Abstract
Dysregulation of RKIP and NRF2 has been widely involved in the therapy resistance of multiple malignances, however, their relation and the corresponding mechanisms, especially in radiation response, have not been elucidated. In this study, we revealed that RKIP could negatively regulate the expression of NRF2 in nasopharyngeal carcinoma (NPC) cells. Depletion or ectopic expression of NRF2 countered the pro- or anti- radioresistant effects of RKIP knockdown or overexpression on NPC cells, respectively, both in vitro and in vivo. Furthermore, our results indicated that NQO1 was positively regulated by NRF2 and served as the downstream effector of RKIP/NRF2 axis in regulation of NPC radioresistance. Mechanistically, miR-450b-5p, being positively regulated by RKIP in NPC cells, could sensitize NPC cells to irradiation by directly targeting and suppressing the level of NRF2. Besides, we analyzed the level of aforementioned molecules in NPC tissues. The results indicated that RKIP was significantly downregulated, NRF2 and NQO1 were notably upregulated in NPC tissues compared with in normal nasopharyngeal mucosa (NNM) tissues. Furthermore, RKIP and miR-450b-5p were remarkably lower, yet NRF2 and NQO1 were notably higher, in radioresistant NPC tissues relative to in radiosensitive NPC tissues. Consistent with the pattern in NPC cells, the RKIP/miR-450b-5p/NRF2/NQO1 axis was significantly correlated in NPC tissues. Downregulation of RKIP and miR-450b-5p, and upregulation of NRF2 and NQO1, positively correlated to malignant pathological parameters such as primary T stage, Lymph node (N) metastasis, and TNM stage. Finally, RKIP and miR-450b-5p served as favorable prognostic indicators, and NRF2 and NQO1 acted as unfavorable prognostic biomarkers in patients with NPC. Collectively, our outcomes reveal that RKIP downregulation promotes radioresistance of NPC by downregulating miR-450b-5p and subsequently upregulating and activating NRF2 and NQO1, highlighting RKIP/miR-450b-5p/NRF2/NQO1 axis as a potential therapeutic target for improving the radiosensitivity of NPC.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is one of most prevalent head and neck cancers in the middle and southeast coastal regions of China. Epstein–Barr (EB) virus infection, host genetics, and environment stresses account for the main etiologies of NPC. Surgical resection is not recommended for NPC treatment for its unreachable anatomical location. Nevertheless, NPC is highly sensitive to ionizing radiation, and radiation therapy is the preferred treatment modality for non-metastatic NPC patients1,2. Although most NPC patients have benefited a lot from radiotherapy, a considerable proportion of NPC patients develop radioresistance either at beginning or after a couple of terms of radiation1,3. Therefore, radioresistance stands for the main cause for the failure of NPC treatment and exploring the molecular mechanisms of radioresistance may present candidate targets for radiation sensitization and eventually improve therapeutic efficacy of NPC.
Overwhelming reactive oxidative stress (ROS), induced by irradiation, is one of big challenges for survival of cancer cells4. Therefore, upregulation of genes/proteins with ROS removal capability seem to be the obliged choice for cancer cells to maintain survival. NRF2 (Nuclear factor erythroid 2-related factor 2), one of basic leucine zipper transcription factors, which serves as an essential activator of genes encoding antioxidant enzymes and phases II detoxifying enzymes such as HO-1 (Heme oxygenase-1), NQO1 (NAD(P)H dehydrogenase, quinone 1), PRDX1(Peroxiredoxin1), and GCLC (glutamate-cystine ligase catalytic subunit)5, is upregulated or activated in various radioresistant cancer cells, including lung cancer6,7, alveolar rhabdomyosarcoma8, hepatoma9, and breast cancer10. The activity of NRF2 is regulated by both KEAP1 (Kelch-like ECH-associated protein1) dependent and independent mechanisms. Under basal conditions, KEAP1 binds and sequesters NRF2 in cytoplasm for proteasome degradation, whereas NRF2 can evade the repression of KEAP1 and translocate into nucleus to initiate the transcription of target genes11. Apart from KEAP1, NRF2 can be regulated by other factors12,13, such as WDR23-DDB1-CUL4 regulatory axis14 and miRNAs related mechanisms15,16. Recent study has shown that NRF2 is the main target of radiosensitive agent salinomycin in NPC cells17, however, the expression pattern of NRF2, and its activation mechanism in NPC remains unknown.
Raf kinase inhibitor protein (RKIP) is a well-known suppressor in carcinogenesis and malignant progression18. Downregulation of RKIP has been observed in various cancers such as prostate cancer19, breast cancer20, lung cancer21, gastric cancer22, esophageal squamous cell carcinoma23, and colorectal cancer24. Reduction of RKIP is almost involved in promotion of each malignant characteristics, especially for metastasis and therapy resistance, by activating oncogenic regulators or signaling axes like NF-κB25, YY126, STAT327, MAPK28, and AKT29. Moreover, the recent study has indicated that hyper-activation of NRF2 accounts for the chemotherapeutic resistance caused by RKIP downregulation in colorectal cancer cells30. Accordingly, our previous studies also demonstrated that RKIP is downregulated in NPC and RKIP reduction promotes invasion, metastasis, and radioresistance of NPC29,31,32. These results present the possibility that NRF2 may be involved in RKIP-regulating radioresistance of NPC.
Therefore, to reveal the relation between RKIP and NRF2 in NPC radioresistance, in this study, we explored the effects of RKIP alteration on NRF2, detected the role of NRF2 in RKIP-regulating radioresistance, and investigated the regulatory mechanisms of NRF2 by RKIP in NPC. Our results indicated that NRF2 is downregulated and negatively regulated by RKIP in NPC. NQO1 is the main effector of NRF2 and NRF2/NQO1 axis mediates the functions of RKIP-regulating radioresistance in NPC. Mechanistically, RKIP could upregulate miR-450b-5p, which is downregulated and can directly target NRF2 in NPC, subsequently suppress the activity of NRF2/NQO1 axis, and eventually improve the radiosensitivity of NPC cells. Taken together, our study presents that RKIP/miR-450b-5p/NRF2/NQO1 axis play vital roles in radioresistance of NPC and severs as a promising targets for improving treatment of radioresistant NPC.
Materials and methods
Cell lines
Radioresistant human NPC cell line CNE2-IR and radiosensitive cell line CNE2 cells were previously established by us29,32,33, and cultured with RPMI-1640 medium containing 10% fetal bovine serum (FBS) (BI, Jerusalem, Israel). Radioresistant CNE2-IR cells were derived from parental CNE2 cells by treating the cells with four rounds of sublethal ionizing radiation33. Radiosensitive CNE2, used as a control, were treated with the same procedure except sham irradiated. Moreover, stable cell lines, including CNE2 shNC, CNE2 shRKIP, CNE2-IR vector, and CNE2-IR RKIP cells, were successfully established by us in previous studies29,32, which were also maintained by RPMI-1640 medium plus 10% FBS.
