Abstract
Molecular oxygen (O2) is essential for most biological reactions in mammalian cells. When the intracellular oxygen content decreases, it is called hypoxia. The process of hypoxia is linked to several biological processes, including pathogenic microbe infection, metabolic adaptation, cancer, acute and chronic diseases, and other stress responses. The mechanism underlying cells respond to oxygen changes to mediate subsequent signal response is the central question during hypoxia. Hypoxia-inducible factors (HIFs) sense hypoxia to regulate the expressions of a series of downstream genes expression, which participate in multiple processes including cell metabolism, cell growth/death, cell proliferation, glycolysis, immune response, microbe infection, tumorigenesis, and metastasis. Importantly, hypoxia signaling also interacts with other cellular pathways, such as phosphoinositide 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) signaling, nuclear factor kappa-B (NF-κB) pathway, extracellular signal-regulated kinases (ERK) signaling, and endoplasmic reticulum (ER) stress. This paper systematically reviews the mechanisms of hypoxia signaling activation, the control of HIF signaling, and the function of HIF signaling in human health and diseases. In addition, the therapeutic targets involved in HIF signaling to balance health and diseases are summarized and highlighted, which would provide novel strategies for the design and development of therapeutic drugs.
Similar content being viewed by others
Introduction
Molecular oxygen is an indispensable component in mammalian cells. In the condition of normal oxygen, mammalian cell consumes oxygen and nutrients to synthesize adenosine 5’-triphosphate (ATP)1 It is also involved in various key biochemical reactions in the cells. Therefore, mammalian cells maintain oxygen balance to ensure their physiological function. Decreased oxygen concentration stimulates a variety of downstream signal responses in the cells. In the presence of hypoxic pressure, mammalian cells will activate a series of downstream pathways, mainly including hypoxia-inducible factor (HIF), autophagy, energy metabolic pathways like the mTOR complex 1 (mTORC1), and cell stress pathways such as ER stress;2,3 these pathways facilitate the cell’s response to the hypoxia stress.
The central pathway of cell response to a low oxygen environment involves HIF transcription factors, which are responsible for sensing the hypoxic environment in the cells, inducing metabolic changes, regulating cell proliferation, and controlling inflammatory response and other functions.1,4 Simultaneously, HIF signal is also proved the association with several diseases, such as cardiovascular, metabolic, inflammatory, and infection-related diseases.5,6,7. The discovery of this pathway provides a complete molecular framework to explicate how cells perceive oxygen changes, mediate downstream signal transduction, and provide new therapeutic targets in various human diseases.
Here, we focused on how cells recognize oxygen changes and mediate signal transduction, especially the role of HIFs in cells’ perception of hypoxia. Additionally, we comprehensively summarized the role of HIF signaling in homeostasis of cells, including the mechanism underlying upstream or downstream activation or signal transduction of HIFs, the cross-talking of HIF pathway, and other cellular pathways. Moreover, the roles of HIFs pathway in human health and diseases, and the advances and development of various drugs targeting HIFs pathway were summarized.
History of HIF pathway
The study on HIF pathway has gained significant achievements in the past 30 years (Fig. 1). In 1991, Semenza et al. demonstrated that in the kidney or liver, hypoxic or ischemic conditions induce the production of nuclear factors that promote erythropoietin (EPO) expression by binding to the enhancer elements located 3’ to the human EPO gene,8 first reported as HIF. Ratcliffe et al. then revealed the ubiquity of this oxygen-sensing system in mammals.9 In their subsequent study, a regulatory effect of HIF on glycolysis was identified. Their studies uncovered that the expression of two genes associated with glycolysis, phosphoglycerate kinase (PGK) along with lactate dehydrogenase (LDHA) are elevated under hypoxia.10 In 1995, Semanza et al. isolated and purified HIF-1 and confirmed that HIF-1 contains two subunits: HIF-1α and HIF-1β.11,12 Other studies reported that HIF-1α accumulation enhances the expression of vascular endothelial growth factor (VEGF), whereas HIF-1α deficiency impairs the process of angiogenesis and eventually causes embryonic death.13,14
Based on the discovery of HIF function in biological process, the exact regulatory mechanism of HIF was elucidated. Kaelin et al. identified a complex formed by von Hippel-Lindau (VHL) tumor suppressor protein (pVHL) with Cullin2 (CUL2), Elongin B, and Elongin C.15 Among these factors, VHL protein has a negative regulatory effect on HIF,16 and the absence of VHL prohibits HIF degradation and promotes tumor initiation.17 Accumulating evidence has clarified the regulatory role of HIF. Under normoxia, HIF-1α undergoes hydroxylation to inhibit the recruitment of transcriptional coactivators,18 while VHL recognizes and binds to the hydroxylation sites and subsequently degrades HIF-1α.19,20 In the next decade 1991–2001, emerging enzymes related to HIF-1α hydroxylation are reported.21,22,23 For their contributions to the discovery of how human and animal cells perceive and adapt to oxygen supply, William Kaelin, Peter Ratcliffe, and Gregg Semenza were awarded the 2019 Nobel Prize in Physiology and Medicine.24
HIFS-mediated signal transduction
HIF family
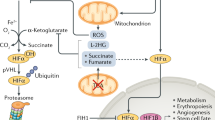
HIFs are the central factors that mediate downstream gene expression in response to hypoxic stress. The HIF family contains two different subunits: α and β. The α part composes of HIF-1α, HIF-2α, and HIF-3α; the β part contains one protein (HIF-1β). HIF-1α is widely expressed in all body tissues, while HIF-2α and HIF-3α are only detected in a few specific tissues.25,26,27 The α-subunit protein is regulated by cellular oxygen levels, whereas the β subunit is constitutively expressed.26,28 Under normoxic conditions, HIF-α proteins (HIF-1α, HIF-2α, and HIF-3α) undergo rapid ubiquitination and sequent degradation by proteasome through hydroxylation of prolyl residues (Fig. 2a). HIF- α proteins contain an oxygen-dependent degradation domain with two proline sites hydroxylated, by the oxygen-dependent proline hydroxylase family (PHDs), including PHD1, PHD2, and PHD3.20,29 Interestingly, this enzymatic activity requires oxygen, iron, and 2-oxo-glutarate.19,29 After hydroxylation, HIF-α interacts with pVHL and then promotes HIF-α ubiquitin-proteasome degradation.19,30 However, under hypoxic conditions, the enzymatic activity of PHD is inhibited, which prevents HIF-α hydroxylation and ubiquitin-mediated proteasome degradation (Fig. 2a). Subsequently, the HIF-α subunit interacts with HIF-1β to form a transcriptional complex dimerization, then entering the nucleus and combining with hypoxia-responsive elements (HREs), inducing the expression of numerous downstream genes.31,32 Notably, HIF-3α exerts an opposite role in the induction of hypoxia-related gene expression. Also, the abundant expression of HIF-3α reduces angiogenesis and restrains cell proliferation.33
The underlying principles of hypoxia and cross-talk of HIF signal with multiple pathways. a Under normoxia, HIFs (α and β subunits) undergo ubiquitination mediated by PHDs (oxygen-dependent proline hydroxylase family) and pVHL (von Hippel–Lindau tumor suppressor protein). The enzymatic activity PHD is prohibited under hypoxia. HIFs are stabilized to promote downstream genes transcription. b The interaction among HIF signal with multiple signaling pathways
Cross-talk of pathways and HIF signal
In addition to the regulation at the protein level, multiple signaling pathways are included in the transcription of HIFs, further affecting the regulatory pathway (Fig. 2b). PI3K-mTOR signaling promotes HIF-α mRNA expression, suggesting its activity upstream of HIF-α.34,35 In addition, the upregulated PI3K-mTOR signaling in cancer cells can facilitate HIF-α activity and induce the angiogenic factors expression.36 Furthermore, signal transducer and activator of transcription 3 (STAT3) was phosphorylated by mTORC1 in a hypoxic environment, thereby inducing HIF-1α RNA expression.37 A study on T cell function showed that the activation of mTOR signal promotes HIF-α to drive metabolic reprogramming and prolongs the T cell survival.38 These studies indicated that PI3K-mTOR signaling regulates the mRNA level of HIF-α.
Mitochondria is a major energy metabolism organelle in a mammalian cell and the powerhouse of oxygen consumption. It plays a crucial role in the modulation of HIF-α via the enrichment of reactive oxygen species (ROS) that enhances HIF stability through inhibition of PHD function.39,40 Reportedly, interleukin-6 (IL-6) accelerates HIF-α expression by activating the downstream Janus kinase (JAK)-STAT3 signaling pathway,41 which is similar to the fact that STAT3 is phosphorylated by mTORC1, upregulating the HIF-1α RNA expression.37 In addition, the activation of pattern recognition receptors (PRRs) can trigger HIF-α transcription. The activation of the Toll-like receptor (TLR) signal drives the downstream NF-κB pathway to promote HIF-α transcription. For example, lipopolysaccharide (LPS) primes TLR4 signaling to induce HIF-1α mRNA expression.42
The ERK pathway is another important pathway that induces HIF-1α expression.43 Reportedly, hyperthermia promotes HIF-1α expression through AKT and ERK pathways.44 Besides, photodynamic therapy (PDT) induces HIF-1α expression through ROS-ERK axis, which enhances the therapy resistance.45 Lastly, the mitogen-activated protein kinase (MAPK) signaling activates of HIF-1α pathway through regulating the p300/CBP protein complex.46 These studies indicated that ERK signaling regulates the mRNA level of HIF-1α to coordinate HIF signal.
In addition to the above signaling pathways, other pathways including Wnt/β-catenin, Notch, and FAT1-ROS are also involved in HIF signals. The Wnt/β-catenin could initiate PI3K/Akt signaling and then adjust HIF-1α function.47 Wnt/β-catenin cooperates with HIF-1α signal in cancer cells,48 while HIF-1α signal also regulates Wnt/β-catenin pathway by calreticulin.49 Emerging studies manifest that the Notch/HIF-1α signaling modulates liver regeneration, angiogenesis, and cancer epithelial-mesenchymal-transition (EMT).50,51,52 The FAT1/ROS/HIF-1α signaling cascade is found to participate in the growth of glioblastoma (GBM).53
Based on the fact that mouse articular chondrocytes promoted HIF-2α expression after treatment with IL-1β, a stimulator of NF-κB pathway, NF-κB pathway could act as an activator to regulate HIF-2α mRNA expression in osteoarthritic.54 Another study found that Icariin modulated NF-κB/HIF-2α axis and reduced inflammation in chondrocyte.55 Since NF-κB and mTOR signaling pathways regulate the expression of HIF-1α, the above investigations imply that HIF-1α and HIF-2α may be modulated by common pathways. Although the constitutive expression of HIF-1β is independent of the cellular oxygen level,28 one interesting study found that NF-κB signaling also promotes HIF-1β expression.56
ER stress is one of the key stress pathways in the host cell in the form of cellular unfolded protein response (UPR) through activating a series of downstream factors, such as protein kinase R-like ER kinase (PERK) and activating transcription factor 6 (ATF6).57,58 ER stress is strongly associated with hypoxia-related pathways. HIF-1α induces ER stress response and promotes alveolar epithelial cell apoptosis.59 Another study revealed that HIF signaling downstream factor VEGF regulates the expression of ATF6 and PERK,60 suggesting a regulatory action of HIF signaling on ER stress. Besides, X-box binding protein 1 (XBP1), a key protein in UPR, is induced in a hypoxia environment and promotes tumor growth,61 implying that hypoxia coupled with ER stress plays certain roles in tumor development. Hypoxic pathway is recently found to interact with ER stress to affect chemoresistance in tumor development.62 In addition, ER stress could reduce the expression of hypoxia-related factors, such as HIFs.63 Therefore, the interaction between hypoxia pathway and ER stress serves an integral function in diverse biological processes.
Biological functions of HIF
HIFs participate in multiple biological processes: metabolism, proliferation, cell growth and survival, glycolysis, immune response, microbe infection, tumorigenesis, and metastasis (Fig. 3). The activation of HIF-1 transcription complex induces significant gene expression,64 including glucose transporter 1,3 (GLUT1,3), LDH-A, VEGF, transforming growth factor-β (TGF-β), matrix metalloproteinases (MMPs), and nitric oxide synthase (NOS), which in turn play a critical part in cell metabolism, tumorigenesis, and many other aspects.65,66,67,68 In addition, HIF signals interact with other cellular pathways and regulate various biological processes.
Cell metabolism by hypoxia
The generation of ATP occurs in the majority of the cells through oxidative phosphorylation. Conversely, HIF-1α stimulates PGK and LDHA in the regulation of the glycolysis process under hypoxia conditions.10 Anaerobic metabolism is also regulated by HIF-1α as it induces anaerobic metabolism shift through multiple enzymes related to glycolysis and glucose transporters, like pyruvate kinase M (PKM), in turn producing energy.69 In addition to glucose consumption and glycolysis, HIF-1α activation underlies lipid metabolism or lipid anabolism,70,71,72 effectuating its pivotal role in the liver and cardiac metabolism.
Cell proliferation by hypoxia
Cell viability and growth are reduced due to deprivation of nutrients and dispossession of oxygen, termed hypoxia. In various cell types, such as hematopoietic stem cells, keratinocytes, lymphocytes, embryonic fibroblasts, embryonic stem cells, and a wide variety of cancer cells, hypoxia inhibits cell proliferation.73 HIF-1α acts biological functions in tumor proliferation and development in hypoxic conditions due to the extreme demands of energy. The tumor survival is mediated by HIF-1α in a hypoxic environment through inhibition of MYC, a transcriptional factor regulating mitochondrial mass and oxygen consumption in several human cancers. HIF-1α decreases the level of MYC by inducing the transcription of MAX interactor 1 (MXI1) (a repressor of MYC) in cancer cells and enhances mitochondrial respiration but increases the glycolysis, leading to tumor growth and survival in a low oxygen environment.74,75,76
Distinguishing to HIF-1α, HIF-2α is unable to compete with MYC for specificity protein 1 (SP1) binding through protein kinase D1 (PKD1)-mediated phosphorylation of HIF-2α.77 In human microvascular endothelial cells, HIF-2 α enhances SP1 activity and also facilitates MYC function to drive IL-8 expression.78 In primary mouse embryo fibroblasts and VHL−/− kidney tumor cells, MYC activity is enhanced by HIF-2α.79,80 Moreover, HIF-2α triggers the activation of MYC by way of the stabilization of the MYC/MAX heterodimer complex under hypoxia. This effect is more exquisite than the degradation of MYC mediated by HIF-1α in cancer cells.81 In cancer cells, MYC regulates the HIF-2α by binding to the HIF-2α gene promoter and such regulation is facilitated by stem cell factors in stem cell renewal and tumor.82
Hypoxia-mediated angiogenesis
HIF-1α plays a vital role in cell metabolism and physiological homeostasis.83 Another major function of HIF-1α is to promote angiogenesis through endothelial cell migration to a hypoxic environment by the transcription of VEGF. A new blood vessel in endothelial cells supplies oxygenated blood to a specific area.84,85
Hypoxia-induced autophagy
The orchestration of multiple stress response pathways including unfolded protein response (UPR), HIF-1 signal, and autophagy, are required for the tumor cells’ adaptation and survival. Hypoxia-induced autophagy performs a certain function in tumor progression.86 Several hypoxia-responsive genes’ transcription is regulated by HIF-1 activation under hypoxia stress. Despite the complexities of regulation, the significance of autophagy-associated HIF-1 in tumor growth has been identified previously.87 Recent evidence suggested that altered expression of many HIF-1 downstream genes regulates both selective and bulk autophagy. Significantly, HIF-1 targets have been shown to have essential autophagic machinery components, such as autophagy related 5 (ATG5), ATG7, and ATG9A.88,89,90
HIF-1 could reprogram glucose metabolism by regulating a cluster of associated genes to indirectly modulate autophagy by modifying glucose metabolism.87,91,92 Autophagy regulates glucose uptake by controlling GLUT1 expression and function during oxygen deprivation. Upon glutamate and oxygen deprivation, PGK1 initiates autophagy via direct binding to ATGL14/VPS34/Beclin1. During tumorigenesis, glycolysis and autophagy are regulated by protein kinase activity of PGK1, which results in Beclin phosphorylation at Ser30.93,94,95 Autophagy is blocked in human T cells deficient in 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) by converting glycolysis to pentose phosphate pathway (PPP), increasing nicotinamide adenine dinucleotide phosphate (NADPH) generation and reducing ROS. On the other hand, the inhibition of PFKFB3 restricts glucose uptake in colon adenocarcinoma cells and induces autophagy.96,97,98 In acute myeloid leukemia (AML), the interaction of pyruvate dehydrogenase kinase 1 (PDK1) between unc-51-like autophagy-activating kinase 1 (UKL1) determines a regulatory manner in autophagy. The inhibition of PDK1 with dichloroacetopenone prevents this interaction and successively suppresses autophagy.99 Besides, hypoxia promotes the location of AKT in mitochondria, increasing phosphorylation of PDK1 on Thr346 and then inhibiting autophagy.100 Autophagy stimulation through hexokinases 2 (HK2)-mediated repression of TORC1 has been reported in glycose starvation neonatal rat ventricular myocytes (NRVMs).101 Lastly, the mTOR together with PP2A controls PHD function and further regulates HIF-1 signal and autophagy.102
Hypoxia in cell death
Programmed cell death (PCD) is a common biological process in organisms that functions in the normal development of cells, maintaining tissue homeostasis against foreign infection, activating immunity, and clearing damaged cells.103,104 Presently, the common ways of programmed cell death include apoptosis, pyroptosis, necrosis, ferroptosis, autophagic death, and necroptosis.105 In addition to affecting cell proliferation, metabolic reprogramming, and autophagy, hypoxia-related pathways regulate the mode of cell death. The function of hypoxia in PCD is discussed below.
Apoptosis
Apoptosis is a classic way of cell death, which play a major role in plentiful biological processes that can be activated by endogenous or exogenous signals.106,107 To date, the role of hypoxia in apoptosis exerts a two-side effect. Hypoxia promotes cell proliferation and inhibits the occurrence of apoptosis. A study reveals that dictamnine decreases the protein expression of HIF-1α and slug to promote cell apoptosis.108 Besides, the HIF-1α-BNIP3 (B-cell lymphoma 2 (BCL2) and adenovirus E1B 19 kDa-interacting protein 3) pathway mediates mitochondrial autophagy to inhibit apoptosis and ROS production, exerting a protective effect in acute renal injury.109 In addition to HIF-1α-reduced apoptosis in hepatoma cell HepG2,110 HIF-2α inhibits apoptosis and autophagy of cervical cancer cells under hypoxia.111 Accumulating evidence demonstrated that hypoxia increases apoptosis. Typically, hypoxia reduces the proliferation of embryonic stem cells and accelerates apoptosis in response to HIF-1α knockdown.112 In addition, the inhibited mitochondrial function under hypoxia promotes ROS production and mitochondrial damage that accelerates apoptosis.32 Notably, these studies suggested that hypoxia can accelerate apoptosis independent of HIFs. Conversely, hypoxia accelerates apoptosis through HIF-dependent pathway. Several studies have identified that Nix and BNIP3, two pro-apoptotic factors, play vital roles in HIF-1 mediated apoptosis.5,113,114 P53 is a crucial tumor suppressor with a key role in apoptosis. HIF-1α promotes p53-dependent apoptosis.115 In this process, HIF-1α stabilizes p53 in dephosphorylated state and regulates p53-dependent apoptosis.116,117
Pyroptosis
A gasdermin (GSDM) family could program another type of cell death called pyroptosis,118 containing five members named GSDMA/B/C/D/E.119 Cell pyroptosis occurs after gasdermin family is cleaved by caspase or other protein, and the N-terminal pore-forming domain is located on cell membrane.120,121,122 Reportedly, hypoxia plays a key role in pyroptosis. Hou et al. demonstrated that hypoxia mediates programmed death ligand 1 (PD-L1) into the nucleus and then induces the expression of GSDMC gene to promote pyroptosis in tumor cells.123 Since the tumor microenvironment is hypoxic, pyroptosis may have varied roles in different tumors. Another study claimed that LPS induces ROS generation to promote inflammasome activation and pyroptosis in H9C2 cells.124 It was also confirmed that hypoxia induces ROS generation to promote pyroptosis in an NF-κB/HIF-1α-dependent pathway.125 Hypoxia/reoxygenation induces cardiomyocyte pyroptosis and IL-18 release, which is mediated by caspase 11-mediated cleavage of GSDMD.126 Strikingly, HIF-1 plays a key role in pyroptosis based on NLRP3 inflammasome.127,128,129,130 Based on the above findings on the role of hypoxia in inducing pyroptosis, hypoxia-induced cell death is speculated as a vital target for disease intervention.
