Abstract
Rapid development of nanotechnology often requires verification of existing phase diagrams, which were suitable for bulk materials. This work presents a new phase map (phase diagram) for Al2O3-ZrO2 crystalline powders including the role of the nanoscale particles. Al2O3-ZrO2 composites are relevant for industry for applications demanding high hardness. The nanopowders were manufactured via co-precipitation process followed by microwave hydrothermal synthesis (MHS) at 270 °C, drying at room temperature and annealing in the temperature range 300–1500 °C. The phase composition was investigated using X-ray diffraction (XRD) and Rietveld refinement analysis. The grain size and size distribution were calculated using Rietveld refinement analysis and using transmission electron microscopy (TEM). A particular feature of the composites was the nanoisolation, separation of different phases on a nanoscale. This feature limited grain growth during annealing and permitted the phase diagram for a nano-enabled system to be determined, which turned out to be different from that of conventional composites. In particular, considerable solubility of Al3+ in ZrO2 was found for temperatures less than 1000 °C.
Similar content being viewed by others
Introduction
Alumina-toughened zirconia (ATZ- ZrO2-Al2O3) and zirconia-toughened alumina (ZTA- Al2O3-ZrO2) are important materials for high-temperature structural1,2,3 and functional4,5,6,7 applications thanks to their exceptional mechanical properties, and high temperature stability.
These ceramic materials can be synthesized using various methods. The most popular methods are: the sol–gel method8, hydrothermal synthesis9 and the co-precipitation followed by calcination10. The above mentioned synthesis routes yield nano- and microsized materials. Recently, we evaluated the advantages of producing ZrO2-Al2O3 materials using 4-step method: (I) co-precipitation of precursors, (II) microwave hydrothermal synthesis (MHS), (III) drying materials in room temperature, and (IV) annealing11,12. This method allows producing highly crystalline nanoparticles with a narrow particle size distribution.
Unique physico-chemical properties of nanomaterials prompted an unprecedented interest in control of their nanostructure and characterization. In most cases bulk material properties differ significantly from those obtained for materials of the same chemical composition but with grain size in the micrometer range. Kimmel et al.13, discussed the role of the grain size on the coexistence of ZrO2-Al2O3 phases. According to Kimmel et al.13, nanoscale grain size leads to formation of phases and structures which do not appear for ZrO2-Al2O3 conventional composites and/or are metastable. Materials obtained in sol-gel process by Kimmel et al.13 were identified as tetragonal zirconia (t-ZrO2), quasi-amorphous zirconia and several spinel-like aluminas. These findings are consistent with several earlier reports14,15,16,17.
In the case of micron-sized ZrO2-Al2O3 powders, at ambient pressure and below 1530 °C, there is no mutual solubility of the two oxides. However the situation changes when the grain size of the oxides is reduced to the nano-range13. In nanopowders dissolution of Al3+ in zirconia can take place13,18,19,20. According to Kimmel et al.13, the Zr-rich side of the binary oxides phase diagram shows quasi-amorphous ZrO2 and t-ZrO2–like structures after annealing of xerogels at low temperatures. In addition, in the region from ZrO2 – 0% Al2O3 up to ZrO2 – 25% Al2O3, temperature up to 1100 °C, and grain size below 50 nm stable t-ZrO2 solid solution was found. After annealing xerogels above 1200 °C, the two oxides separate and stable mixture of the monoclinic phase of ZrO2 (m-ZrO2) and corundum (α-Al2O3) was present.
Thus, phase diagrams for nanomaterials may be different from that of conventional materials. Understanding of nanomaterials phase composition is critical for many industrial applications such for example additive manufacturing or smart nanocomposites. Since the initial MHS products are different than products obtained in sol-gel method it is essential to prepare a new phase diagram of the ZrO2-Al2O3 system for nanosized particles obtained in MHS in a wide annealing temperature and composition range.
Results
The present method of particles preparation ensured that the grain size of particles was in the nano range from room temperature up to 1000 °C for the identified several phases present for the composition range from 0% Al to 100% Al.
