Abstract
Malaria is a major public health problem in India and in the Chhattisgarh state. The diagnosis of malaria presents a major challenge in remote areas The prevalence of malaria in Darbha and Kilepal Community Health Centers (CHCs) of the Jagdalpur district, Chhattisgarh affected by conflict was determined using microscopy and polymerase chain reaction (PCR). In the year 2015, 29.4% and 21.5% cases were found to be positive for malaria at the Darbha and Kilepal CHCs, respectively, by microscopy, and 7.4% and 1.6% of cases had mixed infections, respectively. Among the suspected cases of mixed infections and doubtful diagnoses, 21% had mixed infections with two or more species at the Darbha CHC, and 17% from the Kilepal CHC, as determined by PCR. Both the P. vivax subspecies Pv210 (56%) and Pv247 (44%) and the P. ovale curtisi subspecies were found in this area. The high proportion of mixed malaria parasitic infections detected in this study indicate the need to adequately train health staff involved in diagnosing malaria. This study showed that there is a need for site-specific data to understand the epidemiological picture and to develop appropriate intervention strategies and management guidelines for controlling and eliminating malaria in India.
Similar content being viewed by others
Introduction
Malaria is a major public health problem in India and contributes to 89% of malaria burden in South-East Asia1. A diversity of factors influences the prevalence and incidence of malaria within the malaria-endemic regions of India depending on the presence of vectors, parasite species, drug resistance, socio-economic factors, geographical areas and the types of interventions used2,3. According to the world malaria report (2015), a global decline in the number of malaria cases was observed from an estimated 262 million cases in 2000 to 214 million cases in 2015, which represents an approximately 18% decline. Furthermore, 57 out of 106 countries have shown a sharp reduction of approximately 75% in the incidence of malaria, and an additional 18 countries have shown an estimated 50–75% reduction in the incidence of malaria4.
Malaria is a major health problem in remote forested areas of the country. Recent records show that 46% of malaria, 70% of P. falciparum and 47% of deaths due to malaria are contributed by 7.8% of the population that resides in 124 districts of the country5. The diagnosis of malaria presents a major challenge in remote community health centers (CHCs) that have limited health infrastructure and human resources. In areas affected by conflict, this problem is further compounded by limited resources. Many febrile patients are still treated with antimalarial drugs without a confirmed diagnosis of malaria because the diagnosis of malaria is mainly based on microscopic examination of blood smears6,7. Although rapid diagnostic tests (RDTs) are easy and reliable tools for the diagnosis of P. falciparum and P. vivax, RDTs are often unable to differentiate between mixed infections. Moreover, RDTs are unable to detect a low density of parasites7,8. Triple and quadruple mixed malaria infections have been reported in malaria-endemic areas of Asia9,10,11 and Africa12. The objective of this study is to record the prevalence of malaria in two CHCs of the Jagdalpur district affected by conflict. The findings from the study will provide evidence that can be used for developing region-specific malaria control and elimination strategies in areas that are difficult to reach.
Results
In 2015, a total of 5246 and 3253 blood smears were screened for malaria parasites using microscopy among febrile patients presenting to the malaria clinic in the CHCs of Darbha and Kilepal. Of these cases, 1541 (29.4%) and 698 (21.5%) at the Darbha and Kilepal CHCs were positive for malaria, respectively, as determined by microscopy. Malaria positivity per month varied from 16.5 to 42% (Avg SPR 29·4; 95% CI 28·1–30·6) with 83% P. falciparum infection at Darbha and 10.4 to 32% (Avg SPR 21.5%; 95% CI 20.1–22.9) with 90% P. falciparum infection at Kilepal. Malaria was recorded in all age groups ranging from 1 month to 80 years (mean age 16.12 ± 13.84). There is no difference in malaria positivity between males and females at either CHC. Age-specific and species-specific data on malaria are shown in Table 1. The slide positivity rate was lowest in adults (aged >14 yrs) when compared to all other age groups at both CHCs. At Darbha, the SVR was highest in infants (aged < 1 yrs) and showed a declining trend with increasing age (χ2 trend = 90.6; p < 0·0001). However, SFR was highest in relatively older children >4–8 years when compared to other age groups. A similar trend was found at the Kilepal CHC. The SVR was highest among infants and showed a linear declining trend with increasing age (χ2 trend = 107.5; p < 0·0001), and SFR was highest in relatively older children (>4–8 years) when compared to all other age groups. Mixed infections were 7.4% and 1.6% at the Darbha and Kilepal CHCs, respectively, as determined by microscopy. The proportion of P. falciparum was high (p < 0·0001) in all age groups compared to P. vivax at both CHCs. P. falciparum gametocytes were found more frequently in younger age groups (1–4 years) compared to other age groups at both CHCs.