Necroptosis
Necroptosis is another programmed cell death that could be regulated by hypoxia, which is mediated by cell death receptors and related to many inflammatory diseases.131 HIF-1α accelerated necroptosis in macrophages through miR-210 and miR-383.132 HIF-1α also participates in receptor interacting protein 1 (RIP1)-, RIP3-, and mixed lineage kinase domain-like protein (MLKL)-induced necroptosis and deteriorates ischemic brain injury.133 Conversely, a deficiency of HIF-1α and HIF-2α in the myeloid leads to macrophage necroptosis in a myocardial infarction model.134 These studies suggested varying roles of hypoxia-related factors in necrosis.
Ferroptosis
The typical character of ferroptosis is iron-dependent lipid peroxidation accumulation. Ferroptosis is associated with various diseases, including those of the intestine, kidney, liver, and tumors.135 Increasing evidence demonstrates a highly concerned relationship between hypoxia and ferroptosis. Fan et al. demonstrated that hypoxia restrains ferroptosis in hepatocellular carcinoma (HCC) via HIF-1α/solute carrier family 7 member 11 (SLC7A11) axis.136. Another study showed that sorafenib reduces CCl4-induced liver fibrosis through the induction of ferroptosis in hepatic stellate cells via HIF-1α/SLC7A11 pathway.137 Moreover, hypoxia stimulates SUMO/sentrin-specific peptidase 1 (SENP1) protein to promote deSUMOylation of HIF-1α in H9C2 cells, thereby inhibiting cardiomyocyte ferroptosis.138 Similar to the treatment of di-(2-ethylhexyl) phthalate (DEHP), exposure to MEHP (a major biometabolite of DEHP) results in HIF-1α accumulation and transfer to the nucleus, followed by activation of HIF-1α/HO-1 signaling pathway to promote ferroptosis.139 Altogether, hypoxia-induced cell death is speculated as a major target for disease intervention.
Hypoxia and immune response
The immune system is an extremely complex defense system of the body, responsible for preventing pathogen invasion, recognizing and removing damaged cells, malignant cells, or other harmful components to maintain homeostasis. The immune system is mainly divided into innate and adaptive immunity. Failure to activate or excessive activation of the immune system leads to dysfunction or autoimmune diseases.140 In addition, the hypoxic environment is related to immune response, including innate and adaptive immunity.141,142 In this chapter, the role of hypoxia in immune response is summarized systematically.
Hypoxia in innate immunity
Innate immunity eliminates the infection, responds rapidly, and activates adaptive immunity.142 It is well explored that hypoxia-related factors regulate the innate immunity pathway. NF-κB is a key inflammatory response pathway that promotes HIF-α transcription.42 In turn, HIF-1α promotes LPS-induced NF-κB pathway activation and downstream gene expression in a succinate-dependent manner.143 In addition, pyruvate kinase M2 (PKM2) regulates HIF-1α function to mediate LPS-induced IL-1β expression.144 HIF-1α also regulates the interferon pathway. In hypoxic monocytes, HIF-1α negatively regulates the interferon expression.145 Upon severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, HIF-1α signaling pathway activates the interferon and pro-inflammatory cytokines.146 In a previous study, we revealed that SARS-CoV-2 infection induces HIF-1α expression, thereby promoting viral replication and virus-induced inflammatory responses.147 HIF-1α is widely expressed in different innate immune cells, including macrophages, dendritic cells (DCs), and neutrophils. It also mediates metabolic reprogramming to mainly control innate immune cell activation and immune response.148,149,150
Hypoxia in adaptive immunity
In adaptive immune regulation, HIF-1α affects the differentiation and function of T cell-like innate immune cells, and T cells undergo metabolic reprogramming after activation. Shi et al. illustrated a vital role of HIF-1α-dependent glycolysis pathway in the differentiation of Th17 and Treg cells, whereas loss of HIF-1α reduces Th17 differentiation but enhances Treg cell differentiation.151 Another study showed that HIF-1 promotes the development of Th17 and inhibits the development of Tregs,152 implying varying glycolysis-dependence of the two cell subsets. In addition, Palazon et al. found that HIF-1α is essential for CD8+ T cells in anti-cancer immunity.153 The above studies explored that HIF exerts a regulatory role in different T cell subsets. B cell is an important adaptive immune cell. This phenomenon clarified that hypoxia plays a specific role in B cell differentiation and function in a HIF-1α-dependent glycolysis pathway.154,155 Additionally, HIF-1α stimulates the production of IL-10 in B cells via HIF-1α-mediated glycolysis,156 thus regulating B cell-related autoimmune diseases.
Hypoxia signaling in human diseases
Metabolic diseases
Hypoxia signaling in diabetes
Diabetes, a heterogeneous metabolic disease, is featured by the presence of hyperglycemia because of either defective insulin function, impaired insulin secretion or both.157 Diabetes is rapidly spreading worldwide, and its complications cause kidney failure, blindness, cardiovascular disease risk, and increased mortality in individuals with diabetes.158,159,160 A broad consensus was observed on four categories of diabetes: type 1 diabetes (T1D), T2D, hyperglycemia in pregnancy, and diabetes with a specific etiology that may be genetic defects or secondary to drugs, pancreatic factors, or other illnesses.161,162 Type 1 and T2D are primary forms of diabetes.163 Increasing evidence demonstrates that it is hypoxic in diabetes, wounds, pancreatic islets, and tissues (such as the kidney), indicating that hypoxia is closely involved in the occurrence of diabetes.164,165,166 Next, we described the major mechanisms underlying hypoxia signaling-regulated diabetes and diabetic complications.
Hyperglycemia is a common indicator for diagnosing T1D and T2D. High glucose levels suppress hypoxia-induced stabilization of HIF-1α protein level against degradation in specific cells.167 A series of studies have presented the suppressed stabilization and function of HIF-1α in the kidney, wound, and the heart of animal models of diabetes or diabetes patients.166,168,169 Different cell types decide specific roles of HIF-1α activity and signaling in diabetic kidney diseases. High glucose level activates HIF-1α signaling in glomerular mesangial cells,170 however, in proximal tubular HK-2 cells, HIF-1α signaling is suppressed by high glucose levels.171
Typically, activating HIF-1α signaling prevents the development of diabetic kidney disease in the T2D animal model.172 Inhibited HIF-1α signaling impairs wound healing, while activated HIF-1α signaling increases fibroblast proliferation, migration, and angiogenesis to promote wound healing in the diabetes animal models.168,173,174 Properly activated HIF-1α signaling is critical for diabetic heart disease.175 Pharmacologically, activating HIF-1α signaling restores the hypoxic response and improves functional recovery post-ischemia in diabetic heart diseases.176
Unlike HIF-1α, there are only a few studies focused on HIF-2α in diabetes. Brunt et al. suggested that overexpression of HIF-2α does not alter glucose homeostasis in pancreatic β cells.177 However, recent studies have described a critical role of HIF-2α in hepatic glucose homeostasis.178,179 Taniguchi et al. uncovered that the increased hepatic HIF-2α, but not HIF-1α, improves glucose tolerance and insulin sensitivity to ameliorate diabetes.178 Similarly, Wei et al. demonstrated that increasing hepatic HIF-2α ameliorates dyslipidemia, decreases hepatic gluconeogenesis, and improves glucose tolerance and hepatic insulin sensitivity in a HIF-2α-IRS-2-dependent manner.179
Hypoxia signaling in hypoglycemia
Hypoglycemia is defined by a low plasma glucose level, the development of autonomic or neuroglycopenic symptoms, and symptoms in response to the administration of carbohydrates.180 Interestingly, the deprivation of glucose is capable to lead to numerous cellular effects, including cell cycle arrest, autophagy, and apoptosis.181,182 High level of glucose can weaken HIF-1α signaling in several mammalian cell types.183,184,185 Furthermore, it is important to understand the correlation between hypoxia signaling and glucose deprivation.
Limberg et al. demonstrated that hypoglycemia-impaired cardiovascular and autonomic functions are worsened in adults with type 1 diabetes when hypoglycemia is combined with hypoxia signaling.186 Miro and Tirosh showed that hypoxic treatment has a strong hypoglycemic effect, and cholesterol could regulate a metabolic ketogenic shift to prevent hypoxia-induced hypoglycemia.187 Zamudio et al. demonstrated that altitude-induced hypoxia decreases fetal circulating glucose concentration and consumption, which unrecovered the correlation of hypoglycemia with the derivation of hypoxia-induced decline in human fetal growth.188
Hypoxia signaling in non-alcoholic fatty liver disease (NAFLD)
NAFLD is a kind of the most prevalent chronic liver disease globally,189 characterized by macrovesicular steatosis in hepatocytes (≥5%) in the absence of a secondary cause, such as drugs or alcohol.190 In the absence of overdose alcohol intake, it is a progressive disease that involves lipid accumulation and non-alcoholic steatohepatitis that ultimately causes cirrhosis and hepatocellular carcinoma.191,192,193 It is reported that the pathogenesis of NAFLD has been linked to hypoxia signaling.194,195 HIFs can also regulate cellular metabolism in hypoxia. HIF-1α upregulates the expression of genes encoding glycolytic enzymes (i.e., LDHA) and promotes glucose consumption, while HIF-2α represses the expression of genes associated with oxidative metabolisms (i.e., FAO) and regulates lipid storage.70,196,197,198,199
HIF-1α activation promotes glucose consumption and glycolysis and affects lipid metabolism.70,71 HIF-1α is upregulated in hepatocytes in NAFLD and is also a critical regulator of liver fibrosis in NAFLD.200,201,202 Csak et al. observed that microRNA (miRNA)-122 regulates HIF-1α in hepatocytes and is correlated with fibrosis in methionine-choline-deficient (MCD) diet-induced steatohepatitis. Wang et al. showed that palmitic acid induces HIF-1α and impairs autophagic flux and autophagy via HIF-1α in macrophages.203 HIF-1α also mediates activation of NF-κB and production of monocyte chemoattractant protein-1 (MCP-1), impairs autophagy, and increases IL-1β production. Both MCP-1 and IL-1β contribute to MCD diet-induced non-alcoholic steatohepatitis.203 Asai et al. showed that cholesterol induces HIF-1α activation and liver steatosis, and HIF-1α reduces the expression of hepatic aquaporin 8 (AQP8) and promotes cholesterol gallstone formation.204 The high expression of hepatic HIF-1α is observed in the livers of patients with NAFLD and gallstones than in those without gallstones.204
HIF-1α and −2α affect lipid metabolism; however, HIF-2α is the predominant subunit regulating lipid metabolism, which suppresses fatty acid oxidation and promotes the genes related to fatty acid synthesis and lipid storage.194,205 Knockdown of HIF-2α protein reverses lipid metabolism dysregulation by acute hypoxia in the human hepatocellular carcinoma HepG2 cell line.206 Rankin et al. demonstrated that constitutive HIF-2α activation impairs fatty acid β-oxidation and increases lipid storage capacity, leading to severe fatty liver disease in mice.205 Morello et al. found that HIF-2α activation influences the severity of steatohepatitis and fibrogenesis in human NAFLD by upregulating the expression of histidine-rich glycoprotein (HRGP).194 Qu et al. revealed that HIF-2α activation promotes the developmental progression of steatohepatitis by increasing lipid accumulation, subsequent inflammation, and eventually fibrosis.207
Hypoxia signaling in osteoporosis
Osteoporosis, a common skeletal disease is featured by systemic impairment of bone mass, strength, and microarchitecture, which increases the risk for fragility fractures.208 Oxygen is required for the activity of skeletogenic cells and many fundamental cellular processes that are critical for normal fracture healing.209 In recent years, several studies elucidated the mechanisms by which HIFs (HIF-1α and HIF-2α) impact bone remodeling and pathologies.210 However, the underlying correlations between hypoxia signaling and osteoporosis remain poorly understood.
Miyauchi et al. showed that estrogen receptor α (Erα) decreases HIF-1α protein levels in osteoclasts, and osteoclast formation is blocked by HIF-1α deficiency in hypoxic conditions.211 Importantly, HIF-1α is controlled by estrogen signaling in osteoclasts, and thus, it may be a promising therapeutic target to treat postmenopausal osteoporosis.211 Tando et al. illustrated that mouse HIF-1α protein accumulates in osteoclasts following orchidectomy in vivo and in osteoclasts cultured in hypoxic conditions in vitro.212 The protein level is suppressed by testosterone treatment in osteoclasts cultured in hypoxic conditions, and HIF-1α inhibitor abrogates testosterone deficiency-induced bone loss and osteoclast activation in orchidectomized mice.212 This testosterone deficiency accelerates HIF-1α protein accumulation, thereby promoting the development of male osteoporosis.212 Zhao et al. suggested that the expression of HIF-1α and HIF-2α was suppressed by pVHL in osteoblasts, and HIF signaling activation in osteoblasts might prevent the bone loss induced by ovariectomy and increased angiogenesis and osteogenesis in mice.213 Hence, HIF-1α protein may be a critical therapeutic target for osteoporosis.211,212,213
Infectious diseases
Hypoxia and infectious pneumonia
Infectious pneumonia is an acute inflammation of the lung tissue caused by large-scale pathogens including viral and bacterial infections.214 Patients confirmed with infectious pneumonia are at a high risk of acute lung injury (ALI), especially those with specific types of viral pneumonia,215 including Streptococcus pneumoniae (S. pneumoniae), the most common cause of pneumonia, and influenza virus, frequently leading to viral pneumonia. Notably, S. pneumoniae usually infects nervous system to cause fatal bacterial meningitis, and the course of the infection could be affected by hypoxia and HIF-1.216 Hypoxia is the hallmark of SARS-CoV-2 pneumonia.217 Therefore, hypoxia signaling might be closely associated with the occurrence and progression of SARS-CoV-2 pneumonia. Herein, we described the correlation between coronavirus disease 2019 (COVID-19) and hypoxia signaling (Fig. 4).
Role of HIF-1α in hypixa signaling in COVID-19. When SARS-CoV-2 entering host cells, viral ORF3a protein induces HIF-1α expression through triggering mitochondrial reactive oxygen species (ROS) activation. The accumulated HIF-1α stimulates Ca2+ release, promotes viral replication and enhances glycolytic and inflammatory genes, which leads to a cytokine storm
Serebrovska et al. speculated that the activation of HIF-1α decreases the expression of angiotensin converting enzyme-2 (ACE2) along with transmembrane serine protease 2 (TMPRSS2) while increasing the expression of ADAM metallopeptidase domain 17 (ADAM17) on the surface of alveolocytes under hypoxic conditions, thereby decreasing the invasiveness of SARS-CoV-2.218 The study also concluded that HIF-1α signaling participates in severe hypoxia-induced activation of pro-inflammatory cytokine expression and cytokine storm phase of COVID-19.218 We have recently revealed that SARS-CoV-2 induces expression of HIF-1α and secretion of inflammatory cytokines via ORF3a, and conversely, HIF-1α facilitates SARS-CoV-2 replication and aggravates inflammatory responses.147 HIF-1α also facilitates the infections of other viruses, such as herpes simplex viruses 1 (HSV-1) and vesicular stomatitis virus (VSV).147 Codo et al. showed that SARS-CoV-2 triggers mitochondrial ROS production, which enhances HIF-1α stabilization and sustains SARS-CoV-2 replication in monocytes.219 Mitochondrial ROS-mediated stabilization of HIF-1α also sustains replication of SARS-CoV-2 in monocytes.219 However, Prieto-Fernández et al. have shown that hypoxia reduces the binding of the SARS-CoV-2 spike (S) protein to epithelial cells through decreasing ACE2, neuropilin-1 (NRP1), and cellular heparan sulfate (HS) expression.220
Hypoxia and viral hepatitis
The term viral hepatitis means liver inflammation induced by hepatic viral infections of mainly hepatitis B virus (HBV) and hepatitis C virus (HCV).221 Viral hepatitis is a global public health problem that leads to thousands of patients dying of acute and chronic infections, liver cirrhosis, and cancer.222 In 2000, Lee et al. demonstrated that the expression of HBV X protein (HBx) was elevated when HBV-infected hepatoma cells were cultured under hypoxic conditions. Concurrently, when a reporter plasmid carrying HBV Enh1 was transfected into hepatoma cells under hypoxia, the HBV enhancer 1 (Enh1) activity was augmented.223
In hepatocarcinogenesis, HBx protein may be a critical mediator of hypoxia-induced angiogenesis.223 It increases the transcriptional and translational level and also stabilizes HIF-1α.224,225 Moreover, HBx promotes the HIF-1α transcription by activating MAPK pathway.226 Yoo et al. have shown that HBx protein increases the transcriptional level of metastasis associated 1 (MTA1) and histone deacetylase 1 (HDAC1), thereby enhancing HIF-1α protein in hepatocellular carcinoma cells.227 HBV also induces the HIF-2α expression via HBx protein, conversely, HBx activates NF-κB signaling to increase HIF-2α expression.228
Hallez et al. found that DNase I, a cellular restriction factor of HBV, is induced by HIF-1α.229 Wing et al. found that HIF-1α and HIF-2α promote HBV replication via activating the HBV basal core promoter.230 HIF1α stabilization offers a reservoir for HBV in immune-active patients and impairs NF-κB-mediated A3B induction, which is critical for eliminating HBV covalently closed circular DNA (cccDNA).231 Consequently, HIF-1α is a potential target in anti-HBV strategy in the context of immune-mediated A3B induction.
Furthermore, Ripoli et al. showed that HCV protein expression stabilizes HIF-1α under normoxic conditions, and glycolytic enzymes are upregulated by activated HIF-1α in HCV-infected cells.232 Under hypoxic conditions, HCV core protein enhances HIF-1α protein expression, which then elevates VEGF expression.233 Zhu et al. found that HCV core protein enhances the HIF-1α expression and stabilization, and subsequently, HIF-1α stimulates VEGF expression in Huh7.5.1 cells.234 Both VEGF and HIF-1α are crucial angiogenic factors. Hence, HIF-1α might be a new therapeutical target against HCV-induced HCC.234
Apart from the above bacterial and viral infection, hypoxia is found to be closely related to the pathogenesis of multiple neurological infectious diseases, including enterovirus, mumps, lymphocytic choriomeningitis, and type I and II scab viruses,235 the interconnection between hypoxia and infectious diseases in nervous system is taken under consideration to a potential targeted therapy in the following investigations.
Neoplastic diseases
Hypoxia in colon cancer
Colon cancer is one of the most common cancers worldwide, with the highest mortality rate along with breast, lung, and prostate cancers.236 The colon and rectum are the final portions of the human digestive tract. Colon cancer arises from the colonic epithelial cells that line the lumen of the organ and results from a multistep process of colon neoplasia over several years.237 Hypoxia is a typical feature of solid tumors in common and it is related to the progression and metastasis of colon cancer.238,239,240 For example, the expression of Orai1 is induced by hypoxia in colon cancer, which promotes hypoxia-induced invasion and angiogenesis.241 The correlation between colon cancer and hypoxia is illustrated (Fig. 5).