Patients and tissues samples
Ninety-seven NPC patients without distant metastasis (M0 stage) at the time of diagnosis who were treated by radical radiotherapy alone in the Third Xiangya Hospital of Central South University between February 2013 and December 2018 were enrolled in this study. The radiotherapy was administered for a total dose of 60–70 Gy (2 Gy/fraction, 5 days a week). The neck received 60 Gy for patients without lymph node metastasis and 70 Gy for patients with lymph node metastasis. NPC tissue biopsies were obtained at the time of diagnosis before any therapy, fixed in 4% formalin and embedded in paraffin. Thirty cases of formalin-fixed and paraffin-embedded normal nasopharyngeal mucosa (NNM) tissue specimens were also obtained in the same period. All tumors were histopathologically diagnosed as poorly differentiated squamous cell carcinomas (WHO type III) according to the 1978 WHO classification34. The clinical stage of the patients was classified according to the 2008 NPC staging system of China35.
The radiotherapy response was evaluated clinically for primary lesions based on nasopharyngeal fiberscope and magnetic resonance imaging 1 month after the initiation of radiotherapy according to the following criteria. Radioresistant NPC patients were defined as ones with persistent disease (incomplete regression of primary tumor and/or neck lymphonodes) at >3 months or with local recurrent disease at the nasopharynx and/or neck lymphonodes at ≤12 months after completion of radiotherapy. Radiosensitive NPC patients were defined as ones without the local residual lesions (complete regression) at >3 months and without local recurrent disease at >12 months after completion of radiotherapy29. On the basis of the above criteria, 97 NPC patients comprised 46 radioresistant (incomplete regression (n = 22) and local recurrence (n = 24)), and 51 radiosensitive ones.
The patients were followed up strictly in outpatient clinics: every 3 month for the first year and then every 6 months for the next 2 years, and finally annually. The patients were followed up for a maximum period of 89 months (median time: 68.08 months). Overall survival (OS) was defined as the interval from the initiation of primary radiotherapy to the date of cancer-related death or when censured at the latest date if patients were still alive.
RNA isolation and qPCR detection
The RNA extraction and qPCR assay were performed as described previously by us29,32. Simply, total RNA was extracted from paraffin-embedded tissues and NPC cells using RecoverAll Total Nucleic Acid Isolation Kit (Thermo Fisher, CA, USA) and Trizol reagent (Thermo Fisher, CA, USA), respectively, according to the instructions. Bulge-Loop miRNA qRT-PCR Starter Kit (RiBoBio, Guangzhou, China) was applied to reversely transcribe mRNAs into cDNA and detect the relative expression of miR-450b-5p using commercial primers from RiBoBio Inc (Guangzhou, China). FastKing gDNA Dispelling RT SuperMix Kit (TIANGEN, Beijing, China) was used to transcribe mRNAs into cDNA for qPCR detection of NRF2 mRNA and hnRNA (heterogeneous nuclear RNA) via RealUniversal Color PreMix (SYBR Green) (TIANGEN, Beijing, China), according to the manufacturer’s instructions. The relative quantification of products was analyzed using 2−ΔΔCt method with 5 s and GAPDH as internal controls. The primer sequences were summarized in the Supplementary Table S1. All assays were performed three times in triplicate.
Stable cell lines establishment
Lentivirus particles for expression of NRF2, and short hair RNA (shRNA) of NRF2, which simultaneously express the anti-hygromycin gene, were purchased from Genechem Inc. (Shanghai, China). CNE2 shRKIP and CNE2-IR RKIP cells were infected with shNRF2 and NRF2 lentivirus particles, respectively, and cultured with medium containing hygromycin for 1 week. The survival cells were recognized as CNE2 shRKIP+shNRF2 and CNE2-IR RKIP+NRF2 cell lines whose NRF2 expression level was validated by western blot.
Plasmids, miRNAs, and transient transfection
The expression plasmids, pENTER-NRF2, pENTER-NQO1, and the pENTER-vector, were obtained from Vigenebio Inc. (MD, USA). The siRNAs (small interfering RNAs), siNQO1, siNRF2, and siNC (negative control), and miRNAs, miR-450b-5p mimic, miR-450b-5p inhibitor, and their respective negative controls, were all purchased from Ribobio Inc. (Guangzhou, China). The plasmids, miRNAs and siRNAs, were transfected alone or co-transfected into cells as mentioned in the result part using Lipofectamine 2000 (Thermo Fisher, CA, USA) according to the manufacturer’s instructions. The sequences of siRNAs and miRNAs were listed in the Supplementary Table S2.
Cell viability assay
The CCK-8 assay was adopted to analyze cell viability under 6 Gy irradiation according to our previous description29,32. The experiments were independently performed for three times in triplicate.
Plate clone survival assay
Plate clone survival assay was applied to analyzed cell survival under 6 Gy irradiation as previously described by us32. The experiments were independently repeated in triplicate.
EdU incorporation assay
5-Ethynyl-2’-deoxyuridine (EdU) incorporation assay was carried out to analyze cell proliferation under 6 Gy irradiation as our previous description36. The assay was performed three times in triplicate.
Western blot
The total proteins extraction and western blot were performed as our previous description29,32. In brief, the cells were disrupted by radioimmunoprecipitation assay lysis buffer and the total proteins were collected by centrifugation. The denatured proteins (30 μg per lane) were separated by sodium dodecyl sulfate poly-acrylamide gel electrophoresis and subsequently transmitted into 0.22 μm polyvinylidene difluoride membrane. After blocked with 5% nonfat milk at room temperature for 1 h, the membrane was incubated with rabbit anti-RKIP antibody (D221145, dilution 1:500, BBI, Shanghai, China), rabbit anti-NRF2 antibody (16396-1-AP, dilution 1:50, Proteintech, IL, USA), rabbit anti-NQO1 antibody (D261049, dilution 1:500, BBI, Shanghai, China), rabbit anti-HO-1, rabbit anti-GCLC (D123963, dilution 1:300, BBI, Shanghai, China), and mouse anti-GAPDH antibody (AC002, dilution 1:1000, Abclonal, Wuhan, China), respectively, overnight at 4 °C. Next, after incubated with anti-rabbit or anti-mouse IgG HRP-conjugated secondary antibodies (D110058 or D110098, dilution 1:3000, BBI, Shanghai, China), the protein amounts were visualized by chemiluminescent HRP substrate (EpiZyme, Shanghai, China).