The Boehmite (AlOOH) grain size evaluated by Rietveld refinement analysis for as-synthesized samples was found to be above 100 nm. When it transforms to γ-Al2O3, grain size of that phase was 10 nm and stayed in this range up to 700 °C. At 1000 °C, for γ-Al2O3, the grain size was found to be up to 15 nm. Upon further annealing, when the grain size increases above 15 nm, the phase transition γ- to θ-Al2O3 takes place. The grain size of t-ZrO2 was in the same range as alumina.
Figure 1 presents how the grain size of tetragonal zirconia depends on its composition, for as synthesized samples, annealed at 600 °C, and at 700 °C. The t-ZrO2 grain size is in the range 4–12 nm for as synthesised samples and samples annealed at 600 °C. Increase of annealing temperature up to 700 °C causes minor grain growth up to 13 nm. Annealing at 1000 °C leads to the grain size increase from 20 to 60 nm only and these values were found for 85% and 10 mol.% of Al, respectively. Annealing in the temperature range 1000–1200 °C caused further increase up to 100 nm. For this phase a solid solution of Al3+ in ZrO2 was observed for Al content up to 30 mol% and temperature below 1000 °C. In contrast to the sol-gel products (as-synthesised and after annealing) there was no zirconia-like quasi amorphous phases in any powder obtained in MHS12,13.
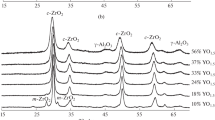
Figure 2 shows the new phase map for ZrO2-Al2O3 nanopowders. The phase map was determined for as-synthesized ZrO2-Al2O3 nanomaterials and powders after annealing for 1 h in whole range of compositions in temperatures range from 300 to 1500 °C. Samples with 1 wt.% or less of a given phase were considered free of that phase. The symbols visible in Fig. 2. represent phase mixtures (i.e. a star: mixture of t-ZrO2, m-ZrO2, θ-Al2O3), as well as single phases (i.e. a square: α-Al2O3). Dotted black lines marked in Fig. 2. represent the temperatures of alumina phase transitions and they were determined using thermal analysis21 and Rietveld refinement.
For better clarity and understanding of presented phase map (Fig. 2) the XRD patterns for chosen compositions are presented in Fig. 3. All existing phases were marked in the figure. It should be noted, that phase map was determinate for crystalline phases. During phase analysis of compositions with high amount of Al we found that after Boehmite (AlOOH) disappearance and from ~700 °C weak, very broad, γ-Al2O3 diffraction lines appear with very high background (Fig. 3c). That may indicate presence of residual amorphous alumina phase.
Since this work is dedicated to size dependent phase map of ZrO2-Al2O3 nanopowders we applied 2 methods to evaluate grain size. For grain sizes above 60 nm Rietveld analysis cannot be applied and TEM investigations were needed to determine particle size distribution for samples annealed at 1500 °C (Fig. 4). The morphology of ZrO2 and Al2O3 nanopowders obtained using the 4-steps method was discussed previously11,12. The first column of images in Fig. 4 shows the change of morphology, the second column shows the elements distribution (EDX), and the third one shows particle size distribution for ZrO2 and Al2O3 particles. The compositions chosen for TEM study were as follows: ZrO2 with 10 mol.% of Al (Fig. 4a–c), ZrO2 with 50 mol.% of Al (Fig. 4d–f), ZrO2 with 70 mol.% of Al (Fig. 4g–i), and ZrO2 with 90 mol.% of Al (Fig. 4j–l). Figure 4 shows that the nano-particles of the component phases formed agglomerates. The agglomerates size depends on composition. It is seen that the individual particles size of ZrO2 is in the range from 50 up to 500 nm for powders containing 90 and 10 mol.% of Al, respectively. Thus, in the whole composition range, up to 1500 °C, the particle size of at least one of the components is in the nano-range. For the annealing temperature lower than 1000 °C all the system components displayed size less than 60 nm. Hence, the phases map studied in the present paper concern a nano-enabled system.
Figure 5a–c presents the change of content in the system of t-ZrO2, m-ZrO2, AlOOH, θ-Al2O3 and α-Al2O3 phases as a function of composition and annealing temperature. Figure 5a presents results for as synthesized materials, while Fig. 5b,c show change of composition for powders after annealing at 1200 °C, and 1500 °C, respectively. The graphs were obtained based on Rietveld refinement analysis performed for XRD patterns.
