Abstract
Background
Malignant pleural mesothelioma (MPM) is an aggressive neoplasm and often acquires chemoresistance by increasing stemness in tumour tissue, thereby generating cancer stem cells (CSCs). CSCs escape treatment by deploying metabolic pathways to trigger dormancy or proliferation, also gaining the ability to exit and re-enter the cell cycle to hide their cellular identity.
Methods
We employed various cellular and biochemical assays to identify the role of the glycolytic enzyme PFKFB3, by knocking it down and pharmacologically inhibiting it with PFK158, to determine its anticancer effects in vitro and in vivo by targeting the CSC population in MPM.
Results
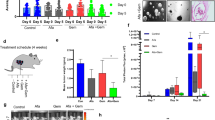
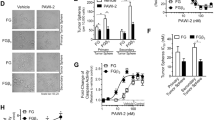
Here, we have identified PFKFB3 as a strategic player to target the CSC population in MPM and demonstrated that both pharmacologic (PFK158) and genetic inhibition of PFKFB3 destroy the FAK-Stat3-SOX2 nexus resulting in a decline in conspicuous stem cell markers viz. ALDH, CD133, CD44, SOX2. Inhibition of PFKFB3 accumulates p21 and p27 in the nucleus by decreasing SKP2. Lastly, PFK158 diminishes tumour-initiating cells (TICs) mediated MPM xenograft in vivo.
Conclusions
This study confers a comprehensive and mechanistic function of PFKFB3 in CSC maintenance that may foster exceptional opportunities for targeted small molecule blockade of the TICs in MPM.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on a reasonable request.
References
De Francesco EM, Sotgia F, Lisanti MP. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem J. 2018;475:1611–34.
Colombo SL, Palacios-Callender M, Frakich N, Carcamo S, Kovacs I, Tudzarova S, et al. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc Natl Acad Sci USA. 2011;108:21069–74.
Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA 2nd, et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461–70.
Shi L, Pan H, Liu Z, Xie J, Han W. Roles of PFKFB3 in cancer. Signal Transduct Target Ther. 2017;2:17044.
Kessler R, Bleichert F, Warnke J-P, Eschrich K. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. J Neuro-Oncol. 2008;86:257–64.
Li H-M, Yang J-G, Liu Z-J, Wang W-M, Yu Z-L, Ren J-G, et al. Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:7.
Bobarykina AY, Minchenko DO, Opentanova IL, Moenner M, Caro J, Esumi H, et al. Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers. Acta Biochim Pol. 2006;53:789–99.
Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, et al. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62:5881–7.
Mondal S, Roy D, Sarkar Bhattacharya S, Jin L, Jung D, Zhang S, et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int J Cancer. 2019;144:178–89.
Sarkar Bhattacharya S, Thirusangu P, Jin L, Roy D, Jung D, Xiao Y, et al. PFKFB3 inhibition reprograms malignant pleural mesothelioma to nutrient stress-induced macropinocytosis and ER stress as independent binary adaptive responses. Cell Death Dis. 2019;10:725.
Yalcin A, Clem BF, Simmons A, Lane A, Nelson K, Clem AL, et al. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J Biol Chem. 2009;284:24223–32.
Gustafsson NMS, Färnegårdh K, Bonagas N, Ninou AH, Groth P, Wiita E, et al. Targeting PFKFB3 radiosensitizes cancer cells and suppresses homologous recombination. Nat Commun. 2018;9:3872.
Li F-L, Liu J-P, Bao R-X, Yan G, Feng X, Xu Y-P, et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508.
Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606.
Jang H, Yang J, Lee E, Cheong JH. Metabolism in embryonic and cancer stemness. Arch Pharm Res. 2015;38:381–8.
Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S. Cancer stem cell metabolism and potential therapeutic targets. Front Oncol. 2018;8:203.
Cancer.Net. Mesothelioma: Statistics, 2019; https://www.cancer.net/cancer-types/mesothelioma/statistics.