Dual luciferase reporter assay
Dual luciferase reporter assay was performed as previous description by us32,37. In brief, a dual luciferase reporter plasmid expressing NRF2 with wild 3′-UTR (untranslated region) or NRF2 with mutant 3′-UTR was co-transfected with miR-450b-5p mimic or mimic control into CNE2-IR cells using Lipofectamine 2000 (Thermo Fisher, CA, USA), respectively. Forty-eight hours later, the cells were harvested and both firefly luciferase and renilla luciferase activities were measured using the dual luciferase reporter assay system (Promega, WI, USA) according to the manufacturer’s instructions, and luciferase activity was estimated using a luminometer (Promega, WI, USA).
In vivo tumor radioresponse assay
In vivo tumor radioresponse assay was performed according to our previous study29,37. Simply, nude female mice (4 weeks old) were raised under specific pathogen-free circumstances. NPC cells were subcutaneously injected into the right flanks of mice at 2 × 106 cells/mouse (n = 5 each group). Tumor volume (in mm3) was measured by caliper measurements performed every 4 days and calculated by using the modified ellipse formula (volume = length × width2/2). Seven days after cell implantation, when the volumes of xenograft tumors reached ~50 mm3, the mice were irradiated at the dose of 8 Gy. Twenty days later, the mice were killed by cervical dislocation, and the xenograft tumors were excised, weighted, and cut in half, with one half fixed and embedded in paraffin for immunohistochemical staining, and the remaining half flash-frozen in liquid nitrogen for reserve.
Immunohistochemistry
Immunohistochemical staining of RKIP, NRF2, NQO1, γH2AX (phospho-S139), a marker for DNA double-strand breaks38, and Ki-67, a marker for cell proliferation, were performed on the formalin-fixed and paraffin-embedded tissue sections according to our previous description29. Simply, after antigen retrieval, tissue sections were incubated with rabbit anti-RKIP antibody (D221145, dilution 1:50, BBI, Shanghai, China), rabbit anti-NRF2 antibody (16396-1-AP, dilution 1:50, Proteintech, IL, USA), rabbit anti-NQO1 antibody (D261049, dilution 1:50, BBI, Shanghai, China), anti-γH2AX (ab2893, dilution 1:50, Abcam, MA, USA) or anti-Ki-67 antibody (ab15580, dilution 1:100, Abcam, MA, USA) overnight at 4 °C, and then were incubated with biotinylated secondary antibody followed by avidin-biotin peroxidase complex (DAKO, Glostrup, Denmark). Finally, tissue sections were incubated with 3′, 3′-diaminobenzidine (Sigma) and counterstained with hematoxylin. Primary antibodies were omitted in negative controls,.
Immunohistochemical staining was evaluated and scored by two independent pathologists base on the staining intensity and area. Discrepancies were resolved by consensus. Staining intensity was categorized: absent staining as 0, weak as 1, moderate as 2, and strong as 3. The percentage of stained cells was categorized as no staining = 0, <30% of stained cells = 1, 30~60% = 2, and >60% = 3. The staining score (ranging from 0 to 6) for each tissue was calculated by adding the area score and the intensity score. A combined staining score of ≤3 was considered to be low expression; and a score of >3 was considered to be high expression. Quantitative evaluation of DNA damaged or proliferation cells was done by examining the sections in 10 random microscopic fields and counting the number of γH2AX or Ki-67-positive nuclei among 1000 carcinoma cells under the microscope. The rate of DNA damaged or proliferation cells was expressed as positive cells per 100 cancer cells.
In situ detection of apoptotic cells
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) was carried out to explore apoptotic cells of formalin-fixed and paraffin-embedded tissue sections of xenografts after irradiation with In Situ Cell Death Detection Kit (Roche, Basel, Switzerland) according to the instruction and our previous description29. Quantitation of apoptotic cells was evaluated by examining the sections in 10 random microscopic fields and counting the number of TUNEL-positive cancer cells among 1000 cells under the microscope. The apoptotic rate was exhibited as positive cells per 100 cancer cells.
Statistical analysis
All experiments were carried out at least three times. Data were presented as the mean±standard deviation (SD). Statistical analysis and charts were processed by IBM SPSS Statistics version 20.0 (IBM, NY, USA) and Prism 8 (GraphPad, CA, USA). The Student t test or Fisher’s exact test were applied to compare statistical significance between two groups. Survival curves were drawn by using Kaplan–Meier method, and comparisons were analyzed by Log-rank test. Univariate and multivariate survival analyses were conducted on all parameters using Cox proportional hazards regression model. The Spearman rank correlation coefficient was used to determine the correlation between two parameters. P values < 0.05 were considered to be statistically significant.
Ethics statement
This study was approved by the Ethics Committee of the Third Xiangya Hospital of Central South University, Hunan, China. Written informed consent was obtained from all participants in the study. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals of Central South University, with the approval of the Scientific Investigation Board of Central South University.
Results
RKIP could regulate NRF2 expression in dependent with Keap1 in NPC
NRF2 has been reported to mediate the pro-chemosensitivity function of RKIP in colorectal cancer30. Therefore, we explored whether NRF2 involved in the regulation of RKIP-associated radioresistance in NPC cells. Western blot and qPCR assays were performed to analyze the expression of NRF2 in NPC cells with RKIP alteration. As Fig. 1a shown, RKIP knockdown increased the protein level of NRF2 in radiosensitive CNE2 cells, whereas, RKIP overexpression suppressed the protein level of NRF2 in radioresistant CNE2-IR cells. Surprisingly, RKIP alteration did not change the level of Keap1, suggesting the regulation NRF2 by RKIP is independent with Keap1 (Fig. 1a). Accordingly, the qPCR results demonstrated that RKIP could negatively regulate the mRNA level of NRF2 as well (Fig. 1b). These results indicated that RKIP could inversely regulate NRF2 level in NPC cells with district radiosensitivity in an independent manner with Keap1, indicating NRF2 may play an important role in RKIP regulated radioresistance in NPC.
a Western blot indicating the levels of NRF2, Keap1, and RKIP in CNE2 shRKIP, CNE2-IR RKIP, and their control cells. b qPCR assay showing the expression of NRF2 in CNE2 shRKIP, CNE2-IR RKIP, and their control cells. c Western blot showing the level of NRF2 in CNE2 shRKIP, CNE2 shRKIP+shNRF2, CNE2-IR RKIP, CNE2-IR RKIP+NRF2, and their control cells. d–e under 6 Gy irradiation, NRF2 silence antagonizes the pro-radioresistant effects of RKIP knockdown in CNE2 cells and NRF2 overexpression counters the anti-radioresistant effects of RKIP overexpression in CNE2-IR cells demonstrating by CCK-8 d, clone survival e, and EdU incorporation f assays. *P < 0.05; **P < 0.01; ***P < 0.001.