HIF-1α was upregulated in colon cancer tissues.242 Santoyo-Ramos et al. showed that HIF-1α and HIF-2α are expressed in human colon cancer cells but not in non-malignant cells under normoxic conditions.243 Jeon et al. revealed that protein S-glutathionylation increases the protein level of HIF-1α in hypoxic colon cancer cells.244 Zheng et al. demonstrated that DJ-1 protein facilitates the survival of human colon cancer cells by the increased HIF-1α protein expression by means of PI3K-AKT signaling pathway.245
Under hypoxic stress, upregulated HIF-1α induces the expression of phospholipase D2 (PLD2) in colon cancer cells, while downregulation of the protein significantly reduces the expression of PLD2 and tumor volume.238 Hypoxia-induced elevated expression of PLD2 facilitates cell proliferation by NF-κB signaling activation to upregulate the expression of Cyclin D1 in colon cancer.246 Du et al. have suggested that annexin A3 (ANXA3) expression is upregulated by HIF-1α under hypoxic stress and promotes tumor growth in colon cancer.247 The expression of HIF-1α and semaphorin 4D (Sema4D) is closely related to lymphatic metastasis and specific histological types in colon cancer. Mechanistically, in colon cancer, tumor-associated macrophages (TAMs) may accelerate cell migration and invasion via upregulation of HIF-1α and Sema4D.248 Costa et al. found that miR-675-5p is overexpressed in metastatic colon cancer patients and is involved in tumor progression by promoting HIF-1α-induced EMT.249 HIF-1α mediates hypoxia-induced apoptosis-inducing factor (AIF) inhibition, and downregulation of AIF contributes to hypoxia-induced EMT of colon cancer.250 In a subset of colon cancers, HIF-1α is a positive factor for non-hypoxia-mediated cell proliferation in vitro and in vivo, and hypoxia-mediated cell proliferation and survival in vitro but does not contribute to the hypoxic tumor compartments in vivo.251
HIF-2α is essential in the inflammatory response and the regeneration and proliferation capacity of the intestine following an acute injury, and its chronic activation enhances the proinflammatory response, intestinal injury, and colorectal cancer.252 Franovic et al. showed that suppression of HIF-2α restrains tumorigenesis and the proliferation of genetically diverse human cancer cells in vivo.253 Xue et al. suggested that HIF-2α activation increases tumor progression in colon cancer, whereas the HIF-2α-induced tumor formation is reduced upon low-iron treatment.254
Experimental evidence highlighted that apart from human colon carcinoma cell lines, HIF-2α is also important for the survival of patient-derived primary colon cancer cells.255 Different from HIF-1α, HIF-2α plays an important role in resistance in colon malignant cells.255 Cyclooxygenase 2 (COX2) expression is dependent on HIF-2α in colon tumors, and its inhibition reduces HIF-2α-induced colon tumor formation.256 Yes-associated protein 1 (YAP1) activity is upregulated by HIF-2α in CRC-derived cell lines and mouse models; HIF-2α also promotes colon cancer growth by upregulating the activity of YAP1.257
Hypoxia signaling in lung cancer (LC)
LC, a kind of malignant tumor and a leading cause of death worldwide, is mostly classified into two categories, namely small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC).258,259,260 NSCLC is the major subtype of LC and accounts for about 80% of all patients with LC.261 The initiation of LC derives from a highly vascularized and oxygenated tumor microenvironment, crucial for tumor progression.262,263 Current studies have found that hypoxia signaling is associated with multiple processes in the occurrence and progression of NSCLC and SCLC,264,265 which are controlled precisely and differentially (Fig. 5).
Hypoxia elevates the HIF-1α level in LC cells.266 Moreover, HIF-1α expression in LC is higher than in normal lungs. NSCLC patients have a higher HIF-1α expression than SCLC patients, while upregulation of HIF-1α is closely related to tumor growth and survival rate of NSCLC.267,268,269 It is reported that long non-coding RNA (lncRNA) PVT1 increases the expression of HIF-1α in NSCLC.270 Wu et al. found that fibroblast growth factor 11 (FGF11) is upregulated in NSCLC tumor tissues and cell lines, and high expression of FGF11 is related to a poor prognostic outcome in NSCLC patients.271 miR-525-5p negatively regulates FGF11 while FGF11 promotes the expression of HIF-1α for NSCLC progression.271 On the other hand, T-lymphokine-activated killer cell-originated protein kinase (TOPK) positively regulates HIF-1α expression and promotes Snail expression, leading to EMT and invasion of NSCLC.272 In response to hypoxia, elevated lncRNA-AC020978 accelerates proliferation and the glycolytic metabolism of NSCLC by regulating PKM2-enhanced HIF-1α transactivation activity.273 Overexpression of miR-622 mediated by forkhead box O3 (FOXO3a) represses HIF-1α to hinder the migration and invasion of LC cells.274 Gamma linolenic acid (GLA) inhibits hypoxia-driven proliferation and invasion of NSCLC cells by inhibition of HIF-1α-VEGF pathway in vitro.275 Subsequently, HIF-1α inhibition suppresses the hypoxia-induced EMT phenotype and increases the efficacy of immune checkpoint blockade in the treatment of NSCLC.276
The study of the correlation between HIF-2α and LC has not been elucidated clearly. Kong et al. showed a higher expression of nuclear paraspeckle assembly transcript 1 (NEAT1) in NSCLC tissues and cells than that in normal controls, and NEAT1 knockdown suppresses cell proliferation, migration, and invasion in NSCLC.277 Interestingly, NEAT1 promotes EMT and NSCLC cell metastasis under hypoxia in a HIF-2α-dependent manner.277 Wang et al. demonstrated that lncRNA HIF2PUT was downregulated in NSCLC tissues and cell lines, and its overexpression inhibits NSCLC proliferation and invasion via HIF-2α pathway.278
Hypoxia signaling in gastric cancer (GC)
GC is a high concern for health globally and the second cause of cancer deaths after LC.279 The causes of GC are multifactorial, although Helicobacter pylori infection is considered the main cause; its effects are modulated by environmental, microbial, and host factors.280 Hypoxia is closely related to the aggressive tumor phenotypes of gastric carcinomas,281,282 including the metastatic ability of cancer cells.283,284 For example, hypoxia increases GC malignancy partially through transcriptional activation of lncRNA-GAPLINC in a HIF-1α-dependent manner.285 Therefore, the factors underlying the correlation between GC and hypoxia need to be investigated further (Fig. 5).
HIF-1α overexpression is a poor prognostic indicator for patients with GC and is highly correlated with histology, depth of invasion, and microvessel density.286 HIF-1α stimulates multidrug resistance in GC cells through stimulating the transcription of miR-27a.287 HIF-1α-induced miRNA-421 promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in GC.288 Liu et al. suggested that HIF-1α and Wnt/β-catenin signaling pathways promote the invasion of hypoxic GC cells.48
Hypoxia increases the migration and invasion of GC cell line BGC-823 by activating HIF-1α and inhibiting N-myc downregulated gene 2 (NDRG2)-associated signaling pathway.289 Xia et al. demonstrated that hypoxia promotes the release of GC exosome and the expression of miR-301a-3p; then, miR-301a-3p-rich exosomes increase HIF-1α accumulation and promote GC malignancy and metastasis.290 Ding et al. showed that collagen triple helix repeat containing 1 (CTHRC1) overexpression increases cell migration and invasion capacity in GC. CTHRC1 upregulated the expression of HIF-1α to increase CXC chemokine receptor 4 (CXCR4) expression, ultimately promoting cell migration and invasion.291 Epigallocatechin gallate (EGCG) induces apoptosis and impedes proliferation in GC SGC7901 cells by downregulating the expression of HIF-1α and VEGF under hypoxia.292 Downregulation of HIF-1α, leading to suppressing the PI3K/AKT pathway and VEGF expression, might inhibit the proliferation, migration, and invasion of GC.293
Hypoxia signaling in breast cancer (BC)
BC is the most common malignant tumor diagnosed in women.294 It is also the leading cause of cancer-related deaths in women globally.279 Hypoxia signaling serves an essential role in BC and an increased level of HIF-1α has been documented in BC.295 Overexpression of HIF-1α is significantly associated with poor disease-free and overall survival in BC patients.296 Sun et al. have shown that HIF-1α is closed to tumor differentiation, lymph node metastasis, and clinical stage with respect to survival in BC patients.297 Next, the correlation between BC and hypoxia was interpreted comprehensively (Fig. 5).
HIF-1α overexpression effectuates via different regulatory pathways in BC: (a) hypoxia induces perinecrotic HIF-1α overexpression with a robust expression of hypoxia-related genes that are responsible for poor prognosis; (b) normoxia induces diffuse HIF-1α overexpression lacking major hypoxia-associated downstream effects, which is a favorable prognosis.298 Marton et al. showed that HIF-1α overexpression indicates an unfavorable prognosis and could serve as an additional prognostic factor in neuroendocrine BCs.299 Dales et al. demonstrated that mRNA expression of HIF-1αTAG splice variant reflects a stage of BC progression and is related to poor prognosis.300 Hoffmann et al. found that hypoxia promotes BC cell invasion through HIF-1α-mediated upregulation of cysteine-rich protein 2 (CSRP2), an invadopodia actin-bundling protein.301 Choi et al. suggested that HIF-1α promotes the MMP-9 expression under hypoxic conditions, which affects BC cell invasion.302 HIF-1α signaling is critical in ATP-driven chemoresistance and may serve as a potential target for BC therapies.303
BC cells display phenotypic diversity in response to hypoxic or normoxic microenvironments. HIF-1α induces the expression of hematopoietic pre-B cell leukemia transcription factor-interacting protein (HPIP) that establishes cell survival and promotes migration and invasion of cells, EMT, and metastatic phenotypes under hypoxia. Accumulation of HPIP stabilizes HIF-1α to support cell growth.304 Jia et al. demonstrated that claudin 6 (CLDN6) functions as a tumor suppressor in BC and is upregulated by HIF-1α under hypoxia.305 Increased CLDN6 weakens the stability of HIF-1α protein by reducing the expression of SENP1 and preventing the deSUMOylation of HIF-1α; the negative feedback loop slows down the hypoxia-induced BC metastasis.305 Hypoxia-responsive miR-141-3p is involved in the progression of BC, which prevents hypoxia-induced BC by inhibiting the high mobility group box 1 (HMGB1)/HIF-1α signaling pathway.306 Breast cancer metastasis suppressor 1 (BRMS1), a novel metastasis suppressor protein without the activity of anti-proliferation, attenuates TGF-β1-induced EMT and invasion of BC cells through suppressing HIF-1α expression.307
Similar to HIF-1α, Wang et al. suggested that HIF-2α expression is significantly correlated with tumor size, lymph node involvement, and metastasis, and high expression of the protein is associated with poor overall survival in BC patients.308 Thus, HIF-2α could be a valuable biomarker of BC progression and patient survival.308 It may promote the migration and invasion of human BC MCF-7 cells under hypoxic conditions by potentiating the Notch3 pathway.309 Bai et al. revealed that the downregulation of HIF-2α suppresses the stemness of human BC MDA-MB-231 cells and promotes apoptosis.310
Hypoxia signaling in pancreatic cancer
Pancreatic cancer is a fatal malignancy, predominantly seen in men at an advanced age of 40–85 years. It ranks first among asymptomatic cancers.311 Pancreatic cancer is extremely difficult to detect as it lacks early signs and spreads rapidly to the surrounding organs.311 The high malignancy of pancreatic cancer is mostly attributed to the hypoxic tumor microenvironment.312,313 Pancreatic cancer is accompanied by HIF-1α overexpression.314,315 Herein, we summarized the mechanism by which hypoxia signaling affects the tumorigenesis and progression of pancreatic cancer (Fig. 5).
HIF-1α is overexpressed in pancreatic cancer patients, and it regulates expression of various genes associated with pancreatic cancer.315,316 HIF-1α overexpression induces EMT in an NF-κB signaling pathway-dependent manner.317 Several findings discovered that high expression of HIF-1α significantly enhances the capacity of anti-apoptosis in pancreatic cancer cells.318,319
Upregulation of autophagy induced by HIF-1α improved the malignancy of pancreatic cancer through potentiating EMT and migration of pancreatic cancer stem cells.320 Yue et al. showed that HIF-1α facilitates the expression of miR-212 and results in the development of pancreatic ductal adenocarcinoma.321 Zeng et al. demonstrated that MTA2 transcriptional regulator lncRNA (MTA2TR) is overexpressed in pancreatic cancer patient tissues compared to paired noncancerous tissues and promotes pancreatic cancer cell proliferation and invasion in vitro and in vivo.322 MTA2TR is transcriptionally regulated by HIF-1α under hypoxic conditions.322 Furthermore, miRNAs regulate HIF-1α on the EMT of pancreatic cancer cells. The level of miR-142 was obviously lower in pancreatic cancer cell lines and tissues than that in normal tissues. Downregulating the expression of miR-142 increases HIF-1α expression to upregulate EMT-related proteins, eventually enhancing the invasion and migration of pancreatic cancer cells.323
Wang et al. showed that the mRNA levels of HIF-1α and HIF-2α were upregulated in pancreatic cancer. However, their protein expression patterns differed markedly with varied roles in pancreatic cancer.324 HIF-1α serves as an unfavorable prognostic indicator, whereas HIF-2α is a favorable prognostic indicator in pancreatic cancer patients.324 MiR-301a was upregulated by HIF-2α-dependent signaling pathway, and it promotes hypoxia-induced EMT of pancreatic cancer cells.325 Yang et al. suggested that HIF-2α promotes EMT by regulating Twist2 binding to the E-cadherin promoter in pancreatic cancer.326 HIF-2α facilitates the formation of vasculogenic mimic in pancreatic cancer by regulating Twist1 binding to VE-cadherin promoter.327
Hypoxia signaling in prostate cancer
Prostate cancer is a major disease in males around the world.328 It is the second most common form of cancer in men, surpassed only by nonmelanoma skin cancer.328 The incidence and mortality of prostate cancer are correlated with the mean age at diagnosis is 66 years.329 Zhong et al. found that expression of HIF-1α increases in human and rat prostate cancer cell lines.330 Hypoxia signaling plays a vital role in the tumorigenesis and progression of prostate cancer. Herein, we illustrated the complex correlation between prostate cancer and hypoxia (Fig. 5).
Hypoxia significantly enhances the invasiveness of prostate cancer PC3 cells by upregulating HIF-1α expression and autocrine tumor necrosis factor (TNF)-α production.331 HIF-1α cooperates with TNF-α and stabilizes Snail, which in turn upregulates the invasiveness-associated genes, MMP9, fibronectin, and vimentin.331 Moreover, HIF-1α expression is associated with an increased risk and clinicopathological significance in prostate cancer patients.332 Xia et al. revealed that protein kinase CAMP-dependent type II regulatory subunit beta (PRKAR2B) increases HIF-1α expression, a key mediator of the Warburg effect.333 Interestingly, PRKAR2B-HIF-1α loop enhances the Warburg effect that provides a growth advantage in prostate cancer.333
Cardiovascular diseases
Cardiovascular diseases are the leading threat to life and health worldwide.334,335 The circulatory system, i.e., the organs and tissues in the body that carry blood, primarily the heart and blood vessels (arteries, veins, and capillaries), is involved in the series of illnesses.336,337 Hypoxia is one of the most important pathogenic factors of cardiovascular diseases.338,339,340,341 It heralds the onset of many cardiovascular diseases, i.e., arteriosclerosis, pulmonary hypertension, and heart failure.342 The occurrence and development of cardiovascular diseases can be induced by sympathetic excitation disorder, oxidative stress, inflammatory response, endothelial injury, abnormal glucose, and lipid metabolism caused by hypoxia.343,344,345,346
HIF-1α is the primary controller of physiological and pathological hypoxia and is widely expressed in cardiovascular diseases.141,216,347 Almost all genes related to hypoxia, including glucose transporter (GLUT), VEGF, glycolytic enzymes, cell survival factors, and cell surface receptors, are directly or indirectly regulated by HIF-1.348 The levels of HIF-1α subunits increase exponentially with the decrease in oxygen concentration to regulate hypoxic adaptive response.349 During an oxidative stress response, ROS promotes HIF-1α expression to activate the transcription of several genes, such as endothelin-1 (ET-1); the expression of ET-1 contributes to cardiovascular diseases.350 Previous studies have shown that the expression of HIF-1α activates a series of profibrotic transcriptional genes, including collagen I, III, IV, and lysyl oxidase, leading to myocardial fibrosis.351,352,353,354 The different expressions of HIF-1α in the cardiovascular cell system, significantly affect the function of these cells and performing a certain part in the diseases including atherosclerosis, pulmonary hypertension, cardiomyopathy, arrhythmia, and congenital heart disease.
Hypoxia in atherosclerosis
Atherosclerosis, as the primary cause of cardiovascular disease, leads to mortality and disability worldwide. It is characterized by chronic inflammatory changes in large and medium-sized arterial walls,355 including lipid deposition, atheromatous plaque formation and rupture, inflammatory cell infiltration, and endothelial function damage.356,357 The formation mechanism of atherosclerosis includes oxidative stress, arterial endothelial injury and dysfunction, foam cell formation, and subsequent lipid deposition and thrombosis.358 Arteriosclerosis begins with endothelial dysfunction that induces mononuclear cell infiltration.359 Cytokines released by mononuclear cells stimulate the proliferation of smooth muscle cells in the media of blood vessels and the new intima.360 In addition, mononuclear cells activate into macrophages, during which smooth muscle cells of the new intima ingest lipids to become foam cells, forming atheromatous plaques.361,362
Atherogenesis is related to hypoxia. Under such conditions, the extracellular nutrients and lipids induce the formation of hypoxic areas in arterial plaques, especially in macrophages, vascular smooth muscle cells, and endothelial cells.363,364 These cells subsequently express HIF in response to hypoxia.364 HIF-1α is expressed in 49% of carotid and 60% of femoral endarterectomy patients, providing evidence of its involvement in atherogenesis.365 In addition, pimidazole is increased in hypoxic zones of atherosclerotic areas, indicating the involvement of hypoxia in atherogenesis.366 ATP-binding cassette transporter A1 (ABCA1) and apolipoprotein A1 (ApoA-1) contribute to monocyte-macrophage infiltration and lipid deposition with plaque formation in the arterial wall, respectively.367 HIF-1α interacts with NF-κB and promotes the expression of ABCA1 to exert an anti-atherosclerotic role in the pathogenesis of atherogenesis in THP-1.368 Once the oxygen concentration in the cells is low, HIF-1α signaling participates in the formation and rupture of atherosclerotic plaques by promoting the expression of VEGF.13 Subsequently, VEGF stimulates neovascularization, promotes atherogenesis, increases plaque instability, and hastens plaque rupture.13
In human vascular smooth muscle cells, the expression of low-density lipoprotein receptor-related protein (LRP1) was upregulated by HIF-1α, promoting the deposition of lipids in plaques.369 Furthermore, lncRNAs are differentially expressed in patients with non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI) through the HIF-1α signaling pathway, which might become a serological marker to distinguish between NSTEMI and STEMI.370 Previous studies have shown that HIF-1α and HIF-2α are increased in atherosclerosis, and lesions aggravate with the increase in HIF.364 Moreover, in a high-fat diet mice model, the selective deficiency of HIF-1α in endothelial cells relieved the lesion formation in 6 weeks.371 In apolipoprotein E knockout mice (ApoE−/−) mice, reduced HIF expression decreased VEGF activity and intimal hyperplasia.372. Furthermore, the deletion of Hif-1α gene in ApoE−/− mice reduced the atherosclerotic lesions, inflammation, and the level of chemokines by upregulating miRNA-19a.371 Folco et al. demonstrated that when exposed to hypoxia, human macrophages and foam cells had increased glucose uptake, especially in macrophage-rich regions of the plaques.373 The studies showed various regulations of atherosclerosis by HIF in different types of cells, although the underlying mechanism needs to be further investigated.
Hypoxia in pulmonary hypertension (PH)
Pulmonary hypertension (PH) is characterized by hypoxia-induced pulmonary vessel contraction, vascular remodeling, and increased pulmonary circulation resistance, which results in elevated pulmonary artery pressure.374 Subsequently, the disrupted pulmonary artery endothelial cells (PAECs) produce substances that induce smooth muscle cell proliferation, resulting in neointima development and increased arterial thickening in PH. Compared to healthy controls, proliferating PAECs generate more vasoconstrictors while producing less nitric oxide (NO) and prostacyclin.375 However, the underlying mechanism is yet unknown. Reportedly, HIF is associated with the pathophysiology of PH. Both heterozygous HIF-1-deficient and HIF-2-deficient mice are protected from chronic hypoxia-induced PH.376,377 The occurrence and development of PH are influenced by inducible nitric oxide synthase (iNOS) and ET-1.378,379 HIF-1 activates and boosts the expression of iNOS and ET-1 under hypoxia,380,381 which might underlie the mechanism of PH.
One of the primary enzymes involved in endothelial cell (EC) proliferation and pulmonary dilation of blood vessels is arachidonate 5-lipoxygenase (ALOX5).382 When human PAECs are exposed to hypoxia, ALOX5 pathway is activated, increasing H2O2 generation and contributing to H2O2-dependent EC proliferation.382 Furthermore, Su et al. found that ALOX5 promoter harbors the potential binding sites for early growth response protein 1 (EGR1) and SP1; both act as coregulators of erythropoietin receptor expression in LC cells in collaboration with HIF.383 Moreover, glucose absorption in idiopathic PAH (IPAH) patients’ lungs and the ECs is dramatically elevated with the decrease in mitochondrial concentration in EC and the increase of EC proliferation,384,385,386 while knockdown of glycolytic regulator PFKFB3 protects the mice against hypoxia-induced PH.384 Consequently, HIF in ECs’ physiology might play a role in PH formation. Notably, the mutual regulation of CD146 and HIF-1α is a key factor in the pathological mechanism of vascular reconstruction, remodeling, and PH formation.387 In addition, CD146 and HIF-1α promote each other’s expression and accelerate vascular remodeling and PH formation.387 Therefore, the regulation of HIF expression might be a potential target for the treatment of PH.
