Abstract
Background
Exposure to acetaminophen and its metabolites in very-preterm infants is partly unknown. We investigated the exposure to acetaminophen and its metabolites upon 10, 15, or 20 mg/kg intravenous acetaminophen in preterm infants.
Methods
In a randomized trial, 59 preterm infants (24–32 weeks’ gestational age, postnatal age <1 week) received 10, 15, or 20 mg/kg acetaminophen intravenously. Plasma concentrations of acetaminophen and its metabolites (glucuronide, sulfate, cysteine, mercapturate, and glutathione) were determined in 293 blood samples. Area under the plasma concentration–time curves (AUC0–500 min) was related to dose and gestational age.
Results
Between 10 and 20 mg/kg dose, median AUCs of acetaminophen, glucuronide, sulfate, and cysteine increased significantly resulting in unchanged ratios of AUC of metabolite to acetaminophen. The AUC ratio of glucuronide to acetaminophen increased with gestational age, that of sulfate decreased, and the ratio of cysteine and mercapturate remained unchanged.
Conclusion
We found a gestational-age-dependent increase in glucuronidation but no evidence for saturation of a specific pathway as there was a proportional increase in exposure of acetaminophen and all metabolites. Compared with adults, very low exposure to glucuronide but higher exposure to sulfate, cysteine, and mercapturate metabolites was found, of which the relevance is not yet known.
Similar content being viewed by others
Main
Preterm infants treated in neonatal intensive care units are often exposed to repetitive or prolonged pain (1, 2). Opioids and nonsteroidal anti-inflammatory drugs potentially relieve the pain but may result in serious side effects and potential harm to critically ill preterm infants (3). Intravenous acetaminophen as an opioid-sparing therapy in adults and children has now been introduced in neonatal intensive care units across the globe (4). However, only very limited data of its use are available in the most preterm infants (5, 6).
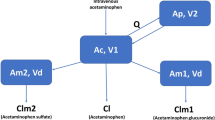
Acetaminophen (N-acetyl-p-amino-phenol) is extensively metabolized in the liver. The main pathways involved are glucuronidation and sulfation, which in adults account for ~55 and 30% of acetaminophen metabolism, respectively (7, 8, 9) (Figure 1). Only 2–5% is excreted unchanged in the urine. Approximately 5–10% of acetaminophen is metabolized by cytochrome P450 (CYP), primarily by the CYP2E1 enzyme (10, 11, 12), to the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) (7, 13, 14, 15). At therapeutic doses, NAPQI is immediately inactivated by conjugation with gluthatione. After formation of acetaminophen–glutathione, acetaminophen–cysteine and acetaminophen–mercapturate are formed consecutively. Without this detoxification route, NAPQI can bind covalently to cellular proteins and form toxic protein adducts, which may cause mitochondrial dysfunction and early oxidant stress (16, 17, 18). This, ultimately, may result in liver cell necrosis (19).
Neonates have a lower total acetaminophen clearance per kg bodyweight compared with adults, with proportionally higher sulfation and lower glucuronidation (20, 21, 22). Acetaminophen sulfation is mainly catalyzed by SULT1A1, 1A3/4, and 1E1 (ref. 23), of which several sulfotransferase enzymes are already widely expressed in fetal tissue (18–25 weeks) (24). Glucuronidation of acetaminophen mainly occurs by UGT-1A6, and to a lesser extent by 1A9 (ref. 25). Low glucuronidation rates have also been shown for morphine in preterm and term infants (26). There are, however, very limited data available on the exact contributions of the different pathways in paracetamol metabolism in preterm infants and particularly with respect to the CYP2E1-mediated oxidation pathway in neonates with a gestational age below 28 weeks.
To gain more insight in the contributions of the different metabolic pathways upon increasing doses of acetaminophen, we studied the exposure to acetaminophen and all of its metabolites in the most premature neonates. To this end, preterm infants were stratified for gestational age (24–28 and 28–32 weeks) and randomized to receive an intravenous dose of 10, 15, or 20 mg/kg acetaminophen.
Methods
Patients
From October 2010 to October 2013, a randomized, two-center trial was performed at the level three Neonatal Intensive Care Units of the Erasmus Medical Center—Sophia Children’s Hospital in Rotterdam and Isala Clinics in Zwolle, the Netherlands. Approval of the Ethics Review Committees of both hospitals and written informed consent from parents/legal guardians were obtained before study initiation (MEC-2009-250, National Trial Register 2290).