Villanova F, Procopio A, Rippo MR. Malignant mesothelioma resistance to apoptosis: recent discoveries and their implication for effective therapeutic strategies. Curr Med Chem. 2008;15:631–41.
Cortes-Dericks L, Carboni GL, Schmid RA, Karoubi G. Putative cancer stem cells in malignant pleural mesothelioma show resistance to cisplatin and pemetrexed. Int J Oncol. 2010;37:437–44.
Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, et al. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget. 2015;6:40822–35.
Okamoto H, Fujishima F, Nakamura Y, Zuguchi M, Ozawa Y, Takahashi Y, et al. Significance of CD133 expression in esophageal squamous cell carcinoma. World J Surg Oncol. 2013;11:51.
Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67.
Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Cañamero M, et al. p27Kip1 directly represses SOX2 during embryonic stem cell differentiation. Cell Stem Cell. 2012;11:845–52.
Jung D, Khurana A, Roy D, Kalogera E, Bakkum-Gamez J, Chien J, et al. Quinacrine upregulates p21/p27 independent of p53 through autophagy-mediated downregulation of p62-Skp2 axis in ovarian cancer. Sci Rep. 2018;8:2487.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Sarkar S, Dutta D, Samanta SK, Bhattacharya K, Pal BC, Li J, et al. Oxidative inhibition of Hsp90 disrupts the super‐chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int J cancer. 2013;132:695–706.
Mandal C, Sarkar S, Chatterjee U, Schwartz-Albiez R, Mandal C. Disialoganglioside GD3-synthase over expression inhibits survival and angiogenesis of pancreatic cancer cells through cell cycle arrest at S-phase and disruption of integrin-beta1-mediated anchorage. Int J Biochem Cell Biol. 2014;53:162–73.
Sarkar S, Mandal C, Sangwan R, Mandal C. Coupling G2/M arrest to the Wnt/β-catenin pathway restrains pancreatic adenocarcinoma. Endocr Relat Cancer. 2014;21:113–25.
Thakur B, Ray P. Cisplatin triggers cancer stem cell enrichment in platinum-resistant cells through NF-kappaB-TNFalpha-PIK3CA loop. J Exp Clin Cancer Res. 2017;36:164.
Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, et al. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018:5416923.
Toledo-Guzman ME, Hernandez MI, Gomez-Gallegos AA, Ortiz-Sanchez E. ALDH as a stem cell marker in solid tumors. Curr Stem Cell Res Ther. 2019;14:375–88.
Clark DW, Palle K. Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann Transl Med. 2016;4:518.
Mori Y, Yamawaki K, Ishiguro T, Yoshihara K, Ueda H, Sato A, et al. ALDH-dependent glycolytic activation mediates stemness and paclitaxel resistance in patient-derived spheroid models of uterine endometrial cancer. Stem Cell Rep. 2019;13:730–46.
Li Z. CD133: a stem cell biomarker and beyond. Exp Hematol Oncol. 2013;2:17.
Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. 2016;35:1926–42.
News in brief. FAK inhibitor kills mesothelioma cells. Cancer Discov. 2014;4:OF3. https://doi.org/10.1158/2159-8290.CD-NB2014-093.
Wang L, Liu X, Ren Y, Zhang J, Chen J, Zhou W, et al. Cisplatin-enriching cancer stem cells confer multidrug resistance in non-small cell lung cancer via enhancing TRIB1/HDAC activity. Cell Death Dis. 2017;8:e2746–e2746.
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–28.
Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336–e1336.
Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9:e84941.
Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843–53.
Shen Y-A, Wang C-Y, Hsieh Y-T, Chen Y-J, Wei Y-H. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14:86–98.
Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, et al. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23:206–19.
Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–85.
Nam K, Oh S, Shin I. Ablation of CD44 induces glycolysis-to-oxidative phosphorylation transition via modulation of the c-Src-Akt-LKB1-AMPKalpha pathway. Biochem J. 2016;473:3013–30.
Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–48.
Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USA. 2011;108:10544–9.