RKIP promotes radiosensitivity of NPC cells by suppressing NRF2 both in vitro and in vivo
To explore the role of NRF2 in RKIP-associated radioresistance regulation in NPC, we successfully knocked down the expression of NRF2 in CNE2 shRKIP cells and overexpressed NRF2 in CNE2-IR RKIP cells by infected cells with respective lentivirus (Fig. 1c). Subsequently, CCK-8, plate clone survival, and EdU incorporation assays were carried out to detect the radiosensitivity of cells with radiation treatment at 6 Gy. Consistent with our previous study29, RKIP depletion significantly enhanced the tolerance of CNE2 cells to radiation exposure. However, the pro-radioresistant effects of RKIP knockdown were nearly abolished in CNE2 cells, when NRF2 was simultaneously repressed, reflected by suppressed cell viability (Fig. 1d, left arm), fewer survival clones (Fig. 1e, left arm), and fewer EdU-labeled cells (Fig. 1f, left arm). Accordingly, ectopic expression of NRF2 could rescue the resistance of CNE2-IR RKIP cells to radiation, indicated by improved cell viability (Fig. 1d, right arm), more survival clones (Fig. 1e, right arm), and more EdU-labeled cells (Fig. 2f, right arm). Thus, these results demonstrated that RKIP knockdown could promote radioresistance of NPC cells via upregulation of NRF2 in vitro.
CNE2 shRKIP, CNE2 shRKIP+shNRF2, CNE2-IR RKIP, CNE2-IR RKIP+NRF2, and their control cells were subcutaneously implanted into nude mice n = 5 each group for xenografts formation, which were irradiated by 8 Gy at 7 day after cell implantation and killed at 20 day after cell implantation. a The photography of mice bearing xenografts and resected xenografts. b The growth curves of xenografts generated by aforementioned cells. c The average weight of xenografts generated by aforementioned cells. d The representative images of TUNEL detection of apoptotic cells in the tumors generated by aforementioned cells (upper). The representative image of immunohistochemical staining of γH2AX in the tumors generated by aforementioned cells (middle). The representative image of immunohistochemical staining of Ki-67 in the tumors generated by aforementioned cells (lower). *P < 0.01, ***P < 0.001. Scale bar, 50 μm.
Moreover, the role of NRF2 in RKIP-related regulation of radioresistance was further validated in vivo via nude mice xenograft model. The aforementioned NPC cells were subcutaneously injected into right flanks of nude mice for xenografts formation, respectively. When the volumes of xenografts came into ~50 mm3, the nude mice were subjected to irradiation treatment (total 8 Gy) and subsequently the respective effects were observed in the following days. In line with the outcomes of in vitro, the tumor volume and weight assays turned out that NRF2 knockdown could deplete the pro-radioresistant effects of RKIP reduction in NPC (Fig. 2a–c, left arms), whereas ectopic expression of NRF2 could antagonize the radiosensitive effects of RKIP overexpression in NPC (Fig. 2a–c, right arms), and these notions were further confirmed by results of TUNEL, γH2AX, and Ki-67 staining assays (Fig. 2d). Taken together, our results demonstrated that RKIP could promote radiosensitivity of NPC cells by suppressing NRF2 both in vitro and in vivo.
NQO1 is the downstream effector of RKIP/NRF2 axis in regulation of NPC radioresistance
As a transcription factor, the functions of NRF2 depend on transcriptional regulation of downstream targets. To address the effector of RKIP/NRF2 axis in NPC, we first analyzed the expressions of the known targets, such as HO-1, NQO1, and GCLC5, of NRF2 in NPC cells with RKIP alteration by qPCR. The results indicated that NQO1 was significantly upregulated in CNE2 shRKIP cells, whereas was notably downregulated in CNE2-IR RKIP cells (Fig. 3a). Meanwhile, the expression of HO-1 and GCLC was slightly influenced in NPC cells by RKIP (Fig. 3a). Accordingly, the proteins level of NQO1, HO-1, and GCLC were identical with the patterns of their mRNAs level (Fig. 3b), indicating NQO1 may be the target of NRF2 in NPC. Indeed, NRF2 knockdown re-decreased NQO1 in CNE2 shRKIP cells and NRF2 overexpression rescued NQO1 in CNE2-IR RKIP (Fig. 3c). Therefore, these results revealed that NQO1 was regulated by NRF2 in NPC.
a qPCR assay showing the expression of HO-1, NQO1, and GCLC in CNE2 shRKIP, CNE2-IR RKIP, and their control cells. b Western blot indicating the levels of HO-1, NQO1, and GCLC in CNE2 shRKIP, CNE2-IR RKIP, and their control cells. c Western blot showing the level of NQO1 in CNE2 shRKIP, CNE2 shRKIP+shNRF2, CNE2-IR RKIP, CNE2-IR RKIP+NRF2, and their control cells. d Western blot showing the level of NQO1 in CNE2 shRKIP, CNE2 shRKIP+shNRF2 transfected with vector plasmids, CNE2 shRKIP+shNRF2 transfected with NQO1 plasmids, CNE2-IR RKIP, CNE2-IR RKIP+NRF2 transfected with siNC, and CNE2-IR RKIP+NRF2 transfected with siNQO1 cells. e–g under 6 Gy irradiation, ectopic expression of NQO1 antagonizes the antiresistant effects of NRF2 knockdown in CNE2 shRKIP cells and NQO1 knockdown counters the pro-resistant effects of NRF2 overexpression in CNE2-IR RKIP cells, demonstrating by CCK-8 e, clone survival f, and EdU incorporation g assays. *P < 0.05; **P < 0.01; ***P < 0.001.
Furthermore, we further explored the roles of NQO1 in RKIP/NRF2-related radiation regulation. First, forced expression of NQO1 successfully rescued the level of NQO1 in CNE2 shRKIP cells with NRF2 knockdown (Fig. 3d, left arm), whereas, NQO1 silence notably re-decreased NQO1 in CNE2-IR RKIP cells with NRF2 overexpression (Fig. 3d, right arm). Subsequently, CCK-8, plate clone survival, and EdU incorporation assays indicated that, under 6 Gy irradiation, ectopic expression of NQO1 could recover the radiation tolerance of CNE2 shRKIP cells with NRF2 knockdown (Fig. 3f–g, left arm); whereas NQO1 depletion could re-sensitize the CNE2-IR RKIP cells with NRF2 overexpression to irradiation (Fig. 5f–g, right arm). Besides, to validate the roles of NRF2 and NQO1 in radioresistance regulation of NPC, we overexpressed NRF2 and NQO1 in CNE2 cells and interfered the expression of NRF2 and NQO1 in CNE2-IR, respectively. The pro-radioresistant effects of NRF2/NQO1 axis own was confirmed (Supplementary Fig. 1). Thus, these results proved that NQO1 could mediate the function of RKIP/NRF2 in regulation of NPC radioresistance.
