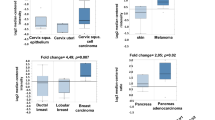
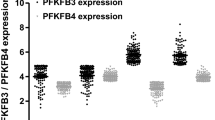
Abstract
The bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBPase-2) controls the glycolytic flux via the allosteric activator fructose 2,6-bisphosphate. Because of its proto-oncogenic character, the PFK-2/FBPase-2 of the PFKFB3 gene is assumed to play a critical role in tumorigenesis. We investigated the PFKFB3 expression in 40 human astrocytic gliomas and 20 non-neoplastic brain tissue specimens. The PFKFB3 protein levels were markedly elevated in high-grade astrocytomas relative to low-grade astrocytomas and corresponding non-neoplastic brain tissue, whereas no significant increase of PFKFB3 mRNA was observed in high-grade astrocytomas when compared with control tissue. In the group of glioblastomas the PFKFB3 protein inversely correlates with EGFR expression. The findings demonstrate that PFKFB3 up-regulation is a hallmark of high-grade astrocytomas offering an explanation for high glycolytic flux and lactate production in these tumors.





Similar content being viewed by others
References
Maher EA, Furnari FB, Bachoo RM et al (2001) Malignant glioma: genetics biology of a grave matter. Genes Dev 15:1311–1333
Kleihues P, Cavenee WK (2000) World Health Organization classification of tumours of the nervous system. IARC Press, Lyon
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Di Chiro G, DeLaPaz RL, Brooks RA et al (1982) Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology 32:1323–1329
Lohle PN, Wurzer HA, Seelen PJ et al (1998) Analysis of fluid in cysts accompanying various primary and metastatic brain tumours: proteins, lactate and pH. Acta Neurochir 140:14–19
Howe FA, Barton SJ, Cudlip SA et al (2003) Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 49:223–232
Boado RJ, Black KL, Pardridge WM (1994) Gene expression of GLUT3 and GLUT1 glucose transporters in human brain tumors. Brain Res Mol Brain Res 27:51–57
Oudard S, Arvelo F, Miccoli L et al (1996) High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer 74:839–845
Meixensberger J, Herting B, Roggendorf W et al (1995) Metabolic patterns in malignant gliomas. J Neurooncol 24:153–161
Oudard S, Boitier E, Miccoli L et al (1997) Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res 17:1903–1911
Van Schaftingen E, Jett MF, Hue L, Hers HG (1981) Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci USA 78:3483–3486
Okar DA, Manzano A, Navarro-Sabate A et al (2001) PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem Sci 26:30–35
Rider MH, Bertrand L, Vertommen D et al (2004) 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J 381:561–579
El-Maghrabi MR, Noto F, Wu N et al (2001) 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: suiting structure to need, in a family of tissue-specific enzymes. Curr Opin Clin Nutr Metab Care 4:411–418
Chesney J, Mitchell R, Benigni F et al (1999) An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc Natl Acad Sci USA 16:3047–3052
Manzano A, Rosa JL, Ventura F et al (1998) Molecular cloning, expression, and chromosomal localization of a ubiquitously expressed human 6-phosphofructo-2-kinase/ fructose-2, 6-bisphosphatase gene (PFKFB3). Cytogenet Cell Genet 83:214–217
Kessler R, Eschrich K (2001) Splice isoforms of ubiquitous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in human brain. Brain Res Mol Brain Res 87:190–195
Chen CY, Shyu AB (1995) AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci 20:465–470
Chesney J (2006) 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Curr Opin Clin Nutr Metab Care 9:535–539
Calvo MN, Bartrons R, Castano E et al (2006) PFKFB3 gene silencing decreases glycolysis, induces cell-cycle delay and inhibits anchorage-independent growth in HeLa cells. FEBS Lett 580:3308–3314
Telang S, Yalcin A, Clem AL et al (2006) Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene 25:7225–7234
Hamilton JA, Callaghan MJ, Sutherland RL et al (1997) Identification of PRG1, a novel progestin-responsive gene with sequence homology to 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Mol Endocrinol 11:490–502
Hirata T, Kato M, Okamura N et al (1998) Expression of human placental-type 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase in various cells and cell lines. Biochem Biophys Res Commun 242:680–684
Atsumi T, Chesney J, Metz C et al (2002) High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res 62:5881–5887
Minchenko OH, Ochiai A, Opentanova IL et al (2005) Overexpression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 in the human breast and colon malignant tumors. Biochimie 87:1005–1010
Nabors LB, Gillespie GY, Harkins L et al (2001) HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine-and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res 61:2154–2161
Ohgaki H (2005) Genetic pathways to glioblastomas. Neuropathology 25:1–7
Fujisawa H, Reis RM, Nakamura M et al (2000) Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest 80:65–72
Wong AJ, Bigner SH, Bigner DD et al (1987) Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA 84:6899–6903
Schlegel J, Merdes A, Stumm G et al (1994) Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer 56:72–77
Minchenko A, Leshchinsky I, Opentanova I et al (2002) Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem 277:6183–6187
Sakakibara R, Kato M, Okamura N et al (1997) Characterization of a human placental fructose-6-phosphate 2-kinase/fructose-2,6-bisphosphatase. J Biochem 122:122–128
Zagzag D, Zhong H, Scalzitti JM et al (2000) Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 88:2606–2618
Zhong H, De Marzo AM, Laughner E et al (1999) Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 59:5830–5835
Dixon DA, Balch GC, Kedersha N et al (2003) Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J Exp Med 198:475–481
Lal A, Kawai T, Yang X et al (2005) Antiapoptotic function of RNA-binding protein HuR effected through prothymosin alpha. EMBO J 24:1852–1862
Kita D, Yonekawa Y, Weller M, Ohgaki H (2007) PIK3CA alterations in primary (de novo) and secondary glioblastomas. Acta Neuropathol 113:295–302
Acknowledgement
This work was supported by the Wilhelm-Sander-Stiftung (2004.010.1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kessler, R., Bleichert, F., Warnke, JP. et al. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. J Neurooncol 86, 257–264 (2008). https://doi.org/10.1007/s11060-007-9471-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9471-7