All preterm neonates with a gestational age ≤32 weeks with an indwelling arterial catheter for clinical purposes, undergoing central venous catheter placement in the first 7 days of life, were eligible for inclusion. Exclusion criteria were as follows: major congenital anomalies, intraventricular hemorrhage ≥grade 3, use of neuromuscular blockers, previous acetaminophen, and maintenance dose of analgesics or more than one loading dose of morphine or midazolam any time before inclusion in the study.
Study Design
Neonates
Sixty neonates were randomly allocated to 10, 15, or 20 mg/kg bodyweight of intravenous acetaminophen (Perfalgan, Bristol-Meyers Squibb, Utrecht, the Netherlands). The dosages were based on extrapolation of the intravenous dosages of the prodrug propacetamol used in older preterm infants (25). Patients were stratified for gestational age—i.e., 30 neonates aged 24–28 weeks’ gestational age and 30 neonates aged 281/7–32 weeks’ gestational age. Acetaminophen was administered via a 15-min infusion before peripheral central venous catheter placement within the first week of life. Five blood samples (0.2 ml per sample) were collected at different sample schedules, which were based on previous pharmacokinetic data on acetaminophen in neonates by Allegaert et al. (6). Samples were randomly taken either at T=0 (before start of acetaminophen administration), 20, 60, 240, and 540 min or at T=15 (after the acetaminophen infusion was completed), 30, 120, 360, and 720 min. Investigators, nursing, and medical staff taking care of the subjects were blinded for the administered dose of acetaminophen.
Drug Assay
Acetaminophen, acetaminophen–glucuronide, acetaminophen–sulfate, acetaminophen–glutathione, acetaminophen–cysteine, and acetaminophen–mercapturate were measured using high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry at the Center for Human Toxicology, University of Utah (Salt Lake City, UT) (27). The assay was linear over 0.05–50 μg/ml for acetaminophen, acetaminophen–glucuronide, and acetaminophen–sulfate and over 0.025–5.0, 0.01–5.0, and 0.01–1.0 μg/ml for acetaminophen–glutathione, acetaminophen–cysteine, and acetaminophen–mercapturate, respectively. The lower limits of the ranges represent the lower limits of quantification (LLOQs) of acetaminophen and its metabolites. The lower limit of detection for acetaminophen–glutathione was 0.1 ng/ml. Intra- and inter-assay accuracies ranged from 80 to 112%, and intra- and inter-assay imprecisions did not exceed 15%.
Data Reporting and Statistical Analysis
Acetaminophen metabolite concentrations were converted to μmol/l molar acetaminophen equivalents using molecular weights for each of the metabolites. Data clearance was performed before AUC calculation; the first time a concentration was below LLOQ, this value was set to 0.5 × LLOQ for that component. If a level was below LLOQ at two consecutive moments, this value was set to 0 μmol/l. The area under the plasma concentration–time curve for acetaminophen and metabolites over 0–500 min (AUC0–500 min) for each individual patient was calculated by noncompartmental analyses using WinNonLin software package (version 6.3; Pharsight, Mountain View, CA) and the linear-log trapezoidal rule.
In a per protocol analysis, Kruskal–Wallis tests with post hoc multiple comparison were applied to test for statistical differences in the median AUC0–500 min values of acetaminophen and metabolites, as well as the median AUC0–500 min ratios of acetaminophen–metabolite to acetaminophen between dose groups. Linear regression analysis was used to identify an association between AUC0–500 min ratios of each metabolite to acetaminophen and gestational age. A multivariate regression analysis was performed to analyze the significance of small for gestational age (SGA) on the AUC0–500 min ratios of the different acetaminophen metabolites to acetaminophen. Therefore, the three dosage groups were converted into two dummy variables. Two-sided P values<0.05 were considered statistically significant. Data were analyzed using SPSS version 22 (IBM, Armonk, NY).
Results
Patients and Data
In total, 266 neonates were assessed for eligibility, of whom 60 were randomized to receive acetaminophen in a dosage of 10, 15, or 20 mg/kg intravenously. Data of one patient in the 20 mg/kg dose group were excluded from analysis because AUC0–500 could not be calculated because of incomplete sampling data. In total, 293 samples were available, of 59 preterm neonates with a median gestational age of 27.9 weeks (range 24.0–31.1 weeks) and a median birth weight of 953 g (range 462–1550 g) treated with acetaminophen (Table 1). One subject in the 10-mg/kg group, who was suspected to be an outlier with high AUCs for all components, may have received a higher dose of acetaminophen, although this could not be confirmed.