Discussion
It is well known, that zirconia exists in three polymorphic phases: monoclinic (m), stable up to 1170 °C, tetragonal (t), stable up to 2370 °C and cubic (c), stable up to 2715 °C12. Usually, a stable at room temperature t-ZrO2 phase is obtained by doping zirconia with some elements i.e. Mg, Ca, Re (Y etc.). Such stabilization is achieved by incorporation of divalent or trivalent cations in the fluorite-type structure by substitution of Zr4+ cations and creation of oxygen vacancies to maintain the local charge balance22. Stabilized zirconia can be processed in a wide temperature range without phase transformations, which is especially important for sintering.
Srdic et al.22, discussed stabilization of t-ZrO2 using Al3+ ions. Authors noticed that development of nanoprocessing techniques in which precursors are mixed on the molecular level have enabled the formation structures, where Al3+ is incorporated into the zirconium-oxygen network12,13,22. One of methods that allows to stabilize t-ZrO2 at room temperature and forms a t-ZrO2 solid solution (t-ZrO2(ss)) is co-precipitation of ZrO2 and Al2O3 precursors together with microwave hydrothermal synthesis, which was reported before11,12. It is frequently claimed22 that doping with ions of lower valency than zirconia leads to vacancy formation to ensure charge compensation, and stabilization of the t-ZrO2 structure. In our opinion for the nano-sized particles’ stabilization is caused not by doping but the small size and surface energy contribution, and this is in agreement with23,24,25. Further, charge compensation effects are hardly imaginable for such high doping levels. It is highly improbable that each Al3+ is accompanied by a vacancy for example 20 or 30 mol. % addition, as there cannot be 20–30% of sites occupied by vacancies. Thus, at nanoscale it is possible to form a regular solid solution of Al3+ in zirconia due to stability of the t-ZrO2 phase caused by the very small grain size.
Discussion on the effect of composition on agglomerates size is outside the scope of the present paper. Here we are concerned only with t-ZrO2 grain size influence. Remarkable is that the aluminum rich and zirconia rich particles are homogeneously dispersed. As discussed in11 this leads to a nanoisolation effect, resulting from the 4-steps synthesis procedure. Nanoisolation means that nanoparticles of zirconia or zirconia rich phases are separated from each other by aluminum-rich phases, and vice-versa. Nanoisolation hinders particle growth due to Oswald ripening and coalescence. It is also a plausible reason for limited particles size growth observed in the present paper, which permitted to construct the nano-enabled phase diagram.
As it can be seen from Fig. 1 the grain size of t-ZrO2 decreases with increasing mol.% of Al. The smallest t-ZrO2 grain size was found for ZrO2 with 90 mol.% and more of Al. The grain of size of t-ZrO2 did not change meaningfully during annealing powders up to 700 °C but increases slightly after annealing at 1000 °C. The biggest t-ZrO2 grain size after treatment at 1000 °C was found for the lowest Al content. For the small particles below 15 nm, Al3+ dissolve in the crystal structure of ZrO2, and segregation of alumina does not take place up to 1000 °C. This is especially visible at high temperatures were isolated in alumina matrix nanosized t-ZrO2 grains do not undergo transformation into m-ZrO2.
Figure 2 presents the phase map for ZrO2-Al2O3 nanocrystalline powders. It can be observed, that t-ZrO2-based solid solution, coexisting with a small amount of m-ZrO2 (up to 20 wt.%) is present up to 30 mol. % of Al, in the annealing temperature range up to 1000 °C. The solid solution formula is Zr(1−x)AlxO(2−x/2), where x~0.45. The phase transformation of solid solution during temperature increase for compositions up to 30 mol.% of Al is presented below (1):
where t-ZrO2(ss) is the solid solution of Al3+ in t-ZrO2. Above 1000 °C the grains start to grow, and at the same time θ-Al2O3 precipitates. At 1200 °C all components of ZrO2-Al2O3 system fully precipitate. These findings are in agreement with our previous work21. Thermal treatment longer than 1 h in the temperature range from 1100 °C up to 1500 °C lead to gradual phases segregation according to the phase diagram for conventional materials. The change of phase composition for this region is in addition presented in Fig. 3(a). It can be seen that for all compositions below 19% of Al after annealing at 1500 °C only m-ZrO2 phase exists.