Hypoxia in cardiomyopathy
Cardiomyopathy is a category of disorders that produces anatomical and functional problems in the heart. It is classified as primary or secondary, with diverse phenotypes, such as dilated, hypertrophic, or restricted.388 However, the prevalence and progression of cardiomyopathy are not well understood. Chen et al. demonstrated that HIF-1α and FoxO3a collectively contribute to increased expression of the death factor BNIP3 and promote cardiac cell apoptosis in response to a combined stimulation of high glucose plus hypoxia.389 Hypoxia-induced mitogenic factor (HIMF) overexpression increases HIF-1α in neonatal rat cardiomyocytes, confirming the role of HIMF in myocardial hypertrophy. Thus, the deletion of HIF-1α reduces cardiomyocyte hypertrophy produced by HIMF and suppresses myocardial hypertrophy, making it a potential target for myocardial hypertrophy therapy.390 Reportedly, HIF-1α and PPAR are major regulators of glycolysis and lipid anabolism; the expression of these molecules is increased in hypertrophic cardiomyopathy. Also, these molecules jointly regulate and participate in the changes in cardiac metabolism, whereas HIF-1 accumulation is limited to pathological cardiac hypertrophy, but not physiological hypertrophy, in humans and mice.72 Some studies demonstrated that long-term intermittent hypoxia (IH) exposure causes continual activation of HIF-1α, which is responsible for the rise in infarct size.391,392 However, sustained heart-specific HIF-1α overexpression is beneficial in mice in the short term, causing cardiac insufficiency with age.393 An increased HIF-1α expression is detected in cardiac samples from cardiomyopathy patients, but a high level of plasma HIF-1α in patients with decompensated heart failure is related to low ejection fraction and survival.393,394,395 Taken together, the current study focuses on HIF-1α in primary cardiomyopathy, which demonstrates that HIF-1α has negative consequences, but its role and mechanism in secondary cardiomyopathy require further exploration.
Hypoxia in arrhythmia
Arrhythmia is an irregular frequency and/or rhythm of heartbeat ascribed to the origin and/or conduction problem of cardiac activity. It comprises a significant category of cardiovascular disorders that can occur alone or in conjunction with other cardiovascular diseases. Atrial fibrillation (AF) is one of the most frequent forms of human arrhythmias, with a significant disability and fatality rate in patients.393,396,397 The etiology of AF is linked to MMP-9; the increased activity of MMP-9 causes atrial fibrosis and induces AF.398 Another study demonstrated that HIF-1α stimulates the downstream factor TGF-β1 by promoting the expression of angiotensin II (Ang II), which causes high expression of MMP-9.399 Conversely, the levels of TGF-β1 and MMP-9 are lowered by inhibited HIF-1α expression, reducing the degree of atrial fibrosis.399 Ogi et al. reported a high HIF-1α level in AF patients. The study also postulated that the subsequent structural remodeling is caused by cardiac hypoxia.400 HIF-1 has been observed in peri-left atrial adipose and linked to fibrotic remodeling, which creates a substrate for AF.401 Xu et al. discovered that patients with permanent or persistent AF had higher levels of HIF-1α expression in the left atrial biopsies compared to patients with paroxysmal AF or patients in sinus rhythm from left atrial samples, implying a significant role of the protein in structural remodeling that supports AF initiation and propagation.402 Also, an increasing number of target genes have been discovered to play a role in various physiological and pathological processes in HIF-mediated AF.403,404
Hypoxia in congenital heart disease (CHD)
CHD is the most common type of congenital deformity, classified into three types based on hemodynamics: no shunt, left to right shunt, and right to left shunt.405,406,407 Patients with cyanotic CHD (CCHD) might have a hypoxic response, which leads to abnormalities in endothelial function, vascular remodeling, and thrombosis after emergency surgery.408 Prolyl-4-hydroxylase2 (PHDP2)/HIF-1α pathway is the key regulator under hypoxia. PHD2 activates HIF-1α oxygen-dependent hydroxylation of the internal oxygen-dependent degradation domain in a normoxic environment. However, this hydroxylation is inhibited during hypoxia, resulting in HIF-1α accumulation and vascular remodeling.409 Thus, it has been demonstrated that Egl-9 family hypoxia-inducible factor 1 (EGLN1) mutation decreases the hypoxic response of CCHD via the PHD2/HIF-1 pathway, which might be a viable target for CCHD therapy.410 Liu et al. discovered that Cited2 functional loss causes abnormalities in the heart and neural tube development, partially due to the regulation of HIF-1α transcriptional activity in the absence of Cited2,411 emphasizing its significant role in the development of CHD.
Neurodegenerative diseases
Neurodegenerative disorders are characterized by the gradual death of susceptible groups of neurons; the frequency of this incidence increases rapidly with age.412 Three major neurodegenerative disorders are Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). Here, we discuss the function of hypoxia in neurodegenerative disorders.
AD is a serious neurodegenerative disease with a convoluted etiology and varying periods of onset, which is one of the most common neurodegenerative disorders.413 AD is distinguished by two key features: amyloid beta-peptide (Aβ) accumulation in the brain and the appearance of neurofibrillary tangles composed of hyperphosphorylated tau protein.414 Cerebral hypoxia is strongly related to AD, which is correlated to cardiovascular risk factors.415 Physical exercise lessens the incidence of AD, featured by functioning of the neurovascular unit.416,417,418 HIF-1α levels in the brain are lower in AD patients, which have been linked to increased phosphorylation of tau protein and production of neurofilament.419 Furthermore, the advancement of neurodegeneration is involved in an increase in the generation of ROS, contributing to decreased expression of genes essential for remaining nerve cell viability and synaptic transmission, especially the HIF-1 gene.414
Another common age-related neurodegenerative disease is PD, which affects the elderly and is characterized by the loss of dopaminergic neurons and α-synuclein’s Lewy bodies (LB) accumulation.420,421,422,423 Accumulating evidence confirmed that mitochondrial malfunction and oxidative stress participate in the etiology of PD.424 Furthermore, HIF-1 is required for differentiation and survival of dopaminergic neuron, and a reduction in its expression results in neuronal death throughout the progression of PD.425 The in vitro and in vivo PD models revealed that the activation of HIF-1 exerts protective effects in neurons via expression of EPO and VEGF genes.197,426,427 Neuroprotective neuropeptide orexin-A induces HIF-1α expression, consequently activating VEGF and EPO in in-vitro PD models. Thus, HIF-1-mediated downstream signaling has the potential for PD treatment. In addition, the regulation of HIF-1 signaling by the ubiquitin-dependent proteasome pathway or HIF-specific prolyl hydroxylases is also able to avoid the neurons injury from oxidative stress, thereby accelerating the progress of PD.401,428,429,430
Amyotrophic lateral sclerosis (ALS) is a chronic neuronal disease caused by the injury to motor neurons in the motor cortex, spinal cord, and sub-brainstem.431 ALS causes gradual muscular weakening and atrophy of the muscles of the limbs, trunk, chest, and abdomen, which affects movement, communication, swallowing, and breathing, leading to death 3–4 years after the initial diagnosis.432,433 The dysregulation of EPO and VEGF accompanied by vascular changes, and blood flow disorder contributes to the pathogenesis of ALS, resulting in the hypoxia of the tissue.434,435 Hypoxia in tissues increases ROS production, leading to cell death.436 Thus, the uncontrolled hypoxia pathway is responsible for motor neuron death in ALS.437 Nomura et al. demonstrated that HIF-1α expression is dynamic in different stages of ALS, indicating the participation of HIF-1α in ALS.438 Dysregulation of the anti-hypoxic pathway induced by impaired HIF-1α activation promotes the motor neuron decline in ALS.439,440 Similar to the role in PD, HIF-1α activation protects the neurons in ALS. In an ALS in vivo model, the induction of HIF-1α decreases hypoxia-caused damage, protecting the neurons, reducing the inflammatory response, and lessening motor neuron degeneration.438 Conversely, decreased HIF-1α expression induced by ONO-1301-MS increases motor neuron generation in the mice model of ALS.441 Nonetheless, these findings need to be investigated further with respect to HIF-1α in ALS.
Target therapeutics based on hypoxia
Oxygen balance ensures the normal progress of life activities. Hypoxia affects the expression of many genes with clinicopathological significance in various human diseases.442 HIF-1 is deemed as the core element in the hypoxia pathway. Based on the advance in human health and diseases involved in hypoxia, researchers have made a great effort to intervene in each step in the hypoxia signaling pathway upon the occurrence of diseases,443 to develop target therapeutics for hypoxia-associated diseases (Table 1). Next, we summarize the hypoxia-targeted therapeutics against major human diseases (Fig. 6).
Hypoxia-targeted therapeutics in cancer and tumor
In the tumor hypoxic microenvironment, HIF functions in many aspects, such as improvement of glucose metabolism and enhancement of VEGF expression for angiogenesis to help the cells adapt to hypoxia. Abnormally high levels of angiogenesis, inflammation, and anaerobic glycolysis promote tumorigenesis and cause neoplastic diseases in the body.444 The stably generated HIF activates the downstream target genes successively, triggering a series of tumor activities. Therefore, HIF is considered one of the therapeutic targets of tumors.445 However, it may have varied roles in different tumor types. For example, the EGLN/HIF axis contributes to tumorigenesis in RCC,446 but has an opposite effect in other types of cancer.447 Thus, elucidating the exact role of HIFs in different conditions in the hypoxia-targeted therapeutics against tumors is recommended.
ccRCC is one of the common kidney cancers. The occurrence of pVHL tumor suppressor inactivation is a major event in ccRCC.448 Inactivation of pVHL stabilizes HIF-1α and HIF-2α. Therefore, several studies have focused on anti-caking agents for HIF-2α. PT2399 is a small-molecule inhibitor that dissociates HIF-2 and inhibits tumorigenesis in 56% of its congeners in human ccRCC cells.449 Compared to untreated controls, the growth of orthotopic tumors treated with PT2399 is arrested and regressed in mice.450 Another HIF2α-specific antagonist, PT2385, also inhibited the expression of HIF-2α target genes in ccRCC cell lines and mouse xenografts tumor model.450 PT2385 demonstrated a favorable safety profile in phase I dose-escalation trial and established the recommended phase II dose (RP2D) of 800 mg twice daily in humans.451 However, some analyses showed that patients are not benefitted clinically from PT2399.452 Belzutifan (MK-6482), a second-generation HIF2α anti-nodal agent, is efficacious in RCC and lung RCC in clinical trials and was subsequently approved for the treatment of VHL-associated diseases in August 2021.453,454,455 Topotecan, a HIF-1α inhibitor,456 exhibits antitumor activity in both in vivo and in vitro assays.457 Thus, it can be used for the treatment of multiple types of cancer, such as SCLC and ovarian cancer.458,459 The obvious decline in tumor blood flow and permeability was observed in 7/10 patients treated with topotecan over one treatment cycle.460.
Bortezomib (PS-341) is a proteasome inhibitor that inhibits HIF-1α activity by inhibiting the recruitment of P300 coactivators.461 A phase II trial showed that Bortezomib is ineffective in metastatic colon cancer but alters tumor response to hypoxia.462 The in vivo experiments of xenograft-bearing mice showed that bortezomib strongly inhibits VEGF production by up to 90%. This effect could be attributed to a decrease in HIF-1 transcriptional activity during treatment.463 RO7070179 is another HIF-1α inhibitor, shown in phase Ib clinical trial to reduce HIF-1α mRNA level in patients with hepatocellular carcinoma, thereby indicating its potential clinical benefit.464 Oxaliplatin, an antitumor drug, was used for the treatment of advanced CRC and GC.465 Several clinical trials have been conducted on oxaliplatin in combination with other drugs.466,467,468 Some studies indicated that the induction of HIF-1α degradation enhances the efficacy of oxaliplatin in CRC therapy.469 In addition, regulating the ubiquitination of HIF-1 is another strategy. Deubiquitinases (DUBs) can remove the ubiquitination of substrates, and the modulation of DUBs has now been identified as a promising drug target.470 USP7, one of the DUB genes, induces tumors by stabilizing HIF-1α.471 However, USP7 inhibitors slowed the tumor development in Lewis LC mice.472
In addition to the regulation of HIFs, applying the hypoxic properties of the tumor microenvironment to enhance the specificity of drugs is another therapeutic strategy. This class of drugs has minimal or no activity normoxically but can undergo bioreduction hypoxically to produce metabolites, known as hypoxia-activated prodrugs (HAPs), that are toxic to the cells.473 Evofosfamide (TH-302) is a HAP,474 which reduces tumor growth in neuroendocrine prostate cancer (NEPC).475 Multiple trials have investigated the antitumor efficacy of TH-302 in combination with other treatments. Data from a phase II trial in advanced pancreatic cancer patients showed that the combination of gemcitabine plus TH-302 significantly improves the progression-free survival (3.6 months in the gemcitabine group vs. 5.6 months in the combination group) and tumor response (3.6 months in the gemcitabine group vs. 5.6 months in the combination group).476 In a transgenic mouse model of adenocarcinoma, the combination of hypoxia-targeted therapy and checkpoint blockade controls tumor progression more significantly than either approach alone.477 The clinical data from another phase II trial of joint use of TH-302 and doxorubicin in advanced soft tissue sarcoma indicated that the combination therapy was superior to other first-line treatments, and TH-302 did not exhibit any hepatic, renal, or cardiac toxicity.478 Nonetheless, phase III data showed that compared to doxorubicin alone, the addition of TH-302 failed to improve the overall survival.479
The ErbB receptor tyrosine kinase family members are considered oncogenes in various cancers.480 Tarloxotinib is also a HAP that effectuates by inhibiting the activation of four members of the ErbB family. Also, it inhibits signaling and cell proliferation in patient-derived cancer cells in vitro and tumor growth in multiple mouse patient-derived xenograft models.481 Importantly, compared to 190 μmol/h/kg to the skin, the total tumor exposure to the metabolite tarloxotinib was 595 μmol/h/kg, indicating the specificity of this drug targeting tumor tissue. However, cancer patients receiving EGFR-targeted HAP therapy eventually develop drug resistance, including pancreatic or metastatic LC.482 Thus, these issues on drug resistance require further exploration.
Hypoxia-targeted therapeutics in cardiovascular diseases
Stabilization of HIF-1α is a prerequisite for normal cardiac development.483 During the disease process, the expression of HIF-1α may be disturbed or inhibited, thereby triggering cardiac dysfunction.484 Ischemic preconditioning and reperfusion are common cardioprotective strategies.485 Also, the modulation of HIF-1α expression with drugs is one of the therapeutic directions, facilitating hydroxylate of HIF-1α and ubiquitin-dependent degradation.486
Molidustat stabilizes HIF-1α and its downstream target genes in T2D cardiomyocytes. In T2D rats, oral administration of molidustat increases the body’s HIF targets and improves the recovery of ischemia-reperfusion by 27%.487 It also reduces fatty acid metabolism in the heart, which is shown as a 70% reduction in myocardial triglycerides.487 Several studies have assessed molidustat for the therapy of chronic kidney disease and anemia.488,489,490 Thus, its potential in the treatment of cardiovascular diseases may be investigated in future studies.
Raynaud’s syndrome is characterized by vasospasm that restricts blood flow leading to hypoxia, with markedly elevated levels of HIF-1α in both monocytes and serum. The combination of prostaglandin E1 (PgE1) and the endothelin-1 blocker bosentan can prevent its increase but not PgE1 administration alone.491 Data from previous studies suggested that PgE1 stimulates neovascularization by upregulating VEGF in patients with ischemic heart disease.492. PgE1 is a pulmonary vasodilator that needs to be evaluated in neonatal hypoxic respiratory failure.493
In addition to removing factors that interfere with HIF-1α expression, exogenous administration of HIF-1α may also achieve therapeutic purposes. A study showed that exosomes (Exo) modified with HIF-1α enhance the proliferation of human umbilical vein endothelial cells injured by hypoxia preconditioning.494. Exo-HIF-1α significantly reduced left ventricular fibrosis area ratio and inner peripheral fibrosis length compared to the Exo group with upregulated pro-angiogenic factors.
Hypoxia-targeted therapeutics in metabolic diseases
Diabetes
HIF-1α plays a vital role in metabolic diseases in tissues or organs.149 Diabetes is one of the most common metabolic diseases, and 90–95% of adults with diabetes worldwide have T2D.495 The regulation of HIF-1α in β-cell reserve and aryl hydrocarbon receptor nuclear translocator expression in islets. When HIF-1α in β cells was disrupted, mice exhibited glucose intolerance and β-cell abnormality; these conditions were improved when HIF-1α levels were restored, suggesting that HIF-1α is a T2D β-cell potential therapeutic target for functional disorders.496 Li et al. reported a HIF-1α stabilizer 1a that induces the activation and accumulation of HIF-1α and its driving genes in a diabetic mouse model.497 Intrarenal hypoxia is detected in diabetic patients, and HIF-1 regulates the occurrence of tubulointerstitial fibrosis. Sodium-glucose cotransporter 2 (SGLT2) inhibitor protects the kidney by inhibiting HIF-1α expression.498 Luseogliflozin, an SGLT2 inhibitor, relieves renal tubular damage and interstitial fibronectin in diabetic mice by inhibiting HIF-1α accumulation that reduces mitochondrial oxygen consumption.499 The treatment with luseogliflozin in mice with inhibited insulin and IGF-1 target receptors showed improved β-cell proliferation and hyperglycemia, but not hyperinsulinemia.500 Empagliflozin is a highly selective SGLT2 inhibitor and well-tolerated in humans.501,502 In T2D patients, the addition of empagliflozin to the standard of care reduces the progression of kidney disease compared to placebo (12.7% of the empagliflozin group vs. 18.8% of the placebo group). Strikingly, renal replacement therapy was initiated in 0.3% of patients receiving empagliflozin, compared to twice as high in the control group.503 Notably, the oxidative stress involved in insulin resistance needs to be considered.504
Chronic kidney disease (CKD)
Erythropoiesis-stimulating agents (ESAs) and prolyl hydroxylase inhibitors (PHIs) are commonly used to treat CKD. However, statistical analysis demonstrated that long-term ESA use might increase the risk of death.505 Therefore, lower doses should be used whenever possible in CKD patients with cancer receiving ESA.506 Unlike ESA requiring injection, PHI is a class of oral medications that reduce the cost and risks of treatment for patients.507 Well-studied PHIs contain vadadustat, roxadustat, and daprodustat. PHIs stabilize HIF and stimulate EPO and erythropoiesis. In a phase III trial, vardarestat was compared to darbepoetin alfa in ESA. The pooled analysis showed that the hazard ratio for major adverse cardiovascular events was 1.17, which did not meet the prespecified non-inferiority of 1.25 but achieved the prespecified non-inferiority for hematologic efficacy.508 Roxadustat has been authorized for China in dialysis-dependent CKD anemia patients’ treatment. A phase II trial showed hemoglobin levels increased by 1.9 ± 1.2 g/dL in patients with CKD in the roxadustat group compared to the baseline mean and a slight decrease in the placebo group.509 The level of total cholesterol was lower in the roxadustat group than that in the placebo group.510 However, patients receiving roxadustat were likely to develop hyperkalemia or metabolic acidosis. A phase III trial in CKD patients with anemia showed that roxadustat had a slightly higher (almost the same) incidence of adverse events than the placebo group, whereas roxadustat significantly reduced the risk of red blood cell transfusion.511 Another phase III trial showed that roxadustat was non-inferior to darbepoetin alfa in maintaining hemoglobin.512 Daprodustat was also non-inferior to darbepoetin alfa in terms of hazard ratios for adverse events and maintenance of hemoglobin levels in anemic patients with or without dialysis.513,514 In the above events, the data from clinical trials of PHIs display the comparative efficacy of ESA.
Hypoxia-targeted therapeutics in infectious diseases
Respiratory system infection
In the most common infectious respiratory diseases caused by influenza virus and coronavirus infection, oxygen tension is considered a non-negligible factor in viral replication.230 As mentioned, HIF-1α facilitates SARS-CoV-2 replication and amplifies inflammatory response,146,147 suggesting that HIF regulation is a promising therapeutic target. However, the roles of HIF vary at different stages of the viral infection in COVID-19 patients. The evidence has shown that the SARS-CoV-2 receptor ACE2 can be reduced by roxadustat through a HIF-1α-dependent pathway, which inhibits virus entry and replication.515 HIF-1α, on the other side, can boost the activity of Cathepsin L which can cleave S protein. Early use of PHD may aid viral replication.516 And it may also participate in the cytokine storm generated by SARS-CoV-2 through its stimulating influence on the expression of macrophage migration inhibitory factor (MIF).517 It is reported that dexamethasone can break the link between HIF and MIF.518 The expression of HIF-1α associated with macrophage inflammation in COVID-19 patients is elevated.519 Upon viral infection, SARS-CoV-2 damages the mitochondria and triggers ROS production, thereby inducing HIF-1α, promoting viral replication, and aggravating the inflammatory response.146,147 In conclusion, HIF-1α may have opposite effects on various aspects of virus invasion activities, and it is necessary to carefully evaluate the measures that need or can be taken according to the conditions of patients.