All available 198 blood samples with doubtful microscopic identification which were diagnosed as P. falciparum infection by microscopy were further confirmed by PCR. Out of 198 samples, 21% were mixed infections with two or more species at the Darbha CHC, including one case that was positive for all four species of the malaria parasite. At the Kilepal CHC, out of 157 samples tested, 17% were mixed infections with two or more species (Fig. 1). Further analysis revealed that mixed infections of P. vivax and P. falciparum were most frequent (16.7%), followed by P. falciparum, P. vivax and P. malariae (2.5%), P. falciparum and P. malariae (1.5%) and P. falciparum, P. vivax, P. malariae and P. ovale (0.51%) at the Darbha CHC (Table 2). At Kilepal, mixed infections with P. falciparum and P. vivax were also highest (16.5%), followed by P. falciparum, P. vivax and P. ovale (0.64%). A total of 66 mixed samples with P. vivax were analyzed for Pv210/Pv247 and, out of these, 56% were Pv210 and 44% were Pv247. The Pv210 was more frequently observed (62%) in the Kilepal CHC, while an almost equal proportion of Pv210 and Pv247 were found at the Darbha CHC. Both P. ovale samples were identified as P. ovale curtisi subspecies. P. knowlesi was not found at either CHC. These mixed infections were mild and did not show any complications. A significantly greater number of malaria cases (χ2 trend = 64.9; p < 0·0001) were recorded at Darbha compared to Kilepal, while there was no significant difference in the prevalence of mixed infection among the CHCs.
Gel image showing the results of molecular diagnosis of Plasmodium species by Polymerase chain reaction (PCR). 1: 100 bp ladder; 2: NC, 3: PC P. falciparum; 4–13: P. falciparum positive results; 14: NC; 15: PC P. vivax; 16–25: P. vivax positive and negative results; 26: 100 bp Ladder; 27: NC; 28: PC P. malariae; 29–38: P. malariae positive and negative results; 39: NC; 40: PC P. ovale; 41–50: P. ovale positive and negative results. NC: Negative control; PC: Positive control.
Discussion
Assessment and analysis of malaria in the local region are a prerequisite for embarking on any control and elimination program. India makes up 61% of malaria cases and 41% of malaria deaths in Southeast Asian countries5. Approximately 91% of India’s population lives in a malaria-affected area, while 14% of the population resides in areas where malaria transmission is high4. Although many cases of mixed malaria infections have been reported in malaria-endemic countries, coincidental infection with more than one species of Plasmodium is rare9,13,14,15,16. An earlier study from India revealed a high proportion of mixed infections (45%) with P. vivax and P. falciparum 13. Another study recorded 13% of mixed infections with P. vivax and P. falciparum 17. However, these authors did not look for P. malariae and P. ovale. Additionally, 17.4% of mixed Plasmodium species infections with 4 Plasmodium species from eight endemic states was also reported recently9. These studies are carried out in areas of the country where malaria is highly endemic. Mixed infections have an epidemiological significance for malaria control and elimination. For example, if P. vivax parasitemia is suppressed by coinfection with P. falciparum, effective control of P. falciparum infection in an area will activate P. vivax transmission in the community18, which is more difficult to control. The frequencies of less common species such as P. malariae and P. ovale are well known to be largely underestimated by microscopy19,20. PCR-based methods are more sensitive and more readily detect mixed species9,13. Using PCR, Snounou and White21 in Thailand found between one-third and one-half of malaria infections to be of mixed species infection. In this study, 21% and 17% of infections were found to be mixed infections at the Darbha and Kilepal CHCs, respectively. The highest number of mixed infections were found to be due to P. falciparum and P. vivax (>16%). However, we recorded only 7.4% and 1.6% of mixed infections with P. falciparum and P. vivax by microscopy at the Darbha and Kilepal CHCs, respectively.