From 36% up to 84 mol.% of Al in as-synthesized samples three phases were present: tetragonal, monoclinic zirconia and the crystalline Boehmite. Boehmite undergoes further transformation to γ-Al2O3 at approximately 300 °C (Fig. 2). These findings are in agreement with our previous studies12. Samples with compositions in the range from 36 to 84 mol. % of Al follow phase transitions (2) as reported in literature11,17,25,26,27.
In addition, Fig. 3(b) shows change of XRD patterns for 36 mol. % of Al as a function of temperature.
Above 36 mol.% of Al at temperatures above 1200 °C segregation of Al2O3 is fast and it is complete at 1500 °C. The crystalline phase transformation of ZrO2-Al2O3 in referred composition range is shown below (3):
In the last region of ZrO2-Al2O3 phase map from 90 to 100% of Al (Fig. 2) m-ZrO2 phase disappears in as-synthesized nanopowders from 90 up to 94 mol.% of Al. From 98 mol.% to 100% of Al pure AlO(OH) phase exists. All Al-based phases transform into alumina transition phases in the way described by Levin et al.17. The change of phases with temperature for 94 mol.% of Al is shown in Fig. 3(c).
Inspection of Fig. 2 shows some remarkable features of the nano-enabled phase map.
-
A solid solution of Al3+ in t-ZrO2 was observed for up to 30 mol.% of Al, and annealing temperature up to 1000 °C.
-
Up to 96 mol.% of Al t-ZrO2 and m-ZrO2 coexisted, with m-ZrO2 less than 20 wt.%, above 96 mol.% of Al, ZrO2 only in the tetragonal structure was found existing from RT up to 1000 °C.
-
At 1000 °C precipitation of θ-Al2O3 started from the above solid solution.
-
Stable γ-Al2O3 phase temperature range was from 300 to 1000 °C.
-
Stable θ-Al2O3 phase temperature range was from 1000 to 1200 °C.
The above nano-enabled phase map is strikingly different from the conventional ZrO2 - Al2O3 phase diagram, where there is no solid solution, the t-ZrO2 phase is not observed below 1170 °C, and the transitions in the Al –structures takes place at temperatures ~100 °C higher than for the bulk system.
Several papers report existence of Zr(1−x)AlxO(2−x/2) solid solutions for nano-sized particles23,24,25. Stefanic and Music28 studied crystallization of amorphous Zr(1−x)AlxO(2−x/2) solid solutions obtained by precipitation. Authors28 showed that metastable t-ZrO2 solid solution crystallizes at 550–1000 °C (4), but not at lower temperature like in our case (270 °C at MHS).
Contrary to our results Stefanic and Music28 showed, that for x > 0.3, the monoclinic phase does not appear at 1000–1100 °C. Similar to our findings Kimmel et al.13 identified solid solution in the same composition range. However, the obtained ZrO2-type nanopowders were quasi-amorphous after drying at room temperature. Our results showed that for compositions with higher Al amount and after thermal treatment up to 600 °C there is a significant amount of amorphous alumina (which also causes problems for the Rietveld analysis). In the phase map (Fig. 2) we refer only to the crystalline phases.
TEM method was used to evaluate particle with sizes above 60 nm, which we expected to obtain for materials after annealing at 1500 °C. The measurements were performed in STEM mode. Figure 4 shows the particle size distributions for ZrO2 and Al2O3 for chosen compositions after annealing at 1500 °C. The smallest particles were observed for ZrO2 with 10 mol.%Al (Fig. 4c), where average size was ~75 nm. Most of them are ZrO2 particles as confirmed by EDX studies (Fig. 4b). The average ZrO2 and Al2O3 particles size distribution for the composition containing higher amount of Al (50% and 70 mol.% of Al) were found to be 409 nm (Fig. 4f), and 337 nm (Fig. 4i). The nanocomposite ZrO2 with 90 mol.% of Al is characterized by 126 nm average ZrO2 and Al2O3 particle size distribution (Fig. 4l). For all compositions above 60 mol.% of Al, and after annealing at 1500 °C ZrO2 particles stay in nano-range in alumina matrix, what we discussed previously11 (Fig. 4). This finding confirms our results described above.