The studies in influenza A virus (IAV)-infected mice showed that after knockout of HIF-1α in lung epithelial cells, the mice exhibited severe lung inflammation.520 Tissue macrophages produce inflammatory mediators during pathogen infection, which is regulated by β-catenin-HIF-1α signaling, and Wnt promotes the interaction between these two signaling molecules. Data from a mouse model of influenza virus pneumonia showed that β-catenin-mediated inflammation in macrophages increases acute host morbidity.519 Therefore, the role of HIF-1α in different tissues should be reconsidered when targeting HIF-1α therapeutically.
Digestive system infection
Hypoxic environment is not conducive to virus replication, but studies have found that HBV can use hypoxia signaling pathway to generate in hypoxic environment,521 Chronic stabilization of HIF exhibits deleterious effects on the body.522 As mentioned above, in liver cancer cells, the activity of HBV enh1 is enhanced.223 Therefore, in addition to using HIF inhibitors to reduce the expression of HIF, this specific activity can also be used to construct a specific expression system for targeted gene therapy.
Helicobacter pylori (H. pylori) is associated with a large number of gastrointestinal diseases,523 and is known as one of the leading factors affecting the development of GC.524,525 Therefore, the treatment of H. pylori is crucial for preventing GC. H. pylori infection may trigger duodenal ulcers, a type of peptic ulcer that is more common than gastric ulcers. Some studies demonstrated that in the process of duodenal ulcer, ischemia induces HIF-1α expression and angiogenesis factors production including VEGF.526 Clinical trials demonstrated H. pylori eradication for the treatment of H. pylori-associated duodenal ulcers.527 Reportedly, H. pylori infection increases the expression of HIF-1α.528 Consequently, the hypoxia signaling pathway may be one of the targets of treatment and illuminates the research on the treatment of other diseases caused by H. pylori. Therapies targeting the hypoxic pathway may be useful in the treatment of pathogens infections of the digestive system.
Nervous system infection
Hypoxia takes part in the pathogenesis of many neurological diseases.235 Meningitis, encephalitis, and even Alzheimer’s disease is a group of diseases caused by infection or autoimmunity.529 Among most cases of viral infection, enterovirus is the main agent,530 and mumps, lymphocytic choriomeningitis, and type I and II scab viruses are also common pathogens.531 Enterovirus 71 (EV71) is a common enterovirus that causes neurological diseases in severe cases, and hypoxia may be one of the participants in the neuropathogenesis of EV71.532 In a consistent study, the constructed immunocompetent or immunodeficient mouse models have white plaques in the muscles after infection with EV71, which are related to hypoxia.533 Progressive multifocal leukoencephalopathy (PML) is a kind of organic brain disease caused by Polyomavirus JC (JCV). HIF-1α activates the JCV virus promoter, implying a cure for the occurrence of PML.534
Furthermore, neurological diseases are also caused by bacterial infection. For example, S. pneumoniae infection can cause fatal bacterial meningitis.535 HIF-1α inhibitor echinomycin can improve blood-brain barrier function and increase the survival in S. pneumoniae-infected mice.536 In neuroinfection events, the investigation of the role of HIF-1α might help to understand the neuropathogenesis and develop treatment options.
Prospects in therapeutics of hypoxia-associated diseases
Hypoxia signaling participates in events of cellular viability and activity to respond to oxygen deprivation. HIF-1 is the central regulator modulated from upstream signals or stimuli and induces downstream gene transcription, which has been implicated in several human diseases. Owing to its control of various diseases, HIF-1 (mainly HIF-1α and HIF-1β) is preferred in the development of targeted therapy. Several strategies are available for therapeutics against hypoxia-associated diseases (Fig. 7). (1) Alteration of HIF-1 transcription by the upstream signals or stimuli; (2) Regulation of HIF-1 stability via interfering protein modification, such as deSUMOylation and deubiquitination; (3) Control of HIF-1 function by disturbing related enzyme activity in the complex. The above strategies are attributed to HIF intervention either directly (the expression and activity) or indirectly (co-activator and repressor). Therefore, the scope of drug screening or repurposing in the development of therapeutics against hypoxia-associated diseases has been clarified.
Hypoxia is the status of the microenvironment in the body.537 HIF-1 regulates various target genes in corresponding diseases. Typically, the hypoxia-targeted therapies are discrepant and may also have opposite effects in the treatment of various diseases. As a result, the effect of hypoxia-targeted therapy interventions on spatiotemporal behaviors in diseases is yet to be investigated. One possible way to improve the efficiency of hypoxia-targeted therapy could be the combination of specific drugs against the diseases. The advantages of this approach are improving the drug effects and eliminating drug resistance. Another aspect may be the modification of the drug and the design of the delivery system, thereby increasing effective hypoxia-targeted therapies. For example, PEGylated biopharmaceuticals are used to improve the physicochemical properties and biological responses of a drug. The use of exosomes as the drug-delivery system would reduce immunogenicity as the therapeutic tool for hypoxia-associated diseases.
The tissue- or disease-specificity of targeted therapy must be considered since improper regulation of HIF or its downstream genes in normal tissues may have harmful consequences in cells or tissues. Agent-targeted therapy does not appear to be very selective, as the same drug can affect multiple organs. Roxadustat, for example, is primarily used to treat anemia in individuals with renal illness, but it has also been proven to affect hepatic lipolysis.538,539 As a result, the danger that other non-diseased tissues may carry during administration should be thoroughly assessed. Even in the same tissue, HIF may play conflicting roles in various disease processes. Stable HIF expression, for example, protects against acute lung injury during hypoxia and promotes pulmonary hypertension development.522 The hypoxic prodrug, on the other hand, has a somewhat higher specificity because its active form requires a certain oxygen concentration to activate. Gene therapy can be also highly tissue-specific. Hypoxia-specific expression system540 constructs an oxygen concentration-dependent gene expression vector541 by inserting the hypoxia response element HRE from different hypoxia-inducible genes into the upstream of the SV40 minimal promoter. This is especially important for solid tumors.
The hypoxic environment is a factor impacting the efficiency of several tumor treatment techniques, yet this characteristic environment is currently being utilized for tumor-targeted therapy. The plasmid will be highly expressed selectively in specific hypoxic locations in this way. Leaky expression is a serious issue that requires immediate care.542 To improve tissue specificity, a promoter that is active exclusively in a certain tissue or place, such as human tumor cells, can be added to the expression system. For instance, the survivin promoter is the sole one,87,543 which could increase the target selectivity of some HIF-1 oxygen-independent cancer therapies.
Among several HIFs, HIF-1α is the primary option to develop target drugs in hypoxia-associated diseases. Most developed drugs in clinical trials are designed on the basis of the direct and indirect regulation of HIF-1α (Table 2). Fortunately, emerging agents targeting HIF-2α are promising anti-tumor therapeutics, providing alternative candidates for hypoxia-targeted drugs when all HIFs beyond HIF-1α are taken into consideration. Finally, with the concerted help of updated basic research on hypoxia-related diseases and advances in multidisciplinary fields, such as structural biology, medicine, chemistry, and pharmacy, therapeutics against hypoxia-associated diseases have novel avenues.
References
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020).
Nakazawa, M. S., Keith, B. & Simon, M. C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673 (2016).
Ratcliffe, P. J. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J. Physiol. 591, 2027–2042 (2013).
Bruick, R. K. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 17, 2614–2623 (2003).
Harris, A. L. Hypoxia-a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 (2002).
Tekin, D., Dursun, A. D. & Xi, L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharm. Sin. 31, 1085–1094 (2010).
Giaccia, A., Siim, B. G. & Johnson, R. S. HIF-1 as a target for drug development. Nat. Rev. Drug Disco. 2, 803–811 (2003).
Semenza, G. L., Nejfelt, M. K., Chi, S. M. & Antonarakis, S. E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. Proc. Natl Acad. Sci. USA 88, 5680–5684 (1991).
Maxwell, P. H., Pugh, C. W. & Ratcliffe, P. J. Inducible operation of the erythropoietin 3’ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc. Natl Acad. Sci. USA 90, 2423–2427 (1993).
Firth, J. D., Ebert, B. L., Pugh, C. W. & Ratcliffe, P. J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3’ enhancer. Proc. Natl Acad. Sci. USA 91, 6496–6500 (1994).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Wang, G. L. & Semenza, G. L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 (1995).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 16, 4604–4613 (1996).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12, 149–162 (1998).
Kibel, A., Iliopoulos, O., DeCaprio, J. A. & Kaelin, W. G. Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science 269, 1444–1446 (1995).
Iliopoulos, O. et al. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc. Natl Acad. Sci. USA 93, 10595–10599 (1996).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Ivan, M. et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Ivan, M. et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl Acad. Sci. USA 99, 13459–13464 (2002).
Ledford, H. & Callaway, E. Biologists who decoded how cells sense oxygen win medicine Nobel. Nature 574, 161–162 (2019).
Wiesener, M. S. et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 17, 271–273 (2003).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Zhe, N. et al. HIF-1alpha inhibition by 2-methoxyestradiol induces cell death via activation of the mitochondrial apoptotic pathway in acute myeloid leukemia. Cancer Biol. Ther. 17, 625–634 (2016).
Kaelin, W. G. Jr. & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Markolovic, S., Wilkins, S. E. & Schofield, C. J. Protein hydroxylation catalyzed by 2-oxoglutarate-dependent oxygenases. J. Biol. Chem. 290, 20712–20722 (2015).
Salceda, S. & Caro, J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272, 22642–22647 (1997).
Wu, D. et al. Structural integration in hypoxia-inducible factors. Nature 524, 303–308 (2015).
Bao, X. et al. The crosstalk between HIFs and mitochondrial dysfunctions in cancer development. Cell Death Dis. 12, 215 (2021).
Barth, D. A. et al. Long-noncoding RNA (lncRNA) in the regulation of hypoxia-inducible factor (HIF) in cancer. Noncoding RNA 6, 27 (2020).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell Biol. 22, 7004–7014 (2002).
Duvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 (2010).
Brugarolas, J. B. et al. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147–158 (2003).
Dodd, K. M. et al. mTORC1 drives HIF-1alpha and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 34, 2239–2250 (2015).
Chisolm, D. A. & Weinmann, A. S. TCR-signaling events in cellular metabolism and specialization. Front. Immunol 6, 292 (2015).
Niecknig, H. et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic. Res 46, 705–717 (2012).
Mansfield, K. D. et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 1, 393–399 (2005).
LaGory, E. L. & Giaccia, A. J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 18, 356–365 (2016).
Rius, J. et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811 (2008).
Malekan, M., Ebrahimzadeh, M. A. & Sheida, F. The role of Hypoxia-Inducible Factor-1alpha and its signaling in melanoma. Biomed. Pharmacother. 141, 111873 (2021).
Wan, J. & Wu, W. Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways. J. Exp. Clin. Cancer Res. 35, 119 (2016).
Lamberti, M. J. et al. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy. PLoS One 12, e0177801 (2017).
Sang, N. et al. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J. Biol. Chem. 278, 14013–14019 (2003).
Lau, M. T., Klausen, C. & Leung, P. C. E-cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via beta-catenin-Egr1-mediated PTEN expression. Oncogene 30, 2753–2766 (2011).
Liu, H. L. et al. Hypoxia-inducible factor-1alpha and Wnt/beta-catenin signaling pathways promote the invasion of hypoxic gastric cancer cells. Mol. Med. Rep. 12, 3365–3373 (2015).
Liu, X. et al. HIF-1-regulated expression of calreticulin promotes breast tumorigenesis and progression through Wnt/beta-catenin pathway activation. Proc. Natl Acad. Sci. USA 118, e2109144118 (2021).
Li, Y. et al. Expression of Notch-Hif-1alpha signaling pathway in liver regeneration of rats. J. Int. Med. Res. 48, 300060520943790 (2020).
Chen, Y. et al. HIF-1-VEGF-Notch mediates angiogenesis in temporomandibular joint osteoarthritis. Am. J. Transl. Res 11, 2969–2982 (2019).
De Francesco, E. M., Maggiolini, M. & Musti, A. M. Crosstalk between Notch, HIF-1alpha and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 19, 2011 (2018).
Li, L. C., Zhang, M., Feng, Y. K. & Wang, X. J. IDH1-R132H suppresses glioblastoma malignancy through FAT1-ROS-HIF-1alpha signaling. Neurol. India 68, 1050–1058 (2020).
Yang, S. et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 16, 687–693 (2010).
Wang, P. et al. Icariin inhibits the inflammation through down-regulating NF-kappaB/HIF-2alpha signal pathways in chondrocytes. Biosci. Rep. 40, BSR20203107 (2020).
van Uden, P. et al. Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS Genet. 7, e1001285 (2011).
Chen, X. & Cubillos-Ruiz, J. R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 21, 71–88 (2021).
Marciniak, S. J., Chambers, J. E. & Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Disco. 21, 115–140 (2022).
Delbrel, E. et al. HIF-1alpha triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci. Rep. 8, 17939 (2018).
Karali, E. et al. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol. Cell 54, 559–572 (2014).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004).
Akman, M. et al. Hypoxia, endoplasmic reticulum stress and chemoresistance: dangerous liaisons. J. Exp. Clin. Cancer Res. 40, 28 (2021).
Mennerich, D. et al. ER-stress promotes VHL-independent degradation of hypoxia-inducible factors via FBXW1A/betaTrCP. Redox Biol. 50, 102243 (2022).
Manalo, D. J. et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105, 659–669 (2005).
Maxwell, P. H. & Ratcliffe, P. J. Oxygen sensors and angiogenesis. Semin Cell Dev. Biol. 13, 29–37 (2002).
Semenza, G. L., Shimoda, L. A. & Prabhakar, N. R. Regulation of gene expression by HIF-1. Novartis Found. Symp. 272, 2–8 (2006).
Palmer, L. A., Semenza, G. L., Stoler, M. H. & Johns, R. A. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am. J. Physiol. 274, L212–L219 (1998).
Lal, A. et al. Transcriptional response to hypoxia in human tumors. J. Natl Cancer Inst. 93, 1337–1343 (2001).
Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 9(Suppl 5), 10–17 (2004).
Shay, J. E. & Celeste Simon, M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin. Cell Dev. Biol. 23, 389–394 (2012).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Krishnan, J. et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 9, 512–524 (2009).
Li, Q., Kluz, T., Sun, H. & Costa, M. Mechanisms of c-myc degradation by nickel compounds and hypoxia. PLoS One 4, e8531 (2009).
Shi, Y. H. & Fang, W. G. Hypoxia-inducible factor-1 in tumour angiogenesis. World J. Gastroenterol. 10, 1082–1087 (2004).
Zagzag, D. et al. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 88, 2606–2618 (2000).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
To, K. K. et al. The phosphorylation status of PAS-B distinguishes HIF-1alpha from HIF-2alpha in NBS1 repression. EMBO J. 25, 4784–4794 (2006).
Florczyk, U. et al. Opposite effects of HIF-1alpha and HIF-2alpha on the regulation of IL-8 expression in endothelial cells. Free Radic. Biol. Med 51, 1882–1892 (2011).
Moeller, B. J. et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 8, 99–110 (2005).
Ito, K. et al. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell 14, 226–237 (2008).
Xue, G. et al. c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2alpha and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene 34, 1393–1406 (2015).
Das, B. et al. MYC regulates the HIF2alpha stemness pathway via nanog and Sox2 to maintain self-renewal in cancer stem cells versus non-stem cancer cells. Cancer Res. 79, 4015–4025 (2019).
Ziello, J. E., Jovin, I. S. & Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 80, 51–60 (2007).
Genbacev, O., Zhou, Y., Ludlow, J. W. & Fisher, S. J. Regulation of human placental development by oxygen tension. Science 277, 1669–1672 (1997).
Richard, D. E., Berra, E. & Pouyssegur, J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J. Biol. Chem. 275, 26765–26771 (2000).
Daskalaki, I., Gkikas, I. & Tavernarakis, N. Hypoxia and selective autophagy in cancer development and therapy. Front. Cell Dev. Biol. 6, 104 (2018).
Masoud, G. N. & Li, W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 5, 378–389 (2015).
Gui, L., Liu, B. & Lv, G. Hypoxia induces autophagy in cardiomyocytes via a hypoxia-inducible factor 1-dependent mechanism. Exp. Ther. Med 11, 2233–2239 (2016).
Abdul Rahim, S. A. et al. Regulation of hypoxia-induced autophagy in glioblastoma involves ATG9A. Br. J. Cancer 117, 813–825 (2017).
Zhou, J. et al. Hypoxia-inducible factor-1alpha-dependent autophagy plays a role in glycolysis switch in mouse granulosa cells. Biol. Reprod. 99, 308–318 (2018).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Denko, N. C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 8, 705–713 (2008).
Qian, X. et al. Phosphoglycerate kinase 1 phosphorylates beclin1 to induce autophagy. Mol. Cell 65, 917–931 e916 (2017).
Qian, X., Li, X. & Lu, Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy 13, 1246–1247 (2017).
Horigome, C. et al. SWR1 and INO80 chromatin remodelers contribute to DNA double-strand break perinuclear anchorage site choice. Mol. Cell 55, 626–639 (2014).
Yang, Z. et al. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med 210, 2119–2134 (2013).
Shi, L. et al. Roles of PFKFB3 in cancer. Signal Transduct. Target Ther. 2, 17044 (2017).
Klarer, A. C. et al. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metab. 2, 2 (2014).
Qin, L. et al. Targeting PDK1 with dichloroacetophenone to inhibit acute myeloid leukemia (AML) cell growth. Oncotarget 7, 1395–1407 (2016).
Chae, Y. C. et al. Mitochondrial Akt regulation of hypoxic tumor reprogramming. Cancer Cell 30, 257–272 (2016).
Roberts, D. J. et al. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol. Cell 53, 521–533 (2014).
Di Conza, G. et al. The mTOR and PP2A pathways regulate PHD2 phosphorylation to fine-tune HIF1alpha levels and colorectal cancer cell survival under. Hypoxia. Cell Rep. 18, 1699–1712 (2017).
Nagata, S. & Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 17, 333–340 (2017).
Lockshin, R. A. & Zakeri, Z. Programmed cell death and apoptosis: origins of the theory. Nat. Rev. Mol. Cell Biol. 2, 545–550 (2001).
Moujalled, D., Strasser, A. & Liddell, J. R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 28, 2029–2044 (2021).
Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007).
Pistritto, G. et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 8, 603–619 (2016).
Wang, J. Y. et al. Dictamnine promotes apoptosis and inhibits epithelial-mesenchymal transition, migration, invasion and proliferation by downregulating the HIF-1alpha and Slug signaling pathways. Chem. Biol. Interact. 296, 134–144 (2018).
Fu, Z. J. et al. HIF-1alpha-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 36, 101671 (2020).
Piret, J. P., Mottet, D., Raes, M. & Michiels, C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann. N. Y Acad. Sci. 973, 443–447 (2002).
Jiang, L. et al. MicroRNA-519d-3p inhibits proliferation and promotes apoptosis by targeting HIF-2alpha in cervical cancer under hypoxic conditions. Oncol. Res. 26, 1055–1062 (2018).
Carmeliet, P. et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Bruick, R. K. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc. Natl Acad. Sci. USA 97, 9082–9087 (2000).
Sowter, H. M. et al. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 61, 6669–6673 (2001).
Levine, A. J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 20, 471–480 (2020).
An, W. G. et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 392, 405–408 (1998).
Suzuki, H., Tomida, A. & Tsuruo, T. Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene 20, 5779–5788 (2001).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Yu, P. et al. Pyroptosis: mechanisms and diseases. Signal Transduct. Target Ther. 6, 128 (2021).
Zhou, Z. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Kovacs, S. B. & Miao, E. A. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 27, 673–684 (2017).
Hou, J. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1264–1275 (2020).
Qiu, Z. et al. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J. Diabetes Res 2019, 8151836 (2019).
Yu, L. M. et al. Hypoxia-induced ROS contribute to myoblast pyroptosis during obstructive sleep apnea via the NF-kappaB/HIF-1alpha signaling pathway. Oxid. Med. Cell Longev. 2019, 4596368 (2019).
Shi, H. et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ. Res 129, 383–396 (2021).