The low sensitivity of microscopy has two major consequences in malaria control efforts. First, low-density parasitemia may serve as a reservoir for infections without the knowledge of program managers. Second, in mixed infections, the tendency of one parasite to dominate the other lowers the efficiency of microscopic detection of the two species in the same sample22. It is also worth mentioning that the person performing the microscopy are often inclined to identify only one species, as microscopic examination is time-consuming and labor-intensive and, as a result, the uncommon species are not detected.
The four Plasmodium species have varying clinical characteristics. Of the four species, P. falciparum causes the most severe symptoms, i.e., severe anemia, cerebral malaria, multiorgan failure and death17,23. P. vivax and P. ovale, though responsible for mild infections, may persist within the liver as hypnozoites, causing relapses even after treatment with blood schizonticides18. P. malariae is also mild and may persist in the human population at a very low density and may cause renal failure24.
The pattern of single or mixed infections is also determined by the ability of the vector species to be infected by different parasite species simultaneously25. In Bastar, 5 efficient vectors were found with variable prevalence and transmission potential26,27,28. It is worth mentioning that the vectors in this area transmit both subspecies (Pv210 and Pv247) of P. vivax, which are normally found in distinct geographically areas29. Although both P. ovale wallikeri and P. ovale curtisi were found in this area in a previously reported study19, in our current study, we only found the P. ovale curtisi subspecies. The quality of intervention measures, i.e., indoor residual spray coverage, distribution of bed nets, regular surveillance and drug distribution, are also very important factors and often affect transmission rates due to the remoteness and ongoing conflicts in the area30,31. Furthermore, health-seeking behaviours among the people, i.e., receiving treatment from quacks (unlicensed professionals) and partial treatment, are other important factors that also play a major role in maintaining reservoirs for infection.
An incorrect diagnosis of malaria is a severe public health concern, as misidentification of malaria parasites could lengthen the time to parasite clearance and can also lead to recrudescence25 and drug resistance32, especially in areas such as India where the treatment of P. falciparum and P. vivax are different. Additionally, incorrect treatment could also lead to changes in sensitivity of the parasite species to the drugs25. The knowledge of mixed infections is important not only for developing appropriate control measures but also for therapeutic options. This study has several limitations. Single infections or negative blood smears were not tested by PCR if parasitemia levels were too low to be detected by microscopy. Furthermore, the study was undertaken in two remote CHCs of a highly endemic district that has limited resources and transport facilities. Patients present to the CHC hospital when they are coming to the market for routine shopping. Detailed studies in different ecosystems in remote areas with larger sample sizes are required for a more accurate picture of mixed infections with common and uncommon parasite species.
In conclusion, the high proportion of mixed infections is a big challenge for the malaria elimination initiative. India launched a National Malaria Elimination Program on 10–11 February 2016 to eliminate malaria from India by 2030. The high proportion of mixed malaria parasite infections detected in this study indicates the need for adequate training of health staff involved in the diagnosis of malaria. This study showed that there is a need for site-specific data to understand the epidemiological picture for developing appropriate intervention strategies and management guidelines.
Methods
Study site and sample collection
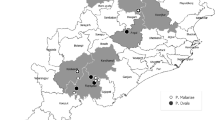
The Chhattisgarh state in Central India is the second highly malarious state in the country contributing to 14% of malaria in the country (NVBDCP - http://nvbdcp.gov.in/Doc/malaria-situation-Feb17.pdf). The Bastar district is known to have highest incidence of malaria in the Chhattisgarh state. Bastar was recently divided into seven districts, i.e., Kanker, Kondagaon, Jagdalpur, Dantewada, Bijapur, Narayanpur and Sukma23. The Jagdalpur district has a population of 125,463, of which 70% are of the ethnic tribe. Additionally, 50% of its geographical area is made up of forests. Jagdalpur has six community health centers (CHCs), of which two, i.e., Darbha and Kilepal, are remote community health centers (CHCs) that were selected for this study (Fig. 2). Darbha (18°51′31.0212″N81°52′7.95″E) is on the border of the Nabrangpur district of Odisha, while Kilepal (18°98′N 81°62′E) is located on the border of the Dantewada district. The villages are inaccessible due to the dense forest and valleys (temperature ranged from 22 °C to 41 °C). The population of Darbha is 79,360, and 83% of this population belong to the tribal community. The population of Kilepal is 49,334, of which 92.3% belong to the tribal community. This region is also experiencing serious problems with insurgency33,34, which also adversely affect health services. The economy of the villagers residing in this area is mainly forest based.