Figure 5 shows the percentage weight proportion of t-ZrO2, m-ZrO2 and AlO(OH) (Al2O3 at higher temperatures) phases in ZrO2-Al2O3 system as a function of composition and annealing temperature. These results were the crucial step for phase map construction. It can be seen from Fig. 5 that the amount of t-ZrO2 varies when the temperature and composition change. For ZrO2 up to 30 mol. % of Al and for materials in as-synthesized state the wt.% of t-ZrO2 is the highest and in a range from 70 to 90 wt.% (Fig. 5a). For compositions above 30 mol. % of Al the amount of t-ZrO2 for as-synthesized nanomaterials drops with increasing Al content. The change of ZrO2 wt.% vary after annealing at 1200 °C (Fig. 5b), where the segregation of Al2O3 already took place. The amount of t-ZrO2 drops to few percent’s in comparison to as-synthesized materials but is rising with increasing content of Al. Annealing at 1500 °C causes further lowering of t-ZrO2 amount (Fig. 5c), and m-ZrO2 becomes ZrO2-predominant phase. Based on the obtained results it is possible to distinguish two cases:
-
1.
In as-synthesized materials, the t-ZrO2 amount decreases and m-ZrO2 increases with increased amount of Al.
-
2.
Above 1200 °C the t-ZrO2 amount increases with increased amount of Al.
The presented above discussion was referring to the ZrO2-Al2O3 system phase composition for amorphous and nanocrystalline materials. The conventional phase diagram for ZrO2-Al2O3 bulk materials is much simpler than for the nano-enabled system or amorphous materials. Nano-enabled29 phase map (Fig. 2) might be useful for development of commercially viable ZrO2-Al2O3 composites via sintering of nanoparticles.
Conclusions
In this work for the first time a comprehensive study of the ZrO2-Al2O3 system composed of nanoparticles in the wide temperature region and whole composition range was performed and the new nano-enabled phase map for the ZrO2-Al2O3 powders was determined. The map is considerably different comparing to the conventional phase diagram. The main difference is the presence of t-ZrO2 or solid solution coexisting with some m-ZrO2 in the range from room temperature up to ~1000 °C, while for conventional phase diagram m-ZrO2 phase and alumina phases only exist.
A significant role for keeping the particles size in the nano-range for the whole annealing temperatures range played nanoisolation.
We found that t-ZrO2 grain size before high temperature treatment is in the range from 4 to 13 nm. The smallest t-ZrO2 grains were found for ZrO2 with 90 and 94 mol% of Al. After annealing at 1500 °C the size of ZrO2 particles increases, but still remains in nanorange for compositions with high Al content due to zirconia nanoparticles isolation in alumina matrix.
We showed that the weight % amount of t-ZrO2 changes with the temperature and composition. Two trends where found. For as synthesised materials the t-ZrO2 amount declines with increased mol.% of Al. For materials annealed above 1200 °C t-ZrO2 content rises with increased amount of Al.
Presented in this work the nano-enabled phase map for ZrO2-Al2O3 composites is different than for bulk materials and it opens new possibilities for materials processing leading to applications.
Materials and Methods
The procedure for obtaining of as-synthesized ZrO2-Al2O3 nanopowders was described in details elsewhere21,30. The metal oxides precursors used in the process were zirconyl chloride octahydrate (ZrOCl2·8H2O, Sigma-Aldrich (≥99.5%)), and aluminum nitrate nonahydrate (Al(NO3)3·9H2O, CHEMPUR, analytically pure). Microwave reactions took place in a MAGNUM II ERTEC microwave reactor (2.45 GHz, 600 W)30. The procedure of materials thermal treatment was described in details in our previous work11. The annealing process was performed for 10 min, 1 h, 2 h and in some cases more than 10 h. We found that 1 h of annealing allows alumina to segregate from the solid solution in the proper temperature range, and this annealing time was used for the phase map construction.
X-ray diffraction patterns for all ZrO2-Al2O3 compositions were collected using diffractometer (X’Pert PRO, PANalytical) equipped with a copper anode (CuKα1) and an ultra-fast PIXcel1D detector. The analysis took place at room temperature in the 2θ range of 10–100° with a step size of 0.03°. The obtained diffraction data were analyzed using processing methods, such as: Rietveld refinement method31, FullProf software32, and by the DBWS program33.