RKIP suppresses the expression of NRF2 by upregulating miR-450b-5p
Next, we further explored the mechanism accounting for the regulation of NRF2 by RKIP in NPC. As the Fig. 1b demonstrated, RKIP could suppress NRF2 by decreasing its mRNA level. Actually, both transcriptional and post-transcriptional mechanisms may cause alteration of mRNA, which could be distinguished by detection of primary transcription activity32. Thus, to address the detail mechanism, we accessed the level of NRF2 hnRNA in NPC cells with RKIP alteration. The qPCR results showed that RKIP alteration did not significantly change the level of NRF2 hnRNA (Fig. 4a), suggesting post-transcriptional mechanisms must explain the regulation of NRF2 by RKIP.
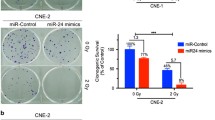
a qPCR showing the level of NRF2 hnRNA in CNE2 shRKIP, CNE2-IR RKIP, and their control cells. b qPCR showing the level of miR-450b-5p in CNE2 shRKIP, CNE2-IR RKIP, and their control cells. c qPCR and d western blot showing the level of NRF2 in CNE2 cells, respectively, transfected with miR-450b-5p inhibitor and inhibitor NC, and CNE2-IR cells, respectively, transfected with miR-450b-5p mimic and mimic NC. e 3’UTR dual luciferase reporter assay. (top) The predicted miR-450b-5p binding sites in the 3’UTR of wild NRF2 and mutant NRF2 3′-UTR are shown; (bottom) luciferase activity of WT and mutant NRF2 3’UTR dual luciferase reporter vector in the HEK293 cells transfected with miR-450b-5p or mimic NC. f–h miR-450b-5p inhibitors enhances the tolerance of CNE2 cells to irradiation (6 Gy), and miR-450b-5p mimics sensitizes the CNE2-IR cells to irradiation (6 Gy), demonstrating by CCK-8 f, clone survival g, and EdU incorporation h assays. *P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance.
Previous studies have demonstrated that RKIP can exert its functions by regulating miRNAs20, which are the most common post-transcriptional regulators. Therefore, the candidate miRNAs of NRF2 and their correlations to NRF2 in cancers were predicted and analyzed by Starbase 2.0, miR-450b-5p was selected for experimental validation owing to its tumor-suppressive function and significantly negative association with NRF2 in head and neck squamous cell carcinoma (data was not shown). Indeed, the qPCR results indicated that the expression of miR-450b-5p was decreased by RKIP depletion in CNE2 cells (Fig. 4b, left arm), whereas, increased by RKIP overexpression in NPC cells (Fig. 4b, right arm), suggesting that RKIP could positively regulate miR-450b-5p in NPC. Subsequently, the qPCR and western blot results indicated that miR-450b-5p inhibitor notably enhanced the level of NRF2 mRNA and protein in CNE2 cells (Fig. 4c, d, left arms), whereas, miR-450b-5p mimic remarkably decreased the level of NRF2 mRNA and protein in CNE2-IR cells (Fig. 4c, d, right arms). Furthermore, the dual luciferase reporter assay presented that the inhibitory effects of miR-450b-5p on NRF2 were abolished when the predicted bind site of NRF2 at 3′-UTR was mutated (Fig. 4e). Thus, these results confirmed that miR-450b-5p, being positively regulated by RKIP, could directly target and inhibit NRF2 in NPC.
miR-450b-5p sensitizes NPC cells to irradiation and mediates the radiosensitive functions of RKIP by targeting NRF2 in NPC
We further evaluated the functions of miR-450b-5p itself and its roles in RKIP-regulated radioresistance in NPC. CCK-8, plate clone survival, and EdU incorporation assays were carried out to detect the cell viability, survival, and proliferation of NPC cells irradiated at 6 Gy. The results indicated that miR-450b-5p inhibitor significantly reinforced the radiant tolerance of CNE2 cells reflected by improved cell viability (Fig. 4f, left arm), more survival clones (Fig. 4g, left arm), and more EdU-labeled cells (Fig. 4h, left arm), whereas miR-450b-5p mimic notably sensitized the CNE2-IR cells to irradiation demonstrated by impaired cell viability (Fig. 4f, right arm), fewer survival clones (Fig. 4g, right arm), and fewer EdU-labeled cells (Fig. 4h, right arm). These outcomes suggested that miR-450b-5p could promote radiosensitivity in NPC.
Besides, we further assessed the roles of whether miR-450b-5p/NRF2 mediated the radiosensitive roles of RKIP in NPC. The western blot indicated that ectopic expression of NRF2 significantly antagonized the inhibitory effects of miR-450b-5p on NRF2 in CNE2 shRKIP cells (Fig. 5a, left arm). Accordingly, NRF2 knockdown notably counteracted the promotive effects of miR-450b-5p inhibitor on NRF2 in CNE2-IR RKIP (Fig. 5a, right arm). Consistently, the results of CCK-8, plate clone survival, and EdU incorporation assays manifested that, under 6 Gy irradiation, ectopic expression of NRF2 remarkably released the radiosensitive functions of miR-450b-5p in CNE2 shRKIP cells (Fig. 5b–d, left arm), whereas, NRF2 depletion significantly eradicated the radioresistant roles of miR-450b-5p inhibitor in CNE-IR RKIP cells (Fig. 5b–d, right arm). Thus, these results suggested miR-450b-5p could mediate the radiosensitive roles of RKIP by targeting NRF2 in NPC.
a Western blot showing the level of NQO1 and NQO1 in CNE2 shRKIP, CNE2 shRKIP co-transfected with miR-450b-5p and vector plasmids, CNE2 shRKIP co-transfected with miR-450b-5p and NRF2 plasmids, CNE2-IR RKIP, CNE2-IR RKIP co-transfected with miR-450b-5p inhibitor and siNC, and CNE2-IR RKIP co-transfected with miR-450b-5p inhibitor and siNRF2. b–d under 6 Gy irradiation, ectopic expression of NRF2 antagonized the anti-radioresistant effects of miR-450b-5p in CNE2 shRKIP cells and siNRF2 addition counters the pro-radioresistant effects of miR-450b-5p inhibitors in CNE2-IR RKIP cells, demonstrating by CCK-8 b, clone survival c, and EdU incorporation d assays. *P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance.
