Abstract
Objective
Gluten-free (GF) diet is the only reliable treatment for patients with celiac disease (CeD), but data on the extent of gluten contamination in GF food available in India is scanty. We evaluated gluten content in labeled, imported, and non-labeled GF food products currently available in the Indian market.
Methods
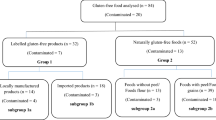
Overall, 794 processed and commercially available packaged GF products (labeled GF (n = 360), imported GF (n = 80), and non-labeled/naturally GF (n = 354)) were collected from supermarkets of National Capital Region of India. Those unavailable in stores were purchased from e-commerce sites or directly from the manufacturers. Gluten level in them was determined by Ridascreen Gliadin sandwich R5 enzyme-linked immunosorbent assay (R-Biopharm AG, Germany). As per Codex Alimentarius and Food Safety and Standard Authority of India, “gluten free” labeled products must not contain > 20 mg/kg of gluten.
Results
Overall, 10.1% of 794 GF products including 38 (10.8%) of 360 labeled and 42 (11.8%) of 354 non-labeled/naturally GF food products had gluten content > 20 mg/kg (range: 24.43–355 and 23.2–463.8 mg/kg, respectively). None of the imported GF products had gluten more than the recommended limits. Contaminated products most commonly belonged to cereal and their products (flours, coarse grains, pasta/macaroni, snack foods) pulse flours, spices, and bakery items.
Conclusions
A substantial proportion (10.1%) of GF food products (both labeled and non-labeled) available in India have gluten content greater than the prescribed limits of <20 mg/kg. Physicians, dietitians, support group, and patients with CeD should be made aware of this fact and regulatory bodies should ensure quality assurance.

Similar content being viewed by others
References
Lindfors K, Ciacci CK, Mearin ML, Lundin KEA, Makharia GK, Mearin ML, et al. Coeliac disease. Nat Rev Dis Prim. 2019;5:1–8.
Niewinski MM. Advances in celiac disease and gluten-free diet. J Am Diet Assoc. 2008;108:661–72.
Codex Alimentarius. CODEX STAN 118–2008: revised version standard for foods for special dietary use for persons intolerant to gluten. 2008. Available from: http://www.fao.org/fao-whocodexalimentarius/standards/en/. Accessed 10 Mar 2020.
Commission Regulation (EC) No.41/2009 concerning the composition and labelling of food stuffs suitable for people intolerant to gluten. Off J Eur Union L. 2009;1620:3–5.
US Government Publishing Office. 78FR47154—food labeling; gluten-free labeling of foods. 2013. Available from: https://www.gpo.gov/fdsys/granule/FR-2013-08-05/2013-18813. Accessed 5 Mar 2020.
Food Safety and Standards Authority of India. Food Safety and Standards (Food Product Standards and Food Additives) Second Amendment Regulation, 2016 and Food Safety and Standards (Packaging and Labelling) First Amendment Regulation, 2016 relating to standards for gluten free food and low gluten food and their labelling requirements. 2016. Available from: http://www.fssai.gov.in/home/fsslegislation/notifications/gazettenotification. Accessed 15 Mar 2020.
Lanzini A, Lanzarotto F, Villanacci V, Mora A, Bertolazzi S, Turini D, et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29:1299–308.
Catassi C, Fabiani E, Iacono G, D’Agate C, Francavilla R, Biagi F, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–66.
Rajpoot P, Makharia GK. Problems and challenges to adaptation of gluten free diet by Indian patients with celiac disease. Nutrients. 2013;5:4869–79.
Wu JH, Neal B, Trevena H, Crino M, Stuart-Smith W, Faulkner-Hogg K, et al. Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br J Nutr. 2015;114:448–54.
Singh J, Whelan K. Limited availability and higher cost of gluten-free foods. J Hum Nutr Diet. 2011;24:479–86.
Gorgitano MT, Sodano V. Gluten-free products: from dietary necessity to premium price extraction tool. Nutrients. 2019;11:1–12.
Bustamante MÁ, Fernández-Gil MP, Churruca I, Miranda J, Lasa A, Navarro V, et al. Evolution of gluten content in cereal-based gluten-free products: an overview from 1998 to 2016. Nutrients. 2017;9:1–12.
Lee HJ, Anderson Z, Ryu D. Gluten contamination in foods labeled as “gluten free” in the United States. J Food Prot. 2014;77:1830–33.
Farage P, Medeiros Nóbrega YKd, Pratesi R, Gandolfi L, Assunção P, Zandonadi RP. Gluten contamination in gluten-free bakery products: a risk for coeliac disease patients. Public Health Nutr. 2017;20:413–16.