Map of India (A), Madhya Pradesh and Chhattisgarh (B), district of Chhattisgarh (C) and the study sites (Darbha and Kilepal CHCs) in the Bastar district (D). The map was generated in-house (the base map was taken from the following website: http://d-maps.com/m/asia/india/chhattisgarh/chhattisgarh66s.gif) and was modified using Adobe software Photoshop_CS3, version 10.0.
In 2015, a malaria clinic of the National Institute for Research in Tribal Health (NIRTH) of Indian Council of Medical Research (ICMR) was established at the Darbha and Kilepal CHCs (30 beds in each hospital) of the Jagdalpur district to study the prevalence of malaria in conflict-affected areas. Symptomatic patients were screened for malaria using the rapid diagnostic test (RDT SD Bioline Malaria Antigen P.f./P.v.) and with microscopy. A blood sample from a finger prick was collected from patients using sterile conditions with disposable equipment after receiving written informed consent. Thick and thin blood smears were prepared and stained with the JSB stain35 and were examined under the microscope. The results of the blood smears were made available in an hour. The person performing the microscopy examined 100 fields in thick smears before declaring the results negative. For quality control purposes, 100% of positive smears and 10% of negative smears were re-examined by the second expert who was unaware of the previous results. Patients were given treatment as per the National Vector Borne disease control program36. Patients having questionable parasite species were identified using molecular methods.
Molecular Characterization
Genomic DNA was isolated from samples by using a commercially available FavorPrep Genomic DNA Mini Kit (Favorgen Biotech Corp., Taiwan). Diagnostic polymerase chain reaction (PCR) was carried out using a standard protocol37. P. knowlesi was also tested in all of the samples using a protocol described by Neomi et al. (2012)38.
The central repeat region of the P. vivax circumsporozoite protein (csp) gene was amplified and sequenced to identify the P. vivax subspecies (Pv210 or Pv247)39. A gene specific to P. ovale (reticulocyte binding protein gene) was also amplified to differentiate among the P. ovale subspecies (P. ovale wallikeri and P. ovale curtisi)19.
Sequencing of PCR products was conducted using the dideoxy chain termination method with forward and reverse primers using the 3730xl genetic analyzer (Applied Biosystems, USA). Sequencing results were analyzed using the sequencing analysis software v5.2 (Applied Biosystems, USA).
Statistical Analysis
Univariate and bivariate statistical tools were used to analyze the prevalence of malaria. The odds ratios were computed to compare the slide positivity rate (SPR), slide P. falciparum rate (SFR) and slide P. vivax rate (SVR) among the different age groups. The chi-square test (χ2) was used to study the association of malaria prevalence among the age groups. The 95% C.I. was also computed for all univariate and bivariate analyses.
Ethical approval
This study was approved by the institutional ethics committee of the National Institute for Research in Tribal Health (NIRTH), Jabalpur. All methods were performed in accordance with the relevant guidelines and regulations. Before collecting the samples, written informed consent was obtained from the patients or from the parents/guardians of the children as per the guidelines of the ICMR. The consent form was also provided and explained to the patients and parents/guardians of children.
Data availability
All data generated or analyzed in this study are included in this published article.
References
World Health Organization. World Malaria Report 2016 http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1 (2016).
Singh, N., Singh, O. P. & Sharma, V. P. Dynamics of malaria transmission in forested and deforested region of Mandla district, Central India, Madhya Pradesh. J Am Mosq Cont Assoc. 12, 225–234 (1996).
Singh, N. et al. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 8, e73730, https://doi.org/10.1371/journal.pone.0073730 (2013).
World Health Organization. World Malaria Report 2015 http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf (2016).