RKIP/miR-450b-5p/NRF2/NQO1 axis is observed in NPC specimens and exhibits significant correlations with NPC progression and prognosis
Finally, we stepped forward to validate whether the RKIP/miR-450b-5p/NRF2/NQO1 axis existed in NPC specimens. At beginning, we detected the level of RKIP, NRF2, and NQO1 in 97 cases of NPC samples, including 46 radioresistant and 51 radiosensitive ones, and 30 cases of NNM samples by immunohistochemistry. Consistent with our previous report, RKIP is significantly downregulated in NPC, particularly in radioresistant NPC (Fig. 6a, Table 1), whereas, the level of NRF2 and NQO1 is remarkably upregulated in NPC specimens (Fig. 6a, Table 1), especially in radioresistant ones (Fig. 6a, Table 1). Subsequently, we detected and compared the relative level of miR-450b-5p between 46 cases of radioresistant and 51 cases of radiosensitive NPC samples by qPCR. Accordingly, miR-450b-5p was notably downregulated in radioresistant NPC specimens (Fig. 6b). Furthermore, the levels of RKIP, miR-450b-5p, NRF2, and NQO1 were notably associated with clinicopathological characteristics such as primary T stage (except for miR-450b-5p and NQO1), lymph node metastasis, and TNM stage, of NPC patients (Table 2), respectively. Next, we further analyzed the prognostic values of RKIP, miR-450b-5p, NRF2, and NQO1 in NPC. Survival analyses demonstrated that low level of RKIP and miR-450b-5p, and high level of NRF2 and NQO1 remarkably correlated with reduced OS of the patients with NPC (Fig. 6c). Moreover, the univariate Cox proportional hazards regression analysis revealed that primary T stage, TNM stage, and the levels of RKIP, miR-450b-5p, NRF2, and NQO1 were significantly related to the OS of patients with NPC (Table 3). The multivariate Cox proportional hazards regression analysis indicated that low expressions of RKIP and miR-450b-5p, and high expressions of NRF2 and NQO1 severed as an independent predictor for the reduced OS of NPC patients (Table 3), respectively. Furthermore, we investigated the relations of expressions among RKIP, miR-450b-5p, NRF2 and NQO1 in NPC patients by spearman correlation analysis. The level of RKIP was positively correlated to that of miR-450b-5p (Fig. 6d, left arm), whereas negatively associated with that of NRF2 (Fig. 6e, left arm) and NQO1 (Fig. 6e, right). Similarly, the level of miR-450b-5p negatively correlated to that of NRF2 in patients with NPC (Fig. 6d, right arm). At last, the level of NRF2 was positively associated with that of NQO1 in NPC patients (Fig. 6f). Thus, these results demonstrated that RKIP/miR-450b-5p/NRF2/NQO1 axis also exists in NPC specimens and exhibits significant correlation with NPC progression and prognosis.
a IHC showing the levels of RKIP, NRF2, and NQO1 in the normal nasopharyngeal mucosa (NNM), radiosensitive NPC, and radioresistant NPC tissues, respectively. b qPCR showing the relative level of miR-450b-5p in radiosensitive NPC and radioresistant NPC tissues. c Kaplan–Meier survival analysis of RKIP, NRF2, NQO1, and miR-450b-5p in 97 NPC patients according to their expression levels. NPC patients with high RKIP and miR-450b-5p expression, and low NRF2 and NQO1 expression, show more favorable overall survival outcome. d The correlation of miR-450b-5p to RKIP and NRF2. MiR-450b-5p positively correlates to RKIP (left) and inversely correlates to NRF2 (right) in patients with NPC. e the correlations among RKIP, NRF2, and NQO1. RKIP shows negative association with NRF2 (left) and NQO1 (right) in patients with NPC. f NRF2 positively correlates to NQO1 in patients with NPC. ***P < 0.001; Scale bar, 50 μm.
Discussion
Downregulation of RKIP and its pro-radiosensitive role has been announced in lung cancer39 and prostate cancer40. We also previously demonstrated that loss of RKIP confers resistance of NPC cells to irradiation29,32. In this study, we further revealed that RKIP could negatively regulated NRF2/NQO1 activity and consequently enhanced the radiosensitivity of NPC cells. Moreover, miR-450b-5p, which is downregulated in radioresistant NPC and positively regulated by RKIP, could sensitize NPC cells to irradiation by directly targeting and suppressing NRF2. Taken together, we originally reveal that RKIP can sensitize NPC cells to radiation by inhibiting NRF2/NQO1 by raising miR-450b-5p.
The vital roles of NRF2 in radiotherapy resistance have been well announced in a plethora of studies5. For example, downregulation of NRF2 is the main reason for the radiosensitive agents, such as cordycepin41, valproic acid42, and brusatol43. The radiosensitive ability of salinomycin also depends on inhibition of NRF2 in NPC cells17, suggesting NRF2 involves in regulating of NPC radioresistance as well. Moreover, RKIP knockdown is capable to activate NRF2 and bestows resistance to chemotherapeutic drugs implying the potential relation between RKIP and NRF2 in regulation of NPC radioresistance30. Consistently, our results show that RKIP can inversely regulate the level of NRF2 in NPC, which accounts for the underlying mechanism of regulatory roles of RKIP in radiation.
Multiple factors are involved in the management of NRF2 activity. Keap1 is the most common negative regulator of NRF2. Serving as an adapter, Keap1 can bind NRF2 and recruit Cullin-3, one of E3 ubiquitin ligase, to initiate proteasomal degradation of NRF2 under basal condition11. Here, RKIP alteration causes notable fluctuation of NRF2 mRNA, but exerts no significant influence on Keap1, suggesting the regulation of NRF2 by RKIP is independent with Keap1-related protein stability mechanism in NPC. miRNAs are one of the regulators for controlling the level and activity of NRF2. Studies have demonstrated that miRNAs, including miR-101-3p44, miR-144-3p45, miR-15346, miR-340-5p47, and miR-140-5p48, are capable to directly bind and inhibit NRF2 in distinct cells. For example, miR-144-3p and miR-340-5p could reverse the resistance to cisplatin of lung cancer45 and hepatocellular carcinoma cells47 by targeting NRF2, respectively. Importantly, RKIP is able to regulate the level of miRNAs such as let-749, miR-9850, and miR-18520, to exert its tumor-suppressive functions in malignances. In this study, inspired by the compared transcription activity of NRF2 before and after RKIP alteration, we predicted and validated the candidate miRNAs targeting NRF2. The results turn out that miR-450b-5p, which is downregulated and positively regulated by RKIP in radioresistant NPC tissues and cells, could directly target NRF2 and sensitize NPC cells to radiation, which not only identifies a new negative regulator of NRF2, but also presents another case for fulfilling regulatory roles of RKIP via miRNAs.