TEM investigations were conducted using an FEI Talos 20–200 transmission microscope at 200 kV, The measurements were performed in TEM mode and also in STEM using dark field (DF) imaging. Energy-dispersive x-ray spectroscopy was used to detect differences in local chemical composition. The specimens for the TEM observations were prepared by dropping the ethanol particle dispersion, created by an ultrasonic technique, on a carbon film supported on a 300-mesh copper grid. TEM tests were used to determine the nanoparticle size distribution. The grain size histograms were obtained by considering a region of a sample having about 250 nanocrystals and approximating the shape of each nanocrystal by a sphere. The obtained histograms were fitted to normal or lognormal distributions.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Chandradass, J., Kim, M. H. & Bae, D. S. Influence of citric acid to aluminium nitrate molar ratio on the combustion synthesis of alumina–zirconia nanopowders. J. Alloy. Compd. 470, L9–L12 (2009).
Wei, Z. et al. Preparation and property investigation of CeO2–ZrO2–Al2O3 oxygen-storage compounds. J. Alloy. Compd. 455, 322–326 (2008).
Wang, J., Taleff, E. M. & Kovar, D. High-temperature deformation of Al2O3/Y-TZP particulate composites. Acta Mater. 51, 3571–3583 (2003).
Yin, W., Meng, B., Meng, X. & Tan, X. Highly asymmetric YSZ hollow fibre membranes. J. Alloy. Compd. 476, 566–570 (2009).
Benzaid, R., Chevalier, J. & Saadaoui, M. Fracture toughness, strength and slow crack growth in a ceria stabilized zirconia–alumina nanocomposite for medical applications. Biomaterials. 29, 3636–3641 (2008).
Chevalier, J., De-Aza, A. H., Fantozzi, G., Schehl, M. & Torrecillas, R. Extending the life time of ceramic orthopaedic implants. Adv. Mater. 12, 1619–1621 (2000).
Naglieri, V. et al. Optimized Slurries for Spray Drying: Different Approaches to Obtain Homogeneous and Deformable Alumina-Zirconia Granules. Materials. 6, 5382–5397 (2013).
Shukla, S., Seal, S., Vij, R. & Bandyopadhyay, S. Effect of HPC and water concentration on the evolution of size, aggregation and crystallization of sol–gel nanozirconia. J. Nanopart. Res. 4, 553–559 (2002).
Begand, S., Oberbach, T. & Glien, W. Corrosion behaviour of ATZ and ZTA ceramic. Bioceramics. 19, 1227–1230 (2007).
Chuang, C.-C., Hsiang, H.-I., Hwang, J. S. & Wang, T. S. Synthesis and characterization of Al2O3-Ce0.5Zr0.5O2 powders prepared by chemical coprecipitation method. J. Alloy. Compd. 470, 387–392 (2009).
Koltsov, I. et al. Mechanism of Reduced Sintering Temperature of Al2O3–ZrO2 Nanocomposites Obtained by Microwave Hydrothermal Synthesis. Materials. 11, 829 (2018).
Malka, I. E., Danelska, A. & Kimmel, G. The Influence of Al2O3 Content on ZrO2-Al2O3 Nanocomposite Formation—The Comparison between Sol-Gel and Microwave Hydrothermal. Methods. Mater. Today Proc. 3, 2713–2724 (2016).
Kimmel, G. et al. XRPD study of phase transformations accompanied with grain growth in the alumina-zirconia system. Z. Kristallogr. Proc. 1, 455–460 (2011).
Sato, T., Osawa, F., Nakamura, T., Watenable, H. & Ikoma, S. Thermal decomposition of zirconium hydroxide. Thermochim. Acta 34, 211–220 (1979).
Gau, G.-Y. & Chen, Y.-L. A nearly pure monoclinic nanocrystalline zirconia. J. Solid State Chem. 178, 1675–1682 (2005).
Zhou, R.-S. & Snyder, R. L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Cryst. B 47, 6117–617 (1991).
Levin, I. & Brandon, D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 81, 1995–2012 (1998).
Tsunekawa, S., Ito, S., Kawazoe, Y. & Wang, J.-T. Critical Size of the Phase Transition from Cubic to Tetragonal in Pure Zirconia Nanoparticles. Nano Lett. 3, 871–875 (2003).
Kikkawa, S., Kijima, A., Hirota, K. & Yamaguchi, O. Soft solution preparation methods in a ZrO2–Al2O3 binary system. Solid State Ionics 151, 359–364 (2002).