Laszlo V, Valko Z, Ozsvar J, Kovacs I, Garay T, Hoda MA, et al. The FAK inhibitor BI 853520 inhibits spheroid formation and orthotopic tumor growth in malignant pleural mesothelioma. J Mol Med. 2019;97:231–42.
Pachter JA, Kolev VN, Schunselaar L, Shapiro IM, Bueno R, Baas P, et al. Abstract 4236: FAK inhibitor VS-6063 (defactinib) targets mesothelioma cancer stem cells, which are enriched by standard of care chemotherapy. Cancer Res. 2015;75:4236–4236.
Kolev VN, Tam WF, Wright QG, McDermott SP, Vidal CM, Shapiro IM, et al. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget. 2017;8:51733–47.
Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. 2016;35:1926–42.
Zhu W, Ye L, Zhang J, Yu P, Wang H, Ye Z, et al. PFK15, a small molecule inhibitor of PFKFB3, induces cell cycle arrest, apoptosis and inhibits invasion in gastric cancer. PLoS ONE. 2016;11:e0163768.
Banerjee K, Keasey MP, Razskazovskiy V, Visavadiya NP, Jia C, Hagg T. Reduced FAK-Stat3 signaling contributes to ER stress-induced mitochondrial dysfunction and death in endothelial cells. Cell Signal. 2017;36:154–62.
Visavadiya NP, Keasey MP, Razskazovskiy V, Banerjee K, Jia C, Lovins C, et al. Integrin-FAK signaling rapidly and potently promotes mitochondrial function through Stat3. Cell Commun Signal. 2016;14:32.
Xiao F, Connolly DC. Abstract 2095: FAK mediates Stat3 activation, migration and invasion in ovarian carcinoma cells. Cancer Res. 2014;74:2095–2095.
Thakur R, Trivedi R, Rastogi N, Singh M, Mishra DP. Inhibition of Stat3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep. 2015;5:10194.
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab T, Lin J. Stat3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH+/CD133+ stem cell-like human colon cancer cells. Biochem Biophys Res Commun. 2011;416:246–51.
Won C, Kim BH, Yi EH, Choi KJ, Kim EK, Jeong JM, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62:1160–73.
Garg N, Bakhshinyan D, Venugopal C, Mahendram S, Rosa DA, Vijayakumar T, et al. CD133+ brain tumor-initiating cells are dependent on Stat3 signaling to drive medulloblastoma recurrence. Oncogene. 2017;36:606–17.
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, et al. Stat3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25:931–8.
Acknowledgements
We sincerely acknowledge Dr. Tobias Peikert, Mayo Clinic, Rochester, MN for providing the EMMeso cell line. Our acknowledgement towards the personnel of Microscopy and Cell Analysis Core and Pathology Research Cores Mayo Clinic, Rochester, MN.
Funding
This work is supported (in part) by the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (JM and VS) and a generous gift from Samuel and Ilda Conde to JM—Mayo Clinic, Rochester.
Author information
Authors and Affiliations
Contributions
Conceptualisation: SSB and VS; formal analysis: SSB; funding acquisition: VS and JRM; investigation: SSB and VS; methodology: SSB and PT; project administration: VS; supervision: JRM and VS; writing—original draft: SSB; writing—review and editing: SSB, PT, JS and VS.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Mayo Clinic Institutional Review Board (IRB).
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar Bhattacharya, S., Thirusangu, P., Jin, L. et al. PFKFB3 works on the FAK-STAT3-SOX2 axis to regulate the stemness in MPM. Br J Cancer 127, 1352–1364 (2022). https://doi.org/10.1038/s41416-022-01867-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01867-7
- Springer Nature Limited
This article is cited by
-
PFKFB3 in neovascular eye disease: unraveling mechanisms and exploring therapeutic strategies
Cell & Bioscience (2024)
-
Functional Roles of CD133: More than Stemness Associated Factor Regulated by the Microenvironment
Stem Cell Reviews and Reports (2024)