Sharma, R. K. et al. Malaria situation in India with special reference to tribal areas. Indian J Med Res. 141, 537–545 (2015).
Bell, D. R., Wilson, D. W. & Martin, L. B. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg. 73, 199–203 (2005).
White, N. J. et al. Malaria. Lancet. 383, 723–735 (2014).
World Health Organization. NewPerspectives: Malaria Diagnosis. Report of a joint WHO/USAID informal consultation. October 25–27, 1999 http://apps.who.int/iris/bitstream/10665/66321/1/WHO_CDS_RBM_2000.14.pdf (2016).
Krishna, S. et al. Detection of Mixed Infections with Plasmodium spp. by PCR, India, 2014. Emerg Infect Dis. 21, 1853–1857 (2015).
Krishna, S., Bhandari, S., Bharti, P. K., Basak, S. & Singh, N. A rare case of quadruple malaria infection from highly malaria endemic area of Bastar, Chhattisgarh India. PLoS Negl Trop Dis., https://doi.org/10.1371/journal.pntd.0005558 (2017).
Purnomo, S. A., Gomez-Saladin, E. & Bangs, M. J. Rare quadruple malaria infection in Irian Jaya Indonesia. J Parasitol. 85, 574–579 (1999).
Mehlotra, R. K. et al. Random distribution of mixed species malaria infections in Papua New Guinea. Am J Trop Med Hyg. 62, 225–231 (2000).
Gupta, B. et al. High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Trop Med Int Health. 15, 819–824 (2010).
Mohapatra, M. K., Dash, L. K., Barih, P. K. & Karua, P. C. Profile of mixed species (Plasmodium vivax and falciparum) malaria in adults. J Assoc Physicians India. 60, 20–24 (2012).
Haanshuus, C. G. et al. A High Malaria Prevalence Identified by PCR among Patients with Acute Undifferentiated Fever in India. PLoS One. 7, 11:e0158816, https://doi.org/10.1371/journal.pone.0158816 (2016).
Oki, M. et al. A case of quadruple malaria infection imported from Mozambique to Japan. Am J Trop Med Hyg. 90, 1098–1101 (2014).
Jain, V. et al. Burden of cerebral malaria in central India (2004–2007). Am J Trop Med Hyg. 79, 636–642 (2008).
Steenkeste, N. et al. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri province, Cambodia: implication for malaria elimination. Malar J. 9, 108, https://doi.org/10.1186/1475-2875-9-108 (2010).
Chaturvedi, N. et al. Sympatric distribution of Plasmodium ovale curtisi and P. ovale wallikeri in India: implication for the diagnosis of malaria and its control. Trans R Soc Trop Med Hyg. 109, 352–354 (2015).
Bharti, P. K. et al. Emergence of a new focus of Plasmodium malariae in forest villages of district Balaghat, Central India: implications for the diagnosis of malaria and its control. Trop Med Int Health. 18, 12–17 (2013).
Snounou, G. & White, N. J. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 20, 333–339 (2004).
Sethabutr, O. et al. Detection of Plasmodium falciparum by polymerase chain reaction in a field study. J Infect dis. 166, 145–148 (1991).
Jain, V. et al. Burden of Complicated Malaria in a Densely Forested Bastar Region of Chhattisgarh State (Central India). PLoS One. 9, e115266, https://doi.org/10.1371/journal.pone.0115266 (2014).
Neri, S., Pulvirenti, D., Patamia, I., Zoccolo, A. & Castellino, P. Acute renal failure in Plasmodium malariae infection. Neth J Med. 66, 166–168 (2008).
Mayxay, M., Pukrittayakamee, S., Newton, P. N. & White, N. J. Mixed-species malaria infections in humans. Trends Parasitol. 20, 233–240 (2004).
Kulkarni, S. M. Density pattern of anophelines and their relation to malaria in Bastar district, Madhya Pradesh. Indian J Malariol. 27, 187–194 (1990).
Singh, N., Kataria, O. & Singh, M. P. The changing dynamics of Plasmodium vivax and P. falciparum in central India: trends over a 27-year period (1975–2002). Vector Borne Zoonotic Dis. 4, 239–248 (2004).