Li, G., Li, W., Zhang, M. & Tao, K. Characterization and catalytic application of homogeneous nano-composite oxides ZrO2–Al2O3. Catalysis Today 93-95, 595–603 (2004).
Koltsov, I., et al. Thermal and physical properties of ZrO2–AlO(OH) nanopowders synthesised by microwave hydrothermal method. J Therm Anal Calorim. 131 (3), https://doi.org/10.1007/s10973-017-6780-8 (2017).
Srdic, V. V. & Winterer, M. Aluminum-Doped Zirconia Nanopowders: Chemical Vapor Synthesis and Structural Analysis by Rietveld Refinement of X-ray Diffraction Data. Chem. Mater. 15, 2668–2674 (2003).
Srivistawa, A. K. Oxide Nanostructures: Growth, Microstructures, and Properties, April 9, 2014 by Pan Stanford, CRC Press, ISBN 9789814411356 - CAT# N10742.
Yamaguchi, O., Shirai, M. & Yoshinaka, M. Formation and Transformation of Cubic ZrO2 Solid Solutions in the System ZrO2—Al2O3. J. Am. Ceram. Soci. 71(12), C-510–C-512, https://doi.org/10.1111/j.1151-2916.1988.tb05821.x (1988).
Inamura, H., Miyamoto, Y., Imaida, M., Takagawa, K. & Hirota, O. Formation and hot isostatic pressing of ZrO2 solid solution in the system ZrO2-Al2O3. J. Mater. Sci. 29(18), 4913–4917 (1994).
Souza Santos, P., Souza Santos, H. & Toledo, S. P. Standard Transition Aluminas. Electron Microscopy Studies. Mater. Res. 3(4), 104–114 (2000).
Navrotsky, A. & Ushakov, S. V. Thermodynamics of Oxide Systems Relevant to Alternative Gate Dielectrics, A. A. Demkov & A. Navrotsky (eds), Materials Fundamentals of Gate Dielectrics, Springer. Printed in the Netherlands, https://doi.org/10.1007/1-4020-3078-9_3, 57–108. (2005).
Stefanic, G. & Music, S. Factors influencing the stability of low temperature tetragonal ZrO2. Croatica Chem. Acta. 75(3), 727–767 (2002).
ISO/TS 80004-1:2015, Nanotechnologies — Vocabulary — Part 1: Core terms. Geneva, Switzerland, 2015.
Wojnarowicz, J. et al. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomat. 2016, ID 2789871, 15, https://doi.org/10.1155/2016/2789871 (2016)
Rietveld, H. M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1967).
J. Rodrigez-Carvajal: Fullprof, Program for Rietveld refinement, Laboratories Leon Brillouin (CEA-CNRS), Saclay, France, 1997
Young, R. A., Sakthivel, A., Moss, T. S. & Paiva-Santos, C. O. DBWS-9411—An upgrade of the DBWS*.* programs for Rietveld refinement with PC and mainframe computers. J. Appl. Crystallogr. 28, 366–367 (1995).
Acknowledgements
This research was funded by Polish National Science Centre grant number: UMO-2013/11/D/ST8/03429- “Sonata 6”. The TEM study was carried out at the Biological and Chemical Research Centre, University of Warsaw, established within the project co-financed by European Union from the European Regional Development Fund under the Operational Programme Innovative Economy, 2007–2013 and Panda2 501-D312-56-0000002. Authors are indebted to Maria Małysa for help in annealing process.
Author information
Authors and Affiliations
Contributions
I.K. conceived and designed experiments, wrote the paper, synthesized materials, performed annealing process, analyzed results, and prepared XRD-linked figures; G.K. carried out Rietveld refinement analysis, S.S. performed XRD analysis, K.S. performed TEM imaging, particle size distribution and prepared TEM-linked figure, W.L. contributed to the scientific discussion and project conception. This publication is part of Iwona Koltsov’s habilitation thesis. All authors contributed to the discussion and manuscript edition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koltsov, I., Kimmel, G., Stelmakh, S. et al. The new nano-enabled phase map of ZrO2-Al2O3. Sci Rep 9, 5540 (2019). https://doi.org/10.1038/s41598-019-42058-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42058-4
- Springer Nature Limited