Hong, Z. et al. The ROS/GRK2/HIF-1alpha/NLRP3 pathway mediates pyroptosis of fibroblast-like synoviocytes and the regulation of monomer derivatives of paeoniflorin. Oxid. Med Cell Longev. 2022, 4566851 (2022).
Yuan, D. et al. HIF-1alpha aggravated traumatic brain injury by NLRP3 inflammasome-mediated pyroptosis and activation of microglia. J. Chem. Neuroanat. 116, 101994 (2021).
Yang, K. et al. Hypoxia and Porphyromonas gingivalis-lipopolysaccharide synergistically induce NLRP3 inflammasome activation in human gingival fibroblasts. Int Immunopharmacol. 94, 107456 (2021).
Jiang, Q. et al. Hypoxia inducible factor-1alpha (HIF-1alpha) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience 448, 126–139 (2020).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Karshovska, E. et al. HIF-1alpha (Hypoxia-Inducible Factor-1alpha) promotes macrophage necroptosis by regulating miR-210 and miR-383. Arterioscler Thromb. Vasc. Biol. 40, 583–596 (2020).
Yang, X. S. et al. Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci. Rep. 7, 5818 (2017).
DeBerge, M. et al. Hypoxia-inducible factors individually facilitate inflammatory myeloid metabolism and inefficient cardiac repair. J. Exp. Med. 218, e20200667 (2021).
Xu, S. et al. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 12, 289 (2021).
Fan, Z. et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2-dependent silencing of SLC7A11. J. Cell Mol. Med 25, 10197–10212 (2021).
Yuan, S. et al. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1alpha/SLC7A11 pathway. Cell Prolif. 55, e13158 (2022).
Bai, Y. T. et al. Hypoxia protects H9c2 cells against Ferroptosis through SENP1-mediated protein DeSUMOylation. Int J. Med Sci. 18, 1618–1627 (2021).
Wu, Y. et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1alpha/HO-1 signaling pathway in mouse testes. J. Hazard Mater. 426, 127807 (2022).
Vivier, E. & Malissen, B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat. Immunol. 6, 17–21 (2005).
Taylor, C. T. & Colgan, S. P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 17, 774–785 (2017).
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Peng, T., Du, S. Y., Son, M. & Diamond, B. HIF-1alpha is a negative regulator of interferon regulatory factors: Implications for interferon production by hypoxic monocytes. Proc. Natl Acad. Sci. USA 118, e2106017118 (2021).
Codo, A. C. et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 32, 437–446 e435 (2020).
Tian, M. et al. HIF-1alpha promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct. Target Ther. 6, 308 (2021).
Cramer, T. et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
McGettrick, A. F. & O’Neill, L. A. J. The role of HIF in immunity and inflammation. Cell Metab. 32, 524–536 (2020).
Shi, L. Z. et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Dang, E. V. et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Palazon, A. et al. An HIF-1alpha/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32, 669–683 e665 (2017).
Burrows, N. & Maxwell, P. H. Hypoxia and B cells. Exp. Cell Res. 356, 197–203 (2017).
Kojima, H. et al. Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine B cell development in bone marrow. J. Immunol. 184, 154–163 (2010).
Meng, X. et al. Hypoxia-inducible factor-1alpha is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat. Commun. 9, 251 (2018).
Punthakee, Z., Goldenberg, R. & Katz, P. Diabetes Canada Clinical Practice Guidelines Expert, C. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 42(Suppl 1), S10–S15 (2018).
Fedeli, U. et al. Time series of diabetes attributable mortality from 2008 to 2017. J. Endocrinol. Invest. 45, 275–278 (2022).
Cole, J. B. & Florez, J. C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16, 377–390 (2020).
Cho, N. H. et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 138, 271–281 (2018).
American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1), S81–S90 (2014).
American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 36(Suppl 1), S67–S74 (2013).
Hex, N. et al. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet. Med. 29, 855–862 (2012).
Persson, P. & Palm, F. Hypoxia-inducible factor activation in diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 26, 345–350 (2017).
Sato, Y. et al. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J. Biol. Chem. 286, 12524–12532 (2011).
Botusan, I. R. et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl Acad. Sci. USA 105, 19426–19431 (2008).
Catrina, S. B. et al. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes 53, 3226–3232 (2004).
Gu, H. F. et al. Impact of the hypoxia-inducible factor-1 alpha (HIF1A) Pro582Ser polymorphism on diabetes nephropathy. Diabetes Care 36, 415–421 (2013).
Marfella, R. et al. Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes 53, 2383–2391 (2004).
Isoe, T. et al. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int. 78, 48–59 (2010).
Garcia-Pastor, C. et al. Mechanism and consequences of the impaired Hif-1alpha response to hypoxia in human proximal tubular HK-2 cells exposed to high glucose. Sci. Rep. 9, 15868 (2019).
Sugahara, M. et al. Prolyl hydroxylase domain inhibitor protects against metabolic disorders and associated kidney disease in obese type 2 diabetic mice. J. Am. Soc. Nephrol. 31, 560–577 (2020).
Duscher, D. et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 112, 94–99 (2015).
Zhu, Y. et al. Roxadustat promotes angiogenesis through HIF-1alpha/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regen. 27, 324–334 (2019).
Sousa Fialho, M. D. L., Abd Jamil, A. H., Stannard, G. A. & Heather, L. C. Hypoxia-inducible factor 1 signalling, metabolism and its therapeutic potential in cardiovascular disease. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 831–843 (2019).
Dodd, M. S. et al. Fatty acids prevent hypoxia-inducible factor-1alpha signaling through decreased succinate in diabetes. JACC Basic Transl. Sci. 3, 485–498 (2018).
Brunt, J. J. et al. Overexpression of HIF-2alpha in pancreatic beta cells does not alter glucose homeostasis. Islets 6, e1006075 (2014).
Taniguchi, C. M. et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 19, 1325–1330 (2013).
Wei, K. et al. A liver Hif-2alpha-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat. Med. 19, 1331–1337 (2013).
Diabetes Canada Clinical Practice Guidelines Expert, C., Yale, J. F., Paty, B. & Senior, P. A. Hypoglycemia. Can. J. Diabetes 42(Suppl 1), S104–S108 (2018).
Carraway, K. R. et al. Hypoxia and Hypoglycemia synergistically regulate mRNA stability. RNA Biol. 14, 938–951 (2017).
Bursch, W. et al. Cell death and autophagy: cytokines, drugs, and nutritional factors. Toxicology 254, 147–157 (2008).
Gao, W. et al. Glucose attenuates hypoxia-induced changes in endothelial cell growth by inhibiting HIF-1alpha expression. Diab. Vasc. Dis. Res. 11, 270–280 (2014).
Staab, A. et al. Modulation of glucose metabolism inhibits hypoxic accumulation of hypoxia-inducible factor-1alpha (HIF-1alpha). Strahlenther. Onkol. 183, 366–373 (2007).
Malhotra, R. et al. Glucose uptake and adenoviral mediated GLUT1 infection decrease hypoxia-induced HIF-1alpha levels in cardiac myocytes. J. Mol. Cell Cardiol. 34, 1063–1073 (2002).
Limberg, J. K. et al. Effect of hypoxia on heart rate variability and baroreflex sensitivity during hypoglycemia in type 1 diabetes mellitus. Clin. Auton. Res. 25, 243–250 (2015).
Miron, N. & Tirosh, O. Cholesterol prevents hypoxia-induced hypoglycemia by regulation of a metabolic ketogenic shift. Oxid. Med. Cell Longev. 2019, 5829357 (2019).
Zamudio, S. et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One 5, e8551 (2010).
Isaza, S. C. et al. Hypoxia and non-alcoholic fatty liver disease. Front. Med. (Lausanne) 7, 578001 (2020).
Maurice, J. & Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. (Lond.) 18, 245–250 (2018).
Younossi, Z. M. Non-alcoholic fatty liver disease - A global public health perspective. J. Hepatol. 70, 531–544 (2019).
Calzadilla Bertot, L. & Adams, L. A. The natural course of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 17, 774 (2016).
Fujii, H. & Kawada, N. Inflammation and fibrogenesis in steatohepatitis. J. Gastroenterol. 47, 215–225 (2012).
Morello, E. et al. Hypoxia-inducible factor 2alpha drives nonalcoholic fatty liver progression by triggering hepatocyte release of histidine-rich glycoprotein. Hepatology 67, 2196–2214 (2018).
Paschetta, E. et al. OSAS-related inflammatory mechanisms of liver injury in nonalcoholic fatty liver disease. Mediators Inflamm. 2015, 815721 (2015).
Wu, R. et al. Cardiac-specific ablation of ARNT leads to lipotoxicity and cardiomyopathy. J. Clin. Invest 124, 4795–4806 (2014).
Semenza, G. L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev. Pathol. 9, 47–71 (2014).
Hu, C. J. et al. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell Biol. 23, 9361–9374 (2003).
Scortegagna, M. et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat. Genet 35, 331–340 (2003).
Carabelli, J. et al. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J. Cell Mol. Med. 15, 1329–1338 (2011).
Mesarwi, O. A. et al. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One 11, e0168572 (2016).
Moczydlowska, J. et al. HIF-1 alpha as a key factor in bile duct ligation-induced liver fibrosis in rats. J. Invest Surg. 30, 41–46 (2017).
Wang, X. et al. Macrophage-specific hypoxia-inducible factor-1alpha contributes to impaired autophagic flux in nonalcoholic steatohepatitis. Hepatology 69, 545–563 (2019).
Asai, Y. et al. Activation of the hypoxia inducible factor 1alpha subunit pathway in steatotic liver contributes to formation of cholesterol gallstones. Gastroenterology 152, 1521–1535 e1528 (2017).
Rankin, E. B. et al. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell Biol. 29, 4527–4538 (2009).
Cao, R. et al. Hypoxia induces dysregulation of lipid metabolism in HepG2 cells via activation of HIF-2alpha. Cell Physiol. Biochem. 34, 1427–1441 (2014).
Qu, A. et al. Hypoxia-inducible transcription factor 2alpha promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 54, 472–483 (2011).
Rachner, T. D., Khosla, S. & Hofbauer, L. C. Osteoporosis: now and the future. Lancet 377, 1276–1287 (2011).
Lu, C. et al. The role of oxygen during fracture healing. Bone 52, 220–229 (2013).
Knowles, H. J. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia (Auckl.) 3, 73–82 (2015).
Miyauchi, Y. et al. HIF1alpha is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc. Natl Acad. Sci. USA 110, 16568–16573 (2013).
Tando, T. et al. Hif1alpha is required for osteoclast activation and bone loss in male osteoporosis. Biochem. Biophys. Res. Commun. 470, 391–396 (2016).
Zhao, Q. et al. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone 50, 763–770 (2012).
Lapa, S. A. et al. Multiplex PCR for identification of bacterial pathogens of infectious pneumonia. Russ. J. Bioorg. Chem. 46, 859–861 (2020).
Kojicic, M. et al. Risk factors for the development of acute lung injury in patients with infectious pneumonia. Crit. Care 16, R46 (2012).
Devraj, G., Beerlage, C., Brune, B. & Kempf, V. A. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 19, 144–156 (2017).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513 (2020).
Serebrovska, Z. O. et al. Hypoxia, HIF-1alpha, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharm. Sin. 41, 1539–1546 (2020).
Codo, A. C. et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 32, 498–499 (2020).
Prieto-Fernandez, E. et al. Hypoxia reduces cell attachment of SARS-CoV-2 spike protein by modulating the expression of ACE2, neuropilin-1, syndecan-1 and cellular heparan sulfate. Emerg. Microbes Infect. 10, 1065–1076 (2021).
Yi, Z., Chen, J., Kozlowski, M. & Yuan, Z. Innate detection of hepatitis B and C virus and viral inhibition of the response. Cell Microbiol. 17, 1295–1303 (2015).
Pisano, M. B. et al. Viral hepatitis update: Progress and perspectives. World J. Gastroenterol. 27, 4018–4044 (2021).
Lee, S. W. et al. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis. Biochem. Biophys. Res Commun. 268, 456–461 (2000).
Yoo, Y. G., Cho, S., Park, S. & Lee, M. O. The carboxy-terminus of the hepatitis B virus X protein is necessary and sufficient for the activation of hypoxia-inducible factor-1alpha. FEBS Lett. 577, 121–126 (2004).
Moon, E. J. et al. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 18, 382–384 (2004).
Yoo, Y. G. et al. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J. Biol. Chem. 278, 39076–39084 (2003).
Yoo, Y. G. et al. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene 27, 3405–3413 (2008).
Hu, J. L. et al. Hepatitis B virus induces hypoxia-inducible factor-2alpha expression through hepatitis B virus X protein. Oncol. Rep. 35, 1443–1448 (2016).
Hallez, C. et al. Hypoxia-induced human deoxyribonuclease I is a cellular restriction factor of hepatitis B virus. Nat. Microbiol. 4, 1196–1207 (2019).
Wing, P. A. C. et al. Hypoxia inducible factors regulate hepatitis B virus replication by activating the basal core promoter. J. Hepatol. 75, 64–73 (2021).
Riedl, T. et al. Hypoxia-inducible factor 1 alpha-mediated RelB/APOBEC3B down-regulation allows hepatitis B virus persistence. Hepatology 74, 1766–1781 (2021).
Ripoli, M. et al. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 84, 647–660 (2010).
Abe, M. et al. Hepatitis C virus core protein upregulates the expression of vascular endothelial growth factor via the nuclear factor-kappaB/hypoxia-inducible factor-1alpha axis under hypoxic conditions. Hepatol. Res. 42, 591–600 (2012).
Zhu, C. et al. Hepatitis C virus core protein induces hypoxia-inducible factor 1alpha-mediated vascular endothelial growth factor expression in Huh7.5.1 cells. Mol. Med. Rep. 9, 2010–2014 (2014).
Burtscher, J., Mallet, R. T., Burtscher, M. & Millet, G. P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 68, 101343 (2021).
Labianca, R. et al. Colon cancer. Crit. Rev. Oncol. Hematol. 74, 106–133 (2010).
Markowitz, S. D., Dawson, D. M., Willis, J. & Willson, J. K. Focus on colon cancer. Cancer Cell 1, 233–236 (2002).
Liu, M. et al. Hypoxia-inducible factor 1-alpha up-regulates the expression of phospholipase D2 in colon cancer cells under hypoxic conditions. Med. Oncol. 32, 394 (2015).
Bristow, R. G. & Hill, R. P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 8, 180–192 (2008).
Sung, J. J. et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 6, 871–876 (2005).
Liu, X. et al. Hypoxia-induced upregulation of Orai1 drives colon cancer invasiveness and angiogenesis. Eur. J. Pharm. 832, 1–10 (2018).
Sun, Y. et al. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 146, 1139–1152 (2020).
Santoyo-Ramos, P. et al. Hypoxia-inducible factors modulate the stemness and malignancy of colon cancer cells by playing opposite roles in canonical Wnt signaling. PLoS One 9, e112580 (2014).
Jeon, D., Park, H. J. & Kim, H. S. Protein S-glutathionylation induced by hypoxia increases hypoxia-inducible factor-1alpha in human colon cancer cells. Biochem. Biophys. Res. Commun. 495, 212–216 (2018).
Zheng, H. et al. DJ-1 promotes survival of human colon cancer cells under hypoxia by modulating HIF-1alpha expression through the PI3K-AKT pathway. Cancer Manag. Res. 10, 4615–4629 (2018).
Liu, M., Du, K., Jiang, B. & Wu, X. High expression of phospholipaseD2 induced by hypoxia promotes proliferation of colon cancer cells through activating NF- kappa Bp65 signaling pathway. Pathol. Oncol. Res 26, 281–290 (2020).
Du, K. et al. ANXA3 is upregulated by hypoxia-inducible factor 1-alpha and promotes colon cancer growth. Transl. Cancer Res. 9, 7440–7449 (2020).
Mu, L. et al. Hypoxia-inducible factor-1alpha and semaphorin4D genes involved with tumor-associated macrophage-induced metastatic behavior and clinical significance in colon cancer. Chin. Med. J. (Engl.) 127, 3568–3575 (2014).
Costa, V. et al. MiR-675-5p supports hypoxia induced epithelial to mesenchymal transition in colon cancer cells. Oncotarget 8, 24292–24302 (2017).
Xiong, Z. et al. Downregulation of AIF by HIF-1 contributes to hypoxia-induced epithelial-mesenchymal transition of colon cancer. Carcinogenesis 37, 1079–1088 (2016).
Dang, D. T. et al. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 66, 1684–1936 (2006).
Ramakrishnan, S. K. & Shah, Y. M. Role of intestinal HIF-2alpha in health and disease. Annu Rev. Physiol. 78, 301–325 (2016).
Franovic, A., Holterman, C. E., Payette, J. & Lee, S. Human cancers converge at the HIF-2alpha oncogenic axis. Proc. Natl Acad. Sci. USA 106, 21306–21311 (2009).
Xue, X. et al. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 72, 2285–2293 (2012).
Saint-Martin, A. et al. Functional interaction of hypoxia-inducible factor 2-alpha and autophagy mediates drug resistance in colon cancer cells. Cancers (Basel). 11, 755 (2019).
Xue, X. & Shah, Y. M. Hypoxia-inducible factor-2alpha is essential in activating the COX2/mPGES-1/PGE2 signaling axis in colon cancer. Carcinogenesis 34, 163–169 (2013).
Ma, X., Zhang, H., Xue, X. & Shah, Y. M. Hypoxia-inducible factor 2alpha (HIF-2alpha) promotes colon cancer growth by potentiating Yes-associated protein 1 (YAP1) activity. J. Biol. Chem. 292, 17046–17056 (2017).
Tan, S. X. et al. Methylation of PRDM2, PRDM5 and PRDM16 genes in lung cancer cells. Int J. Clin. Exp. Pathol. 7, 2305–2311 (2014).
Bokobza, S. M. et al. Combining AKT inhibition with chloroquine and gefitinib prevents compensatory autophagy and induces cell death in EGFR mutated NSCLC cells. Oncotarget 5, 4765–4778 (2014).
Gadgeel, S. M., Ramalingam, S. S. & Kalemkerian, G. P. Treatment of lung cancer. Radio. Clin. North Am. 50, 961–974 (2012).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Liu, R., Wei, S., Chen, J. & Xu, S. Mesenchymal stem cells in lung cancer tumor microenvironment: their biological properties, influence on tumor growth and therapeutic implications. Cancer Lett. 353, 145–152 (2014).
Wood, S. L., Pernemalm, M., Crosbie, P. A. & Whetton, A. D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 40, 558–566 (2014).
Li, Y. et al. Status of hypoxia-inducible factor-1alpha expression in non-small cell lung cancer. Pharmazie 76, 404–411 (2021).
Bryant, J. L., Meredith, S. L., Williams, K. J. & White, A. Targeting hypoxia in the treatment of small cell lung cancer. Lung Cancer 86, 126–132 (2014).
Wei, D. F. et al. Effect of hypoxia inducible factor-1 alpha on brain metastasis from lung cancer and its mechanism. Sichuan Da Xue Xue Bao Yi Xue Ban 50, 188–192 (2019).
Takasaki, C. et al. Expression of hypoxia-inducible factor-1alpha affects tumor proliferation and antiapoptosis in surgically resected lung cancer. Mol. Clin. Oncol. 5, 295–300 (2016).
Yang, S. L., Ren, Q. G., Wen, L. & Hu, J. L. Clinicopathological and prognostic significance of hypoxia-inducible factor-1 alpha in lung cancer: a systematic review with meta-analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 36, 321–327 (2016).
Ren, W. et al. The expression of hypoxia-inducible factor-1alpha and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss Med. Wkly 143, w13855 (2013).
Wang, C., Han, C., Zhang, Y. & Liu, F. LncRNA PVT1 regulate expression of HIF1alpha via functioning as ceRNA for miR199a5p in nonsmall cell lung cancer under hypoxia. Mol. Med. Rep. 17, 1105–1110 (2018).
Wu, X. et al. Fibroblast growth factor 11 (FGF11) promotes non-small cell lung cancer (NSCLC) progression by regulating hypoxia signaling pathway. J. Transl. Med. 19, 353 (2021).
Park, J. H., Moon, M., Kim, J. S. & Oh, S. M. TOPK mediates hypoxia-induced epithelial-mesenchymal transition and the invasion of nonsmall-cell lung cancer cells via the HIF-1alpha/snail axis. Biochem. Biophys. Res. Commun. 534, 941–949 (2021).
Hua, Q. et al. Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1alpha axis. Theranostics 10, 4762–4778 (2020).