Exposure of Acetaminophen and its Metabolites in the Three Dose Groups
The AUC0–500 min of acetaminophen and its glucuronide, sulfate, and oxidative metabolites in the three dose groups are shown in Figure 2. As concentrations for acetaminophen–glutathione were low, AUC0–500 min for this metabolite could not be calculated (59 samples above limit of detection in 27 neonates; 1 above LLOQ). For the other analytes, the percentage of samples below LLOQ was less than 10%, except for acetaminophen–glucuronide with 15% below LLOQ. The median AUC0–500 min of acetaminophen and its metabolites increased significantly with dose, except for acetaminophen–mercapturate (Figure 2). To correct the AUC0–500 min of the metabolite for the AUC0–500 of acetaminophen in each specific subject, ratios of median AUC0–500 min acetaminophen metabolite to acetaminophen were calculated. No significant difference in AUC ratio for any of the metabolites was found between the dose groups (Figure 3).
Boxplots of AUC0–500 min in preterm neonates (n=59) receiving either 10, 15, or 20 mg/kg of intravenous acetaminophen. (a) Acetaminophen and acetaminophen-sulfate. (b) Acetaminophen glucuronide, acetaminophen–cysteine, and acetaminophen-mercapturate. The boxes indicate the interquartile ranges, and the whiskers indicate the minimum and maximum ranges. Significance of Kruskal–Wallis test with post hoc multiple comparison was indicated with *P<0.05; **P<0.01; ***P<0.001.
Boxplot of AUC0–500 min ratio of sulfate, glucuronide, cysteine, and mercaptopurine metabolite to acetaminophen in preterm neonates (n=59) receiving either 10, 15, or 20 mg/kg of intravenous acetaminophen. The boxes indicate the interquartile ranges, and the whiskers indicate the minimum and maximum ranges. Significance of Kruskal–Wallis test with post hoc multiple comparison was indicated with *P<0.05; **P<0.01; ***P<0.001.
Metabolism of Acetaminophen in Relation to Gestational Age
Figure 4 shows the AUC0–500 min ratios of acetaminophen–metabolite to acetaminophen vs. gestational age. A positive association was found between acetaminophen–glucuronide and gestational age (slope=0.0054, P=0.020, R2=0.089). For acetaminophen–sulfate, a negative association with gestational age was found (slope=−0.093, P=0.001, R2=0.174). Paracetamol–cysteine and mercapturate were not associated with gestational age: P=0.638 and P=0.124, respectively (Figure 4). Subjects with suspected outlying values were checked, but none could be explained. Therefore, all were kept in the analyses.
Multivariate regression analysis showed that SGA was not a significant covariate for any of the ratios of AUC0–500 min acetaminophen–metabolite to acetaminophen. SGA showed a trend toward significance for the ratio of AUC0–500 min acetaminophen–sulfate to acetaminophen (P=0.075, B=−0.219).
Discussion
In this study, we have quantified the exposure to acetaminophen and its metabolites in very-preterm infants with a gestational age ranging from 24 to 32 weeks after a single dose of either 10, 15, or 20 mg intravenous acetaminophen per kg bodyweight. Analysis showed that the higher the dose, the higher the exposure, which theoretically can also result in higher efficacy. Importantly, a dose-related increase in exposure was found for none of the acetaminophen metabolites when corrected for the exposure to acetaminophen in each patient. Furthermore, no saturation was noticed for sulfation even after administration of 20 mg of acetaminophen per kg bodyweight. This is particularly of clinical relevance because in preterm neonates the glucuronidation capacity is still low (28) and the oxidative CYP2E1 pathway is potentially hepatotoxic. The contributions of both the nontoxic sulfation and the glucuronidation pathway in relation to the administered dose are important from a safety point of view because it is the CYP2E1 pathway that is involved in liver toxicity (18).
The finding that with advancing gestational age the contribution of glucuronidation to acetaminophen metabolism increased, while sulfation decreased, might indicate intrauterine maturation of glucuronidation between 24 and 32 weeks, as postnatal age at the time of dose was comparable at different gestational ages. Glucuronidation capacity is low after birth (29) and increases with bodyweight and/or postnatal age as was shown for morphine (21, 26). Theoretically, it could be postulated that the increase in glucuronidation of acetaminophen with gestational age is related to decreased sulfation. However, given that this increase in glucuronidation capacity was also shown for morphine, the main driver of these changes seems to be the increasing glucuronidation capacity after birth. In addition, in adults, in whom glucuronidation is at the maximum capacity, sulfation is of less relevance. Another reason for lower exposure to acetaminophen sulfate with gestational age could be an increased renal elimination clearance of acetaminophen sulfate (30).