Nanda, N. et al. Prevalence and incrimination of Anopheles fluviatilis species S (Diptera: Culicidae) in a malaria endemic forest area of Chhattisgarh state, central India. Parasit Vectors. 5, 215, https://doi.org/10.1186/1756-3305-5-215 (2012).
Rodriguez, M. H. et al. Different prevalences of Plasmodium vivax phenotypes VK210 and VK247 associated with the distribution of Anopheles albimanus and Anopheles pseudopunctipennis in Mexico. Am. J. Trop. Med. Hyg. 62, 122–127 (2000).
Singh, R. et al. First report of detection and molecular confirmation of Plasmodium ovale from severe malaria cases in Central India. Trop Med Int Health. 18, 1416–1420 (2013).
Solberg, K. E. Health crises amid the moist insurgency in India. Lancet. 371, 1323–1324 (2008).
De Roode, J. C., Culleton, R., Bell, A. S. & Read, A. F. Competitive release of drug resistance following drug treatment of mixed Plasmodium chabaudi infections. Malar J. 3, 33, https://doi.org/10.1186/1475-2875-3-33 (2004).
Khan, S. Over 200 jawans battling malaria in Chhattisgarh’s Maoist zone. Mail Today. 13 December 2014 http://indiatoday.intoday.in/story/naxals-maoists-malaria-jawans-bastar-chhattisgarh-cpim/1/406652.html (2016).
Balagopal, K. Chhattisgarh: Physiognomy of Violence. Economic & Political Weekly. 3, 2183–2186 (2006).
Singh, J. & Bhattacharji, L. M. Rapid staining of malarial parasites by a water soluble stain. Indian Med Gaz. 79, 102–104 (1944).
NVBDCP. Diagnosis and treatment of malaria 2013. http://www.nvbdcp.gov.in/Doc/Diagnosis-Treatment-Malaria-2013.pdf (2016).
Snounou, G., Viriyakosol, S., Jarra, W., Thaithong, S. & Brown, K. N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 58, 283–292 (1993).
Lucchi, N. W. et al. A New Single-Step PCR Assay for the Detection of the Zoonotic Malaria Parasite Plasmodium knowlesi. PLoS ONE. 7, e31848, https://doi.org/10.1371/journal.pone.0031848 (2012).
Imwong, M. et al. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar J. 4, 20, https://doi.org/10.1186/1475-2875-4-20 (2005).
Acknowledgements
We are grateful to the study participants and guardians for their cooperation during patient enrollment. We are also thankful to our field staff for their hard work in a remote area. This study was funded by the Indian Council of Medical Research, New Delhi, India as part of the Tribal Health Research Unit.
Author information
Authors and Affiliations
Contributions
N.S. and P.K.B. conceived and designed the experiments; S.K., A.Y., S.B.1. and A.K.V. collected the data; P.B., P.L.M. and S.B.2. performed the clinical examinations and patient care management; S.K., A.Y., S.B.1., A.K.V. and P.K.B. performed the experiments and analyzed the sequencing data; S.K., S.B.1., A.Y., A.K.V., and A.A. analyzed the data; R.K.S. performed the statistical analysis; N.S., S.B.2., P.K.B. and R.K.S. wrote the paper; N.S., S.B.2., P.B., P.L.M., P.K.B. and R.K.S. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krishna, S., Yadav, A., Bhandari, S. et al. Prevalence of malaria in two highly endemic Community Health Centers in the Bastar district, Chhattisgarh showing mixed infections with Plasmodium species. Sci Rep 7, 16860 (2017). https://doi.org/10.1038/s41598-017-16974-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16974-2
- Springer Nature Limited
This article is cited by
-
Malaria misdiagnosis in the routine health system in Arba Minch area district in southwest Ethiopia: an implication for malaria control and elimination
Malaria Journal (2023)
-
Neglected malaria parasites in hard-to-reach areas of Odisha, India: implications in elimination programme
Malaria Journal (2021)
-
Comparison of Plasmodium ovale curtisi and Plasmodium ovale wallikeri infections by a meta-analysis approach
Scientific Reports (2021)
-
Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in four malaria endemic states of India
Malaria Journal (2021)
-
Prevalence and proportion of Plasmodium spp. triple mixed infections compared with double mixed infections: a systematic review and meta-analysis
Malaria Journal (2020)