011).
Kuro-o, M. A potential link between phosphate and aging–lessons from Klotho-deficient mice. Mech. Ageing Dev. 131, 270–275 (2010).
Ohnishi, M. & Razzaque, M. S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. Faseb. J. 24, 3562–3571 (2010).
Crimmins, E., Vasunilashorn, S., Kim, J. K. & Alley, D. Biomarkers related to aging in human populations. Adv. Clin. Chem. 46, 161–216 (2008).
Zee, R. Y., Michaud, S. E., Germer, S. & Ridker, P. M. Association of shorter mean telomere length with risk of incident myocardial infarction: a prospective, nested case-control approach. Clin. Chim. Acta. 403, 139–141 (2009).
Sampson, M. J., Winterbone, M. S., Hughes, J. C., Dozio, N. & Hughes, D. A. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 29, 283–289 (2006).
Demissie, S. et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 5, 325–330 (2006).
Willeit, P. et al. Telomere length and risk of incident cancer and cancer mortality. JAMA 304, 69–75 (2010).
Cassidy, A. et al. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 91, 1273–1280 (2010).
Mirabello, L. et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell 8, 405–413 (2009).
Valdes, A. M. et al. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664 (2005).
Tzanetakou, I. P., Katsilambros, N. L., Benetos, A., Mikhailidis, D. P. & Perrea, D. N. “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res. Rev. 11, 220–229 (2012).
Song, Z. et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell 9, 607–615 (2010).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 (2004).
Kurz, D. J. et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 117, 2417–2426 (2004).
Moore, L. W., Nolte, J. V., Gaber, A. O. & Suki, W. N. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 102, 444–453 (2015).
Obeid, O. A. Low phosphorus status might contribute to the onset of obesity. Obes. Rev. 14, 659–664 (2013).
Wojcicki, J. M. Hyperphosphatemia is associated with anemia in adults without chronic kidney disease: results from the National Health and Nutrition Examination Survey (NHANES): 2005–2010. BMC Nephrol. 14, 178 (2013).
Håglin, L., Lindblad, Å. & Bygren, L. O. Hypophosphataemia in the metabolic syndrome. Gender differences in body weight and blood glucose. Eur. J. Clin. Nutr. 55, 493 (2001).
Onufrak, S. J. et al. Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am. J. Epidemiol. 169, 67–77 (2009).
de Boer, I. H., Rue, T. C. & Kestenbaum, B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney. Dis. 53, 399–407 (2009).
Burnett-Bowie, S.-A. M., Mendoza, N. & Leder, B. Z. Effects of gonadal steroid withdrawal on serum phosphate and FGF-23 levels in men. Bone 40, 913–918 (2007).
Yeap, B. B. et al. Epidemiological and Mendelian Randomization Studies of Dihydrotestosterone and Estradiol and Leukocyte Telomere Length in Men. J. Clin. Endocr. Metab. 101, 1299–1306 (2016).
Lee, D.-C., Im, J.-A., Kim, J.-H., Lee, H.-R. & Shim, J.-Y. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei. Med. J. 46, 471–479 (2005).
Calado, R. T. et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114, 2236–2243 (2009).
Author information
Authors and Affiliations
Contributions
Zhe-Yu Yang contributed to the design of the study, was responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, drafted the initial manuscript. Zhe-Yu Yang, Tung-Wei Kao, Tao-Chun Peng, Yuan-Yuei Chen, Hui-Fang Yang, Chen-Jung Wu, Wei-Liang Chen decided upon the data collection methods. Zhe-Yu Yang and Wei-Liang Chen were also responsible for the data analysis decisions. Wei-Liang Chen conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors meet the ICMJE criteria for authorship.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, ZY., Kao, TW., Peng, TC. et al. Examining the association between serum phosphate levels and leukocyte telomere length. Sci Rep 10, 5438 (2020). https://doi.org/10.1038/s41598-020-62359-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62359-3
- Springer Nature Limited