Cheng, C. W. et al. Foxo3a-mediated overexpression of microRNA-622 suppresses tumor metastasis by repressing hypoxia-inducible factor-1alpha in ERK-responsive lung cancer. Oncotarget 6, 44222–44238 (2015).
Wang, Y., Shi, J. & Gong, L. Gamma linolenic acid suppresses hypoxia-induced proliferation and invasion of non-small cell lung cancer cells by inhibition of HIF1alpha. Genes Genomics 42, 927–935 (2020).
Luo, F. et al. HIF-1alpha inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Lett. 531, 39–56 (2022).
Kong, X. et al. Overexpression of HIF-2alpha-Dependent NEAT1 promotes the progression of non-small cell lung cancer through miR-101-3p/SOX9/Wnt/beta-catenin signal pathway. Cell Physiol. Biochem. 52, 368–381 (2019).
Wang, J. et al. Overexpression of the long noncoding RNA HIF2PUT inhibits non-small cell lung cancer cell proliferation and invasion through HIF-2a pathway. Cancer Biother. Radiopharm. https://doi.org/10.1089/cbr.2020.4629. Online ahead of print (2021).
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Correa, P. Gastric cancer: overview. Gastroenterol. Clin. North Am. 42, 211–217 (2013).
Xing, F. et al. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene 30, 4075–4086 (2011).
Beltrao Hde, B. et al. Investigation of two outbreaks of suspected oral transmission of acute Chagas disease in the Amazon region, Para State, Brazil, in 2007. Trop. Doct. 39, 231–232 (2009).
Lunt, S. J., Chaudary, N. & Hill, R. P. The tumor microenvironment and metastatic disease. Clin. Exp. Metastasis 26, 19–34 (2009).
Zhong, H. et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 59, 5830–5835 (1999).
Liu, L. et al. Hypoxia promotes gastric cancer malignancy partly through the HIF-1alpha dependent transcriptional activation of the long non-coding RNA GAPLINC. Front. Physiol. 7, 420 (2016).
Isobe, T. et al. Clinicopathological significance of hypoxia-inducible factor-1 alpha (HIF-1alpha) expression in gastric cancer. Int J. Clin. Oncol. 18, 293–304 (2013).
Zhao, Q. et al. HIF-1alpha induces multidrug resistance in gastric cancer cells by inducing MiR-27a. PLoS One 10, e0132746 (2015).
Ge, X. et al. MicroRNA-421 regulated by HIF-1alpha promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget 7, 24466–24482 (2016).
Ou, X. W., Wang, R. X., Kang, M. F. & Shi, J. Q. Hypoxia promotes migration and invasion of gastric cancer cells by activating HIF-1alpha and inhibiting NDRG2 associated signaling pathway. Eur. Rev. Med. Pharm. Sci. 22, 8237–8247 (2018).
Xia, X. et al. Hypoxic gastric cancer-derived exosomes promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1alpha positive feedback loop. Oncogene 39, 6231–6244 (2020).
Ding, X. et al. CTHRC1 promotes gastric cancer metastasis via HIF-1alpha/CXCR4 signaling pathway. Biomed. Pharmacother. 123, 109742 (2020).
Fu, J. D. et al. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1alpha and VEGF under a hypoxic state. Eur. Rev. Med Pharm. Sci. 23, 155–161 (2019).
Zhang, J., Xu, J., Dong, Y. & Huang, B. Down-regulation of HIF-1alpha inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression. Biosci. Rep. 38, BSR20180741 (2018).
James, F. R. et al. Obesity in breast cancer-what is the risk factor? Eur. J. Cancer 51, 705–720 (2015).
Bos, R. et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 93, 309–314 (2001).
Shamis, S. A. K., McMillan, D. C. & Edwards, J. The relationship between hypoxia-inducible factor 1alpha (HIF-1alpha) and patient survival in breast cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 159, 103231 (2021).
Sun, G., Wang, Y. & Hu, W. Correlation between HIF-1alpha expression and breast cancer risk: a meta-analysis. Breast J. 20, 213–215 (2014).
Vleugel, M. M. et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J. Clin. Pathol. 58, 172–177 (2005).
Marton, I. et al. Immunohistochemical expression and prognostic significance of HIF-1alpha and VEGF-C in neuroendocrine breast cancer. Anticancer Res. 32, 5227–5232 (2012).
Dales, J. P. et al. Hypoxia inducible factor 1alpha gene (HIF-1alpha) splice variants: potential prognostic biomarkers in breast cancer. BMC Med. 8, 44 (2010).
Hoffmann, C. et al. Hypoxia promotes breast cancer cell invasion through HIF-1alpha-mediated up-regulation of the invadopodial actin bundling protein CSRP2. Sci. Rep. 8, 10191 (2018).
Choi, J. Y., Jang, Y. S., Min, S. Y. & Song, J. Y. Overexpression of MMP-9 and HIF-1alpha in breast cancer cells under hypoxic conditions. J. Breast Cancer 14, 88–95 (2011).
Yang, H. et al. Extracellular ATP promotes breast cancer chemoresistance via HIF-1alpha signaling. Cell Death Dis. 13, 199 (2022).
Khumukcham, S. S. et al. A reciprocal feedback loop between HIF-1alpha and HPIP controls phenotypic plasticity in breast cancer cells. Cancer Lett. 526, 12–28 (2022).
Jia, Y. et al. A SUMOylation-dependent HIF-1alpha/CLDN6 negative feedback mitigates hypoxia-induced breast cancer metastasis. J. Exp. Clin. Cancer Res. 39, 42 (2020).
Sun, S. et al. Hypoxia-responsive miR-141-3p is involved in the progression of breast cancer via mediating the HMGB1/HIF-1alpha signaling pathway. J. Gene Med. 22, e3230 (2020).
Cho, K. H. et al. Breast cancer metastasis suppressor 1 (BRMS1) attenuates TGF-beta1-induced breast cancer cell aggressiveness through downregulating HIF-1alpha expression. BMC Cancer 15, 829 (2015).
Wang, H. X. et al. HIF-2alpha as a prognostic marker for breast cancer progression and patient survival. Genet Mol. Res. 13, 2817–2826 (2014).
Wang, J. G. & Yuan, L. HIF-2alpha/Notch3 pathway mediates CoCl2-induced migration and invasion in human breast cancer MCF-7 cells. Sheng Li Xue Bao 68, 783–789 (2016).
Bai, J. et al. HIF-2alpha regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J. Stem Cells 12, 87–99 (2020).
Goral, V. Pancreatic cancer: pathogenesis and diagnosis. Asian Pac. J. Cancer Prev. 16, 5619–5624 (2015).
Dauer, P., Nomura, A., Saluja, A. & Banerjee, S. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology 17, 7–12 (2017).
Koong, A. C. et al. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 48, 919–922 (2000).
Kumar, S. et al. HIMF (Hypoxia-Induced Mitogenic Factor)-IL (Interleukin)-6 signaling mediates cardiomyocyte-fibroblast crosstalk to promote cardiac hypertrophy and fibrosis. Hypertension 73, 1058–1070 (2019).
Ye, L. Y. et al. Hypoxia-inducible factor 1alpha expression and its clinical significance in pancreatic cancer: a meta-analysis. Pancreatology 14, 391–397 (2014).
Jin, F. et al. Impairment of hypoxia-induced angiogenesis by LDL involves a HIF-centered signaling network linking inflammatory TNFalpha and angiogenic VEGF. Aging (Albany NY) 11, 328–349 (2019).
Cheng, Z. X. et al. Nuclear factor-kappaB-dependent epithelial to mesenchymal transition induced by HIF-1alpha activation in pancreatic cancer cells under hypoxic conditions. PLoS One 6, e23752 (2011).
Jiang, Y. et al. The effect of silencing HIF-1alpha Gene in BxPC-3 cell line on glycolysis-related gene expression, cell growth, invasion, and apoptosis. Nutr. Cancer 67, 1314–1323 (2015).
He, G., Jiang, Y., Zhang, B. & Wu, G. The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac. J. Clin. Nutr. 23, 174–180 (2014).
Zhu, H. et al. Upregulation of autophagy by hypoxia-inducible factor-1alpha promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol. Rep. 32, 935–942 (2014).
Yue, H., Liu, L. & Song, Z. miR-212 regulated by HIF-1alpha promotes the progression of pancreatic cancer. Exp. Ther. Med. 17, 2359–2365 (2019).
Zeng, Z. et al. LncRNA-MTA2TR functions as a promoter in pancreatic cancer via driving deacetylation-dependent accumulation of HIF-1alpha. Theranostics 9, 5298–5314 (2019).
Lu, Y. et al. MiR-142 modulates human pancreatic cancer proliferation and invasion by targeting hypoxia-inducible factor 1 (HIF-1alpha) in the tumor microenvironments. Biol. Open 6, 252–259 (2017).
Wang, M., Chen, M. Y., Guo, X. J. & Jiang, J. X. Expression and significance of HIF-1alpha and HIF-2alpha in pancreatic cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 35, 874–879 (2015).
Zhang, K. D. et al. MiR-301a transcriptionally activated by HIF-2alpha promotes hypoxia-induced epithelial-mesenchymal transition by targeting TP63 in pancreatic cancer. World J. Gastroenterol. 26, 2349–2373 (2020).
Yang, J. et al. HIF-2alpha promotes epithelial-mesenchymal transition through regulating Twist2 binding to the promoter of E-cadherin in pancreatic cancer. J. Exp. Clin. Cancer Res. 35, 26 (2016).
Yang, J. et al. HIF-2alpha promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget 8, 47801–47815 (2017).
Nguyen-Nielsen, M. & Borre, M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl. Med. 46, 484–490 (2016).
Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 10, 63–89 (2019).
Zhong, H. et al. Increased expression of hypoxia inducible factor-1alpha in rat and human prostate cancer. Cancer Res. 58, 5280–5284 (1998).
Lv, L. et al. Stabilization of Snail by HIF-1alpha and TNF-alpha is required for hypoxia-induced invasion in prostate cancer PC3 cells. Mol. Biol. Rep. 41, 4573–4582 (2014).
Huang, M. et al. The association of HIF-1alpha expression with clinicopathological significance in prostate cancer: a meta-analysis. Cancer Manag. Res. 10, 2809–2816 (2018).
Xia, L. et al. PRKAR2B-HIF-1alpha loop promotes aerobic glycolysis and tumour growth in prostate cancer. Cell Prolif. 53, e12918 (2020).
Van Camp, G. Cardiovascular disease prevention. Acta Clin. Belg. 69, 407–411 (2014).
Noutsias, M. & Maisch, B. Treatment of cardiovascular diseases in cancer patients. Herz 36, 340–345 (2011).
Soulaidopoulos, S. et al. Cardiovascular disease in the systemic vasculitides. Curr. Vasc. Pharm. 18, 463–472 (2020).
Meng, J. & Yang, B. Protective effect of ganoderma (Lingzhi) on cardiovascular system. Adv. Exp. Med. Biol. 1182, 181–199 (2019).
Ullah, K. & Wu, R. Hypoxia-inducible factor regulates endothelial metabolism in cardiovascular disease. Front. Physiol. 12, 670653 (2021).
Li, X. et al. Oxygen homeostasis and cardiovascular disease: A role for HIF? Biomed. Pharmacother. 128, 110338 (2020).
Takahashi, K. et al. Chronic intermittent hypoxia-mediated renal sympathetic nerve activation in hypertension and cardiovascular disease. Sci. Rep. 8, 17926 (2018).
Semenza, G. L. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev. Physiol. 76, 39–56 (2014).
Liu, M. et al. Novel therapeutic targets for hypoxia-related cardiovascular diseases: the role of HIF-1. Front. Physiol. 11, 774 (2020).
Demandt, J. A. F. et al. The hypoxia-sensor carbonic anhydrase IX affects macrophage metabolism, but is not a suitable biomarker for human cardiovascular disease. Sci. Rep. 11, 425 (2021).
Aldossary, H. S. et al. G-Protein-Coupled Receptor (GPCR) signaling in the carotid body: roles in hypoxia and cardiovascular and respiratory disease. Int. J. Mol. Sci. 21, 6012 (2020).
Bartman, C. M. & Eckle, T. Circadian-hypoxia link and its potential for treatment of cardiovascular disease. Curr. Pharm. Des. 25, 1075–1090 (2019).
Beaudin, A. E. et al. Vascular responses to hypoxia are not impaired in obstructive sleep apnoea patients free of overt cardiovascular disease. Exp. Physiol. 104, 580–600 (2019).
Tirpe, A. A. et al. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 20, 6140 (2019).
Mylonis, I., Simos, G. & Paraskeva, E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells. 8, 214 (2019).
Shukla, S. D. et al. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 25, 53–63 (2020).
Belaidi, E. et al. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharm. Ther. 168, 1–11 (2016).
Li, Q. O. Y., Soro-Arnaiz, I. & Aragones, J. Age-dependent obesity and mitochondrial dysfunction. Adipocyte 6, 161–166 (2017).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Sun, K. et al. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol. Cell Biol. 33, 904–917 (2013).
Halberg, N. et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 29, 4467–4483 (2009).
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011).
Ahmadi, A. et al. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 74, 1608–1617 (2019).
Kasikara, C., Doran, A. C., Cai, B. & Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Invest. 128, 2713–2723 (2018).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Lin, X. et al. Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis. Arch. Biochem. Biophys. 715, 109098 (2022).
Tomas, L., Prica, F. & Schulz, C. Trafficking of mononuclear phagocytes in healthy arteries and atherosclerosis. Front. Immunol. 12, 718432 (2021).
Yuan, Y., Li, P. & Ye, J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 3, 173–181 (2012).
Ouimet, M. et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 13, 655–667 (2011).
Knutson, A. K., Williams, A. L., Boisvert, W. A. & Shohet, R. V. HIF in the heart: development, metabolism, ischemia, and atherosclerosis. J. Clin. Invest. 131, e137557 (2021).
Sluimer, J. C. et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J. Am. Coll. Cardiol. 51, 1258–1265 (2008).
Gao, L., Chen, Q., Zhou, X. & Fan, L. The role of hypoxia-inducible factor 1 in atherosclerosis. J. Clin. Pathol. 65, 872–876 (2012).
Jain, T., Nikolopoulou, E. A., Xu, Q. & Qu, A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharm. Ther. 183, 22–33 (2018).
Mogilenko, D. A. et al. Endogenous apolipoprotein A-I stabilizes ATP-binding cassette transporter A1 and modulates Toll-like receptor 4 signaling in human macrophages. FASEB J. 26, 2019–2030 (2012).
Bogomolova, A. M. et al. Hypoxia as a factor involved in the regulation of the apoA-1, ABCA1, and complement C3 gene expression in human macrophages. Biochem. (Mosc.) 84, 529–539 (2019).
Castellano, J. et al. Hypoxia stimulates low-density lipoprotein receptor-related protein-1 expression through hypoxia-inducible factor-1alpha in human vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 31, 1411–1420 (2011).
Zhong, Z. et al. Differential expression of circulating long non-coding RNAs in patients with acute myocardial infarction. Med. (Baltim.) 97, e13066 (2018).
Akhtar, S. et al. Endothelial hypoxia-inducible factor-1alpha promotes atherosclerosis and monocyte recruitment by upregulating MicroRNA-19a. Hypertension 66, 1220–1226 (2015).
Christoph, M. et al. Local inhibition of hypoxia-inducible factor reduces neointima formation after arterial injury in ApoE-/- mice. Atherosclerosis 233, 641–647 (2014).
Folco, E. J. et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J. Am. Coll. Cardiol. 58, 603–614 (2011).
Stenmark, K. R., Fagan, K. A. & Frid, M. G. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ. Res. 99, 675–691 (2006).
Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Invest. 122, 4306–4313 (2012).
Brusselmans, K. et al. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J. Clin. Invest. 111, 1519–1527 (2003).
Yu, A. Y. et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J. Clin. Invest 103, 691–696 (1999).
Jin, Q. et al. C1q/TNF-related protein-9 ameliorates hypoxia-induced pulmonary hypertension by regulating secretion of endothelin-1 and nitric oxide mediated by AMPK in rats. Sci. Rep. 11, 11372 (2021).
Hu, R., Dai, A. & Tan, S. Hypoxia-inducible factor 1 alpha upregulates the expression of inducible nitric oxide synthase gene in pulmonary arteries of hyposic rat. Chin. Med. J. (Engl.) 115, 1833–1837 (2002).
Jin, Y. et al. Modulatory effect of silymarin on pulmonary vascular dysfunction through HIF-1alpha-iNOS following rat lung ischemia-reperfusion injury. Exp. Ther. Med. 12, 1135–1140 (2016).
Hisada, T. et al. Statin inhibits hypoxia-induced endothelin-1 via accelerated degradation of HIF-1alpha in vascular smooth muscle cells. Cardiovasc Res. 95, 251–259 (2012).
Porter, K. M. et al. Chronic hypoxia promotes pulmonary artery endothelial cell proliferation through H2O2-induced 5-lipoxygenase. PLoS One 9, e98532 (2014).
Su, T. et al. EtaIotaF1alpha, EGR1 and SP1 co-regulate the erythropoietin receptor expression under hypoxia: an essential role in the growth of non-small cell lung cancer cells. Cell Commun. Signal 17, 152 (2019).
Cao, Y. et al. PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension. Proc. Natl Acad. Sci. USA 116, 13394–13403 (2019).
Fijalkowska, I. et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am. J. Pathol. 176, 1130–1138 (2010).
Tuder, R. M. et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J. Pathol. 195, 367–374 (2001).
Luo, Y. et al. CD146-HIF-1alpha hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nat. Commun. 10, 3551 (2019).
Brieler, J., Breeden, M. A. & Tucker, J. Cardiomyopathy: an overview. Am. Fam. Physician 96, 640–646 (2017).
Chen, Y. F. et al. Synergistic effect of HIF-1alpha and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell Physiol. 233, 3660–3671 (2018).
Kumar, S. et al. Hypoxia-induced mitogenic factor promotes cardiac hypertrophy via calcium-dependent and hypoxia-inducible factor-1alpha mechanisms. Hypertension 72, 331–342 (2018).
Belaidi, E. et al. Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int J. Cardiol. 210, 45–53 (2016).
Bekeredjian, R. et al. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS One 5, e11693 (2010).
Holscher, M. et al. Unfavourable consequences of chronic cardiac HIF-1alpha stabilization. Cardiovasc Res. 94, 77–86 (2012).
Bourdier, G. et al. Intermittent hypoxia triggers early cardiac remodeling and contractile dysfunction in the time-course of ischemic cardiomyopathy in rats. J. Am. Heart Assoc. 9, e016369 (2020).
Li, G. et al. Admission hypoxia-inducible factor 1alpha levels and in-hospital mortality in patients with acute decompensated heart failure. BMC Cardiovasc Disord. 15, 79 (2015).
Babapoor-Farrokhran, S., Gill, D., Alzubi, J. & Mainigi, S. K. Atrial fibrillation: the role of hypoxia-inducible factor-1-regulated cytokines. Mol. Cell Biochem. 476, 2283–2293 (2021).
Ma, Z. et al. Doxycycline improves fibrosis-induced abnormalities in atrial conduction and vulnerability to atrial fibrillation in chronic intermittent hypoxia rats. Med. Sci. Monit. 26, e918883 (2020).
Nakano, Y. et al. Matrix metalloproteinase-9 contributes to human atrial remodeling during atrial fibrillation. J. Am. Coll. Cardiol. 43, 818–825 (2004).
Su, F. et al. Significance of hypoxia-inducible factor-1alpha expression with atrial fibrosis in rats induced with isoproterenol. Exp. Ther. Med. 8, 1677–1682 (2014).
Ogi, H. et al. Is structural remodeling of fibrillated atria the consequence of tissue hypoxia? Circ. J. 74, 1815–1821 (2010).
Abe, I. et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 15, 1717–1727 (2018).
Xu, Y., Sharma, D., Du, F. & Liu, Y. The role of Toll-like receptor 2 and hypoxia-induced transcription factor-1alpha in the atrial structural remodeling of non-valvular atrial fibrillation. Int. J. Cardiol. 168, 2940–2941 (2013).
Dengler, V. L., Galbraith, M. & Espinosa, J. M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 49, 1–15 (2014).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Kameny, R. J. et al. Right ventricular nitric oxide signaling in an ovine model of congenital heart disease: a preserved fetal phenotype. Am. J. Physiol. Heart Circ. Physiol. 309, H157–H165 (2015).
Inai, K. Can pulmonary vasodilator therapy expand the operative indications for congenital heart disease? Int. Heart J. 56(Suppl), S12–S16 (2015).