Downregulation of miR-450b-5p and its tumor-suppressive roles have been indicated in several cancer types. For example, loss of miR-450b-5p upregulates SOX2 and subsequently contributes to maintain stemness and chemoresistance of colorectal cancer cells51. Downregulation of miR-450b-5p serves as an indicator for poor prognosis of patients with hepatocellular carcinoma (HCC) and promotes malignant progression of HCC cells by activating KIF26B51. Accordingly, in current study, we confirm miR-450b-5p is downregulated in radioresistant NPC, positively correlates with favorable prognosis, and exerts pro-radiosensitive roles in NPC. Moreover, we reveal that RKIP is a positive upstream regulator of miR-450b-5p in NPC, however, the underlying mechanisms of how RKIP regulates miR-450b-5p remains unknown. Based on the existing studies, the suppressive roles of RKIP on transcription factors, like STAT327,31, YY126, and NF-κB25, may involve the regulation of miR-450b-5p in NPC, which needs to be validated in the future work.
As a phase II antioxidant enzyme, NQO1, being regulated by NRF2, is aberrantly upregulated in multiple cancers such as pancreatic cancer52, glioblastoma53, lung cancer54, and breast cancer55, and contributes a lot to therapy resistance, especially for radioresistance. β-lapachone, an NQO1-targeting prodrug, are potent to selectively sensitize cancer cells to radiation by aggravating the degree of ROS stress and evoking cell apoptosis in cancers with upregulation of NQO1, including head and neck cancer56 and non-small cell lung cancer54. In this study, in line with head and neck cancer, NQO1 is also upregulated and negatively correlated with RKIP in NPC. RKIP alteration can cause inverse fluctuation of NQO1 in an NRF2-dependent manner. Meanwhile, RKIP alteration shows no significant influence on the expression of other NRF2-regulated antioxidant enzymes, like HO-1 and GCLC5, confirming that the mechanism for pro-radioresistant functions of NRF2 is context-dependent.
In summary, our results demonstrate that RKIP could enhance radiosensitivity of NPC cells by suppressing NRF2/NQO1 axis. Mechanistically, we reveal that RKIP exerts its inhibitory effect on NRF2/NQO1 by upregulating miR-450b-5p, which directly binds and inhibits NRF2 in NPC. Therefore, RKIP/miR-450b-5p/NRF2/NQO1 axis has vital roles in regulation of radioresistance and serves as a promising therapy target for improving the efficacy of clinical treatment in NPC.
Change history
31 May 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41419-023-05861-6
References
Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet 394, 64–80 (2019).
Tsao, S. W., Tsang, C. M. & Lo, K. W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160270 (2017).
Suarez, C. et al. Current treatment options for recurrent nasopharyngeal cancer. Eur. Arch. Otorhinolaryngol. 267, 1811–1824 (2010).
Kim, W. et al. Cellular stress responses in radiotherapy. Cells 8, 1105 (2019).
Zhou, S., Ye, W., Shao, Q., Zhang, M. & Liang, J. Nrf2 is a potential therapeutic target in radioresistance in human cancer. Crit. Rev. Oncol. Hematol. 88, 706–715 (2013).
Jeong, Y. et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 7, 86–101 (2017).
Singh, A., Bodas, M., Wakabayashi, N., Bunz, F. & Biswal, S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox Signal. 13, 1627–1637 (2010).
Marampon, F. et al. NRF2 orchestrates the redox regulation induced by radiation therapy, sustaining embryonal and alveolar rhabdomyosarcoma cells radioresistance. J. Cancer Res. Clin. Oncol. 145, 881–893 (2019).
You, X., Cao, X. & Lin, Y. Berberine enhances the radiosensitivity of hepatoma cells by Nrf2 pathway. Front. Biosci. (Landmark Ed.) 24, 1190–1202 (2019).
Nioi, P. & Nguyen, T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem. Biophys. Res. Commun. 362, 816–821 (2007).
Bellezza, I., Giambanco, I., Minelli, A. & Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res 1865, 721–733 (2018).
Lu, M. et al. GSK3beta-mediated Keap1-independent regulation of Nrf2 antioxidant response: a molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 26, 101275 (2019).
Chatterjee, N., Tian, M., Spirohn, K., Boutros, M. & Bohmann, D. Keap1-independent regulation of Nrf2 cactivity by protein acetylation and a BET bromodomain protein. PLoS Genet. 12, e1006072 (2016).
Lo, J. Y., Spatola, B. N. & Curran, S. P. WDR23 regulates NRF2 independently of KEAP1. PLoS Genet. 13, e1006762 (2017).
Teimouri, M. et al. Inhibiting miR-27a and miR-142-5p attenuate nonalcoholic fatty liver disease by regulating Nrf2 signaling pathway. IUBMB life. 72, 361–372 (2019).
Kurinna, S. & Werner, S. NRF2 and microRNAs: new but awaited relations. Biochem. Soc. Trans. 43, 595–601 (2015).
Zhang, G. et al. Salinomycin overcomes radioresistance in nasopharyngeal carcinoma cells by inhibiting Nrf2 level and promoting ROS generation. Biomed. Pharmacother. 91, 147–154 (2017).
Zaravinos, A., Bonavida, B., Chatzaki, E. & Baritaki, S. RKIP: a key regulator in tumor metastasis initiation and resistance to apoptosis: therapeutic targeting and impact. Cancers 10, 287 (2018).
Zhu, C. X. et al. Tumor suppressor RKIP inhibits prostate cancer cell metastasis and sensitizes prostate cancer cells to docetaxel treatment. Neoplasma 65, 228–233 (2018).
Zou, Q. et al. RKIP suppresses the proliferation and metastasis of breast cancer cell lines through up-regulation of miR-185 targeting HMGA2. Arch. Biochem. Biophys. 610, 25–32 (2016).
Raquel-Cunha, A., Cardoso-Carneiro, D., Reis, R. M. & Martinho, O. Current status of Raf kinase inhibitor protein (RKIP) in lung cancer: behind RTK signaling. Cells 8, 442 (2019).
Martinho, O. et al. Absence of RKIP expression is an independent prognostic biomarker for gastric cancer patients. Oncol. Rep. 29, 690–696 (2013).
Kim, H. S. et al. Reduced expression of Raf-1 kinase inhibitory protein predicts regional lymph node metastasis and shorter survival in esophageal squamous cell carcinoma. Pathol. Res. Pract. 208, 292–299 (2012).
Koelzer, V. H. et al. Geographic analysis of RKIP expression and its clinical relevance in colorectal cancer. Br. J. Cancer 108, 2088–2096 (2013).
Jing, S. H., Gao, X., Yu, B. & Qiao, H. Raf kinase inhibitor protein (RKIP) inhibits tumor necrosis factor-alpha (TNF-alpha) induced acdhesion molecules expression in vascular smooth muscle bells by suppressing (nuclear transcription factor-kappaB (NF-kappaB) pathway. Med. Sci. Monit. 23, 4789–4797 (2017).