Thompson T, Lee AR, Grace T. Gluten contamination of grains, seeds, and flours in the united states: a pilot study. J Am Dietetic Assoc. 2010;110:937–40.
Koerner TB, Cleroux C, Poirier C, Cantin I, La Vieille S, Hayward S, et al. Gluten contamination of naturally gluten-free flours and starches used by Canadians with celiac disease. Food Addit Contam A. 2013;30:2017–21.
Halmos EP, Clarke D, Pizzey C, Tye-Din JA. Gluten in “gluten-free” manufactured foods in Australia: a cross-sectional study. Med J Aust. 2018;209:448–49.
Verma AK, Gatti S, Galeazzi T, Monachesi C, Padella L, Baldo GD, et al. Gluten contamination in naturally or labeled gluten-free products marketed in Italy. Nutrients. 2017;9:1–10.
Raju N, Joshi AKR, Vahini R, Deepika T, Bhaskarachari K, Devindra S. Gluten contamination in labelled and naturally gluten-free grain products in southern India. Food Addit Contam A. 2020;37:531–38.
Mehtab W, Singh N, Malhotra A, Makharia GK. All that a physician should know about gluten-free diet. Indian J Gastroenterol. 2018;37:392–401.
Amit SK, Uddin MM, Rahman R, Rezwanul Islam SM, Khan MS. A review on mechanisms and commercial aspects of food preservation and processing. Agric Food Secur. 2017;6:1–22.
Koerner TB, Cleroux C, Poirier C, Cantin I, Alimkulov A, Elamparo H. Gluten contamination in the Canadian commercial oat supply. Food Addit Contam. 2011;28:705–10.
Hassan H, Elaridi J, Bassil M. Evaluation of gluten in gluten-free-labeled foods and assessment of exposure level to gluten among celiac patients in Lebanon. Int J Food Sci Nutr. 2017;68:881–86.
Dudeja P, Dudeja A, Singh G, Mukherji S. Regulatory framework for “gluten-free” foods in India: magic bullet for celiac disease patients. Med J DY Patil Vidyapeeth.2016;9:680–3.
Halmos EP, Di Bella CA, Webster R, Deng M, Tye-Din JA. Gluten in “gluten-free” food from food outlets in Melbourne: a cross-sectional study. Med J Aust. 2018;209:42–3.
Atasoy G, Gokhisar OK, Turhan M. Gluten contamination in manufactured gluten-free foods in Turkey. Food Addit Contam A. 2020;37:363–73.
Kaukinen K, Peräaho M, Lindfors K, Partanen J, Woolley N, Pikkarainen P, et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237–45.
Paarlahti P, Kurppa K, Ukkola A, Collin P, Huhtala H, Mäki M, et al. Predictors of persistent symptoms and reduced quality of life in treated coeliac disease patients: a large cross-sectional study. BMC Gastroenterol. 2013;13:1–8.
Lebwohl B, Murray JA, Rubio-Tapia A, Green PH, Ludvigsson JF. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment Pharmacol Ther. 2014;39:488–95.
Lebwohl B, Granath F, Ekbom A, Montgomery SM, Murray JA, Rubio-Tapia A, et al. Mucosal healing and mortality in coeliac disease. Aliment Pharm Ther. 2013;37:332–39.
Acknowledgements
We acknowledge Indian Council of Medical Research, Department of Health Research, Government of India for providing funding for this study. We also acknowledge R-Biopharm, Darmstadt, Germany for providing us the ELISA kits. WM acknowledges the University Grant Commission for providing her the Fellowship.
Author information
Authors and Affiliations
Contributions
WM: market survey and procurement of food products, laboratory work, data analysis, drafting of paper; VS: laboratory testing; AS: laboratory testing, critical review of the paper; SA: statistical analysis; NS: study supervision, critical review of the paper; RM: critical review of the paper; AM: critical review of the paper; VA: study supervision, critical review of the paper; GM: overall guarantor of the paper, designed the study concept, supervised the study, data analysis, and finalization of the paper. All authors revised and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of All India Institute of Medical Sciences, New Delhi (Ref. No. IEC-192/07.4.2017, RP-14/2017). The identity of the samples and their manufacturer had been blinded and were not disclosed on the public forum.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehtab, W., Sachdev, V., Singh, A. et al. Gluten content in labeled and unlabeled gluten-free food products used by patients with celiac disease. Eur J Clin Nutr 75, 1245–1253 (2021). https://doi.org/10.1038/s41430-020-00854-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-00854-6
- Springer Nature Limited
This article is cited by
-
Detection of gluten content in both naturally and labelled gluten-free products available in Morocco
Journal of Consumer Protection and Food Safety (2022)