Myers, P. O., Tissot, C. & Beghetti, M. Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circ. J. 78, 4–11 (2014).
Cordina, R. L. & Celermajer, D. S. Chronic cyanosis and vascular function: implications for patients with cyanotic congenital heart disease. Cardiol. Young-. 20, 242–253 (2010).
Bigham, A. W. & Lee, F. S. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev. 28, 2189–2204 (2014).
Zhou, Y. et al. An EGLN1 mutation may regulate hypoxic response in cyanotic congenital heart disease through the PHD2/HIF-1A pathway. Genes Dis. 6, 35–42 (2019).
Yin, Z. et al. The essential role of Cited2, a negative regulator for HIF-1alpha, in heart development and neurulation. Proc. Natl Acad. Sci. USA 99, 10488–10493 (2002).
Checkoway, H., Lundin, J. I. & Kelada, S. N. Neurodegenerative diseases. IARC Sci. Publ. 163, 407–419 (2011).
Bonda, D. J. et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 10, 275–279 (2011).
Mitroshina, E. V., Savyuk, M. O., Ponimaskin, E. & Vedunova, M. V. Hypoxia-inducible factor (HIF) in ischemic stroke and neurodegenerative disease. Front. Cell Dev. Biol. 9, 703084 (2021).
Hachiya, N. et al. Nuclear envelope and nuclear pore complexes in neurodegenerative diseases-new perspectives for therapeutic interventions. Mol. Neurobiol. 58, 983–995 (2021).
De la Rosa, A. et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 9, 394–404 (2020).
Ahmad, A., Patel, V., Xiao, J. & Khan, M. M. The role of neurovascular system in neurodegenerative diseases. Mol. Neurobiol. 57, 4373–4393 (2020).
Yu, X., Ji, C. & Shao, A. Neurovascular unit dysfunction and neurodegenerative disorders. Front. Neurosci. 14, 334 (2020).
Merelli, A. et al. Understanding the role of hypoxia inducible factor during neurodegeneration for new therapeutics opportunities. Curr. Neuropharmacol. 16, 1484–1498 (2018).
Shahmoradian, S. H. et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 22, 1099–1109 (2019).
Lee, A. & Gilbert, R. M. Epidemiology of parkinson disease. Neurol. Clin. 34, 955–965 (2016).
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 896–912 (2015).
Rodriguez, M. et al. Parkinson’s disease as a result of aging. Aging Cell 14, 293–308 (2015).
Hauser, D. N. & Hastings, T. G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 51, 35–42 (2013).
Mehrabani, M. et al. Protective effect of hydralazine on a cellular model of Parkinson’s disease: a possible role of hypoxia-inducible factor (HIF)-1alpha. Biochem. Cell Biol. 98, 405–414 (2020).
Strowitzki, M. J., Cummins, E. P. & Taylor, C. T. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: unique or ubiquitous? Cells. 8, 384 (2019).
Schofield, C. J. & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 (2004).
Liu, C. et al. Orexin and Parkinson’s disease: A protective neuropeptide with therapeutic potential. Neurochem. Int. 138, 104754 (2020).
Aime, P. et al. The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson’s disease. Neurobiol. Dis. 136, 104725 (2020).
Johansen, J. L. et al. HIF prolyl hydroxylase inhibition increases cell viability and potentiates dopamine release in dopaminergic cells. J. Neurochem. 115, 209–219 (2010).
van Es, M. A. et al. Amyotrophic lateral sclerosis. Lancet 390, 2084–2098 (2017).
Longinetti, E. & Fang, F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr. Opin. Neurol. 32, 771–776 (2019).
Lechtzin, N. et al. Respiratory measures in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 321–330 (2018).
Pronto-Laborinho, A. C., Pinto, S. & de Carvalho, M. Roles of vascular endothelial growth factor in amyotrophic lateral sclerosis. Biomed. Res. Int. 2014, 947513 (2014).
Miyazaki, K. et al. Early and progressive impairment of spinal blood flow-glucose metabolism coupling in motor neuron degeneration of ALS model mice. J. Cereb. Blood Flow. Metab. 32, 456–467 (2012).
Tafani, M. et al. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid. Med. Cell Longev. 2016, 3907147 (2016).
Yamashita, T. et al. Hypoxic stress visualized in the cervical spinal cord of ALS patients. Neurol. Res. 43, 429–433 (2021).
Nomura, E. et al. Imaging hypoxic stress and the treatment of amyotrophic lateral sclerosis with dimethyloxalylglycine in a mice model. Neuroscience 415, 31–43 (2019).
Nagara, Y. et al. Impaired cytoplasmic-nuclear transport of hypoxia-inducible factor-1alpha in amyotrophic lateral sclerosis. Brain Pathol. 23, 534–546 (2013).
Moreau, C. et al. Deregulation of the hypoxia inducible factor-1alpha pathway in monocytes from sporadic amyotrophic lateral sclerosis patients. Neuroscience 172, 110–117 (2011).
Tada, S. et al. Single injection of sustained-release prostacyclin analog ONO-1301-MS ameliorates hypoxic toxicity in the murine model of amyotrophic lateral sclerosis. Sci. Rep. 9, 5252 (2019).
Choudhry, H. & Harris, A. L. Advances in hypoxia-inducible factor biology. Cell Metab. 27, 281–298 (2018).
Kim, H. A., Mahato, R. I. & Lee, M. Hypoxia-specific gene expression for ischemic disease gene therapy. Adv. Drug Deliv. Rev. 61, 614–622 (2009).
Ajith, T. A. Current insights and future perspectives of hypoxia-inducible factor-targeted therapy in cancer. J. Basic Clin. Physiol. Pharm. 30, 11–18 (2018).
Li, T. et al. Epigenetic crosstalk between hypoxia and tumor driven by HIF regulation. J. Exp. Clin. Cancer Res. 39, 224 (2020).
Huang, Y., Lin, D. & Taniguchi, C. M. Hypoxia inducible factor (HIF) in the tumor microenvironment: friend or foe? Sci. China Life Sci. 60, 1114–1124 (2017).
Nakazawa, M. S. et al. Epigenetic re-expression of HIF-2alpha suppresses soft tissue sarcoma growth. Nat. Commun. 7, 10539 (2016).
Choueiri, T. K. & Kaelin, W. G. Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 26, 1519–1530 (2020).
Cho, H. et al. On-target efficacy of a HIF-2alpha antagonist in preclinical kidney cancer models. Nature 539, 107–111 (2016).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Courtney, K. D. et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin. Cancer Res. 26, 793–803 (2020).
Xu, R. et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzo nitrile (PT2977), a Hypoxia-Inducible Factor 2alpha (HIF-2alpha) inhibitor for the treatment of clear cell renal cell carcinoma. J. Med. Chem. 62, 6876–6893 (2019).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in von hippel-lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021).
Choueiri, T. K. et al. Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat. Med. 27, 802–805 (2021).
Deeks, E. D. Belzutifan: first approval. Drugs 81, 1921–1927 (2021).
Beppu, K. et al. Topotecan blocks hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression induced by insulin-like growth factor-I in neuroblastoma cells. Cancer Res. 65, 4775–4781 (2005).
Brogden, R. N. & Wiseman, L. R. Topotecan. A review of its potential in advanced ovarian cancer. Drugs 56, 709–723 (1998).
Hartwell, D. et al. Topotecan for relapsed small cell lung cancer: a systematic review and economic evaluation. Cancer Treat. Rev. 37, 242–249 (2011).
De Placido, S. et al. Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study. J. Clin. Oncol. 22, 2635–2642 (2004).
Kummar, S. et al. Multihistology, target-driven pilot trial of oral topotecan as an inhibitor of hypoxia-inducible factor-1alpha in advanced solid tumors. Clin. Cancer Res. 17, 5123–5131 (2011).
Shin, D. H. et al. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood 111, 3131–3136 (2008).
Mackay, H. et al. A phase II trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin. Cancer Res. 11, 5526–5533 (2005).
Birle, D. C. & Hedley, D. W. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 67, 1735–1743 (2007).
Wu, J. et al. Evaluation of a locked nucleic acid form of antisense oligo targeting HIF-1alpha in advanced hepatocellular carcinoma. World J. Clin. Oncol. 10, 149–160 (2019).
Wang, D. & Lippard, S. J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Disco. 4, 307–320 (2005).
Kang, Y. K. et al. PRODIGY: A Phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J. Clin. Oncol. 39, 2903–2913 (2021).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393, 1948–1957 (2019).
Boku, N. et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann. Oncol. 30, 250–258 (2019).
Wei, T. T. et al. Metabolic targeting of HIF-1alpha potentiates the therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene 39, 414–427 (2020).
Mennerich, D., Kubaichuk, K. & Kietzmann, T. DUBs, hypoxia, and cancer. Trends Cancer 5, 632–653 (2019).
Al-Eidan, A., Wang, Y., Skipp, P. & Ewing, R. M. The USP7 protein interaction network and its roles in tumorigenesis. Genes Dis. 9, 41–50 (2022).
Dai, X. et al. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics 10, 9332–9347 (2020).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011).
Li, Y., Zhao, L. & Li, X. F. The hypoxia-activated prodrug TH-302: exploiting hypoxia in cancer therapy. Front. Pharm. 12, 636892 (2021).
Guo, H. et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat. Commun. 10, 278 (2019).
Borad, M. J. et al. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 33, 1475–1481 (2015).
Jayaprakash, P. et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Invest. 128, 5137–5149 (2018).
Chawla, S. P. et al. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 32, 3299–3306 (2014).
Tap, W. D. et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 18, 1089–1103 (2017).
Wang, Z. ErbB receptors and cancer. Methods Mol. Biol. 1652, 3–35 (2017).
Estrada-Bernal, A. et al. Tarloxotinib is a hypoxia-activated Pan-HER kinase inhibitor active against a broad range of HER-family oncogenes. Clin. Cancer Res. 27, 1463–1475 (2021).
Chong, C. R. & Janne, P. A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 19, 1389–1400 (2013).
Compernolle, V. et al. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 60, 569–579 (2003).
Liu, Y. et al. Suppression of myocardial hypoxia-inducible factor-1alpha compromises metabolic adaptation and impairs cardiac function in patients with cyanotic congenital heart disease during puberty. Circulation 143, 2254–2272 (2021).
Hausenloy, D. J. & Yellon, D. M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 13, 193–209 (2016).
Flamme, I. et al. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One 9, e111838 (2014).
Sousa Fialho, M. D. L. et al. Activation of HIF1alpha rescues the hypoxic response and reverses metabolic dysfunction in the diabetic heart. Diabetes 70, 2518–2531 (2021).
Macdougall, I. C. et al. Effects of molidustat in the treatment of anemia in CKD. Clin. J. Am. Soc. Nephrol. 14, 28–39 (2019).
Zheng, Q. et al. Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: A network meta-analysis. Pharm. Res. 159, 105020 (2020).
Gupta, N. & Wish, J. B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 69, 815–826 (2017).
Heger, L. A. et al. Expression of the oxygen-sensitive transcription factor subunit HIF-1alpha in patients suffering from secondary Raynaud syndrome. Acta Pharm. Sin. 40, 500–506 (2019).
Mehrabi, M. R. et al. Clinical benefit of prostaglandin E1-treatment of patients with ischemic heart disease: stimulation of therapeutic angiogenesis in vital and infarcted myocardium. Biomed. Pharmacother. 57, 173–178 (2003).
Sood, B. G., Delaney-Black, V., Aranda, J. V. & Shankaran, S. Aerosolized PGE1: a selective pulmonary vasodilator in neonatal hypoxemic respiratory failure results of a Phase I/II open label clinical trial. Pediatr. Res. 56, 579–585 (2004).
Sun, J. et al. HIF-1alpha overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 11, 373 (2020).
Henning, R. J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 14, 491–509 (2018).
Cheng, K. et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J. Clin. Invest. 120, 2171–2183 (2010).
Li, G. et al. A small molecule HIF-1alpha stabilizer that accelerates diabetic wound healing. Nat. Commun. 12, 3363 (2021).
Verma, S. & McMurray, J. J. V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61, 2108–2117 (2018).
Bessho, R. et al. Hypoxia-inducible factor-1alpha is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci. Rep. 9, 14754 (2019).
Shirakawa, J. et al. Luseogliflozin increases beta cell proliferation through humoral factors that activate an insulin receptor- and IGF-1 receptor-independent pathway. Diabetologia 63, 577–587 (2020).
Frampton, J. E. Empagliflozin: a review in Type 2 diabetes. Drugs 78, 1037–1048 (2018).
Zannad, F. et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-reduced. Circulation 143, 310–321 (2021).
Wanner, C. et al. Empagliflozin and progression of kidney disease in Type 2 diabetes. N. Engl. J. Med 375, 323–334 (2016).
Panic, A., Stanimirovic, J., Sudar-Milovanovic, E. & Isenovic, E. R. Oxidative stress in obesity and insulin resistance. Exploration Med. 3, 58–70 (2022).
Sakaguchi, Y., Hamano, T., Wada, A. & Masakane, I. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J. Am. Soc. Nephrol. 30, 1037–1048 (2019).
Thavarajah, S. & Choi, M. J. The use of erythropoiesis-stimulating agents in patients with CKD and cancer: a clinical approach. Am. J. Kidney Dis. 74, 667–674 (2019).
Del Vecchio, L. & Locatelli, F. Investigational hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHI) for the treatment of anemia associated with chronic kidney disease. Expert Opin. Investig. Drugs 27, 613–621 (2018).
Chertow, G. M. et al. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N. Engl. J. Med. 384, 1589–1600 (2021).
Dhillon, S. Roxadustat: first global approval. Drugs 79, 563–572 (2019).
Chen, N. et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N. Engl. J. Med. 381, 1001–1010 (2019).
Fishbane, S. et al. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized Phase 3 study. J. Am. Soc. Nephrol. 32, 737–755 (2021).
Akizawa, T. et al. Phase 3, randomized, double-blind, active-comparator (Darbepoetin Alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J. Am. Soc. Nephrol. 31, 1628–1639 (2020).
Singh, A. K. et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N. Engl. J. Med. 385, 2313–2324 (2021).
Singh, A. K. et al. Daprodustat for the treatment of anemia in patients undergoing dialysis. N. Engl. J. Med. 385, 2325–2335 (2021).
Wing, P. A. C. et al. Hypoxic and pharmacological activation of HIF inhibits SARS-CoV-2 infection of lung epithelial cells. Cell Rep. 35, 109020 (2021).
Poloznikov, A. A. et al. HIF prolyl hydroxylase inhibitors for COVID-19 treatment: pros and cons. Front. Pharm. 11, 621054 (2020).
Khaddaj-Mallat, R. et al. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol. Dis. 161, 105561 (2021).
Gaber, T. et al. Macrophage migration inhibitory factor counterregulates dexamethasone-mediated suppression of hypoxia-inducible factor-1 alpha function and differentially influences human CD4+ T cell proliferation under hypoxia. J. Immunol. 186, 764–774 (2011).
Zhu, B. et al. Uncoupling of macrophage inflammation from self-renewal modulates host recovery from respiratory viral infection. Immunity 54, 1200–1218 e1209 (2021).
Zhao, C. et al. Deficiency of HIF-1alpha enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells. Emerg. Microbes Infect. 9, 691–706 (2020).
Huang, H. et al. Active HBV replication in hypoxic pericentral zone 3 is up-regulated by multiple host factors including HIF-1alpha. J. Hepatol. 77, 265–267 (2022).
Lee, J. W., Ko, J., Ju, C. & Eltzschig, H. K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 51, 1–13 (2019).
Kamboj, A. K., Cotter, T. G. & Oxentenko, A. S. Helicobacter pylori: the past, present, and future in management. Mayo Clin. Proc. 92, 599–604 (2017).
Choi, I. J. et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 378, 1085–1095 (2018).
Mera, R. M. et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 67, 1239–1246 (2018).
Szabo, S. et al. New molecular mechanisms of duodenal ulceration. Ann. N. Y Acad. Sci. 1113, 238–255 (2007).
Rauws, E. A. & Tytgat, G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet 335, 1233–1235 (1990).
Valenzuela-Valderrama, M. et al. The helicobacter pylori urease virulence factor is required for the induction of hypoxia-induced factor-1alpha in gastric cells. Cancers (Basel). 11, 799 (2019).
Venkatesan, A. et al. Acute encephalitis in immunocompetent adults. Lancet 393, 702–716 (2019).
Kupila, L. et al. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology 66, 75–80 (2006).
Swanson, P. A. 2nd & McGavern, D. B. Viral diseases of the central nervous system. Curr. Opin. Virol. 11, 44–54 (2015).
Jin, Y. et al. Pathological features of enterovirus 71-associated brain and lung damage in mice based on quantitative proteomic analysis. Front. Microbiol. 12, 663019 (2021).
Liou, A. T. et al. Hypoxia and therapeutic treatment of EV-A71 with an immune modulator TLR7 agonist in a new immunocompetent mouse model. J. Biomed. Sci. 26, 93 (2019).
Pina-Oviedo, S., Khalili, K. & Del Valle, L. Hypoxia inducible factor-1 alpha activation of the JCV promoter: role in the pathogenesis of progressive multifocal leukoencephalopathy. Acta Neuropathol. 118, 235–247 (2009).
Schibler, M. et al. Diagnostic tools to tackle infectious causes of encephalitis and meningoencephalitis in immunocompetent adults in Europe. Clin. Microbiol Infect. 25, 408–414 (2019).
Devraj, G. et al. HIF-1alpha is involved in blood-brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis. Acta Neuropathol. 140, 183–208 (2020).
Singh, R. & Manna, P. P. Reactive oxygen species in cancer progression and its role in therapeutics. Exploration Med. 3, 43–57 (2022).
Gao, Y. et al. Roxadustat, a hypoxia-inducible factor 1alpha activator, attenuates both long- and short-term alcohol-induced alcoholic liver disease. Front. Pharm. 13, 895710 (2022).
Iesari, S. et al. Selective HIF stabilization alleviates hepatocellular steatosis and ballooning in a rodent model of 70% liver resection. Clin. Sci. (Lond.) 135, 2285–2305 (2021).
Choi, U. H. et al. Hypoxia-inducible expression of vascular endothelial growth factor for the treatment of spinal cord injury in a rat model. J. Neurosurg. Spine 7, 54–60 (2007).
Huang, D., Desbois, A. & Hou, S. T. A novel adenoviral vector which mediates hypoxia-inducible gene expression selectively in neurons. Gene Ther. 12, 1369–1376 (2005).
Javan, B. & Shahbazi, M. Hypoxia-inducible tumour-specific promoters as a dual-targeting transcriptional regulation system for cancer gene therapy. Ecancermedicalscience 11, 751 (2017).
Yang, L. et al. Tumor-specific gene expression using the survivin promoter is further increased by hypoxia. Gene Ther. 11, 1215–1223 (2004).
Acknowledgements
This study was sponsored by National Natural Science Foundation of China (81730061 to J.W., 32070148 to Z.L., and 32100738 to P.W.), and China Postdoctoral Science Foundation (2021M691246 to M.T.).
Author information
Authors and Affiliations
Contributions
Z.L., M.T., G.Y., P.W., and J.W.: the conceptualization and design of this study. Q.T., Y.C., G.L., Q.Z., and Y.L.: the investigation and methodology of this study. Z.L., M.T., G.Y., Q.T., Y.C., G.L., Q.Z., and Y.L.: the formal analysis. Z.L., M.T., G.Y., P.W., and J.W.: the validation of the date. Z.L., M.T., P.W., and J.W.: the funding acquisition. J.W.: project administration of this study. Z.L., M.T., G.Y., P.W., and J.W.: writing – original draft. P.W., Z.L., and J.W.: writing – review & editing of the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Z., Tian, M., Yang, G. et al. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Sig Transduct Target Ther 7, 218 (2022). https://doi.org/10.1038/s41392-022-01080-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01080-1
- Springer Nature Limited
This article is cited by
-
Protein–protein interaction network-based integration of GWAS and functional data for blood pressure regulation analysis
Human Genomics (2024)
-
Hypoxia exposure blunts angiogenic signaling and upregulates the antioxidant system in endothelial cells derived from elephant seals
BMC Biology (2024)
-
Role of Exosomes in Cancer and Aptamer-Modified Exosomes as a Promising Platform for Cancer Targeted Therapy
Biological Procedures Online (2024)
-
Nanomaterial-related hemoglobin-based oxygen carriers, with emphasis on liposome and nano-capsules, for biomedical applications: current status and future perspectives
Journal of Nanobiotechnology (2024)
-
Lung injury-induced activated endothelial cell states persist in aging-associated progressive fibrosis
Nature Communications (2024)