Wottrich, S. et al. Inverse correlation between the metastasis suppressor RKIP and the metastasis inducer YY1: Contrasting roles in the regulation of chemo/immuno-resistance in cancer. Drug Resist. Updat. 30, 28–38 (2017).
Yousuf, S. et al. Raf kinase inhibitor protein (RKIP) blocks signal transducer and activator of transcription 3 (STAT3) activation in breast and prostate cancer. PLoS ONE 9, e92478 (2014).
Yang, K. et al. KRAS promotes tumor metastasis and chemoresistance by repressing RKIP via the MAPK-ERK pathway in pancreatic cancer. Int J. Cancer 142, 2323–2334 (2018).
Yuan, L. et al. Reduced RKIP enhances nasopharyngeal carcinoma radioresistance by increasing ERK and AKT activity. Oncotarget 7, 11463–11477 (2016).
Al-Mulla, F., Bitar, M. S., Feng, J., Park, S. & Yeung, K. C. A new model for raf kinase inhibitory protein induced chemotherapeutic resistance. PLoS ONE 7, e29532 (2012).
He, Q. Y. et al. Reduction of RKIP expression promotes nasopharyngeal carcinoma invasion and metastasis by activating Stat3 signaling. Oncotarget 6, 16422–16436 (2015).
Huang, W. et al. MiR-181a upregulation promotes radioresistance of nasopharyngeal carcinoma by targeting RKIP. Onco Targets Ther. 12, 10873–10884 (2019).
Feng, X. P. et al. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res. 70, 3450–3462 (2010).
Shanmugaratnam, K. & Sobin, L. H. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer 71, 2689–2697 (1993).
Pan, J. et al. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. Am. J. Clin. Oncol. 38, 189–196 (2015).
Zheng, Z. et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-kappaB signaling pathway. Cell Death Dis. 8, e2855 (2017).
Qu, J. Q. et al. MiRNA-203 reduces nasopharyngeal carcinoma radioresistance by targeting IL8/AKT signaling. Mol. Cancer Ther. 14, 2653–2664 (2015).
Ivashkevich, A., Redon, C. E., Nakamura, A. J., Martin, R. F. & Martin, O. A. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 327, 123–133 (2012).
Xie, S. Y., Li, G., Han, C., Yu, Y. Y. & Li, N. RKIP reduction enhances radioresistance by activating the Shh signaling pathway in non-small-cell lung cancer. Onco Targets Ther. 10, 5605–5619 (2017).
Woods Ignatoski, K. M. et al. Loss of Raf kinase inhibitory protein induces radioresistance in prostate cancer. Int J. Radiat. Oncol. Biol. Phys. 72, 153–160 (2008).
Wang, Z. et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 10, 2538 (2019).
Chen, X. et al. Valproic acid attenuates traumatic brain injury-induced inflammation in vivo: involvement of autophagy and the Nrf2/ARE signaling pathway. Front. Mol. Neurosci. 11, 117 (2018).
Xiang, Y. et al. Brusatol enhances the chemotherapy efficacy of gemcitabine in pancreatic cancer via the Nrf2 signalling pathway. Oxid. Med. Cell Longev. 2018, 2360427 (2018).
Qin, Z. et al. Zinc-induced protective effect for testicular ischemia-reperfusion injury by promoting antioxidation via microRNA-101-3p/Nrf2 pathway. Aging 11, 9295–9309 (2019).
Yin, Y. et al. miR1443p regulates the resistance of lung cancer to cisplatin by targeting Nrf2. Oncol. Rep. 40, 3479–3488 (2018).
Zhu, X., Zhao, Y., Hou, W. & Guo, L. MiR-153 regulates cardiomyocyte apoptosis by targeting Nrf2/HO-1 signaling. Chromosome Res. 27, 167–178 (2019).
Wu, L. L. et al. NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis. J. Cell Commun. Signal. 13, 99–112 (2019).
Liu, Q. Q. et al. MicroRNA-140-5p aggravates hypertension and oxidative stress of atherosclerosis via targeting Nrf2 and Sirt2. Int J. Mol. Med 43, 839–849 (2019).
Dangi-Garimella, S. et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 28, 347–358 (2009).
Chen, Z. et al. Overexpression of RKIP inhibits cell invasion in glioma cell lines through upregulation of miR-98. Biomed. Res. Int. 2013, 695179 (2013).
Jin, Y. et al. MiR-450b-5p suppresses stemness and the development of chemoresistance by targeting SOX2 in colorectal cancer. DNA Cell Biol. 35, 249–256 (2016).
Lewis, A. M. et al. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol. Carcinog. 56, 1825–1834 (2017).
Luo, S., Lei, K., Xiang, D. & Ye, K. NQO1 is regulated by PTEN in glioblastoma, mediating cell proliferation and oxidative stress. Oxid. Med. Cell Longev. 2018, 9146528 (2018).
Motea, E. A. et al. NQO1-dependent, tumor-selective radiosensitization of non-small cell lung cancers. Clin. Cancer Res. 25, 2601–2609 (2019).
Yang, Y. et al. The NQO1/PKLR axis promotes lymph node metastasis and breast cancer progression by modulating glycolytic reprogramming. Cancer Lett. 453, 170–183 (2019).
Li, L. S. et al. NQO1-mediated tumor-selective lethality and radiosensitization for head and neck cancer. Mol. Cancer Ther. 15, 1757–1767 (2016).
Acknowledgements
This study was supported by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (nos. JY201722), the National Natural Science Foundation of China (nos. 81602686 and 81702924), and the Natural Science Foundation of Hunan Province of China (nos. 2018JJ3811).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by A. Stephanou
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41419-023-05861-6
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, W., Shi, G., Yong, Z. et al. RETRACTED ARTICLE: Downregulation of RKIP promotes radioresistance of nasopharyngeal carcinoma by activating NRF2/NQO1 axis via downregulating miR-450b-5p. Cell Death Dis 11, 504 (2020). https://doi.org/10.1038/s41419-020-2695-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-020-2695-6
- Springer Nature Limited
This article is cited by
-
Clinical Significance of Frequently Down-Regulated Phosphatidylethanolamine-Binding Protein-1 in Gallbladder Cancer
Digestive Diseases and Sciences (2024)
-
Ferroptosis in tumors and its relationship to other programmed cell death: role of non-coding RNAs
Journal of Translational Medicine (2023)
-
Retraction Note: Targeting a novel LncRNA SNHG15/miR-451/c-Myc signaling cascade is effective to hamper the pathogenesis of breast cancer (BC) in vitro and in vivo
Cancer Cell International (2023)
-
Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies
Signal Transduction and Targeted Therapy (2023)
-
Epigenetic modulation of ferroptosis in cancer: Identifying epigenetic targets for novel anticancer therapy
Cellular Oncology (2023)