With sulfation being the main pathway for acetaminophen in preterm neonates, and with glucuronidation increasing after birth, an important question is what the relevance is of the exposure to metabolites mediated by CYP2E1 in preterm infants. Plotting the AUC0–500 min of acetaminophen and its metabolites from our study (standardized to a dose of 15 mg/kg) vs. the AUC0–500 min from a previously published study in adults (standardized to a dose of 1,000 mg corresponding to 14.4 mg/kg) (31) revealed comparable acetaminophen exposure between neonates and adults (P=0.296; Figure 5). In contrast, not only the AUC of acetaminophen–sulfate but also those of acetaminophen–cysteine and acetaminophen mercapturate were higher in neonates (i.e., 2.9-, 3.4-, and 4.6-fold higher (all P<0.001)). As expected, the AUC0–500 min of acetaminophen-glucuronide was 18.5-fold lower in neonates compared with adults (P<0.05). Immature glucuronidation potentially leads to higher exposure to the potentially toxic CYP2E1 metabolites, NAPQI, and protein-derived acetaminophen–cysteine (18, 23). The level of exposure to acetaminophen–glutathione, acetaminophen–cysteine, and acetaminophen–mercapturate has been claimed to be a measure for exposure to the toxic CYP2E1–metabolites, NAPQI, and protein-derived acetaminophen–cysteine (23). We emphasize, however, that in the present study the metabolite concentrations were obtained after a single dose without reaching steady state, which limits the use of exposure for calculating the percentage that is being eliminated through a specific pathway. Next to acetaminophen–cysteine and acetaminophen–mercapturate, however, acetaminophen–glutathione was detected in 27 of the 59 preterm neonates. Although these concentrations could not be quantified within the validated range of the assay and need to be interpreted with caution, acetaminophen–glutathione was undetectable in a study in adults (31). Undetectable acetaminophen–glutathione levels in adults may be the result of faster transformation into acetaminophen–cysteine and subsequently into acetaminophen–mercapturate. Another possible explanation is the fact that in adults a smaller proportion of acetaminophen is metabolized through the oxidative pathway. The detectable concentrations of acetaminophen–glutathione in a substantial number of neonates in the present study add up to the higher exposure of the cysteine and mercapturate metabolites when compared with adults (Figure 5). An important question therefore pertains to the safety of intravenous paracetamol in preterm infants. So far, there is only limited evidence on the safety of acetaminophen in neonates below 32 weeks. Prior studies on (multiple) acetaminophen dosages from 10 to 20 mg/kg in preterm neonates up to 60 mg/kg/day for 9 days have not reported associations with hepatotoxicity (32, 33, 34, 35). Nevertheless, prolonged administration of acetaminophen in these vulnerable neonates may pose a problem. Van Ganzewinkel et al. (33), however, found no depletion of glutathione in very-preterm neonates after five 6-hourly doses of 7.5 mg/kg acetaminophen. As such, it seems too early to draw conclusions from the concentrations of cysteine and mercapturate measured in our study. Future research in preterm neonates should focus on the association between NAPQI protein adducts and hepatotoxicity. Advanced detection of even low-grade hepatotoxicity seems important when further considering acetaminophen as analgesic for preterm neonates. Intravenous acetaminophen in a dose as high as 60 mg/kg per day has also been proposed as an off‐label treatment of patent ductus arteriosus in preterm neonates (35, 36, 37, 38). Clearly, and particularly in those cases, its safety needs to be further studied.
Boxplots of AUC0–500 of acetaminophen and its metabolites in preterm neonates (n=59, 15 mg/kg) compared with data from adults (n=8, 1,000 g or 14.4 mg/kg)(31). The AUC0–500 min of acetaminophen and all of its metabolites in preterm neonates is standardized to a dose of 15 mg/kg vs. the AUC0–500 min in adults standardized to a dose of 1,000 mg (corresponding to 14.4 mg/kg). Acetaminophen AUC0–500 min is comparable between neonates and adults (Mann–Whitney test, P=0.296). In contrast, the AUC0–500 min of acetaminophen-sulfate, acetaminophen–cysteine, and acetaminophen-mercapturate was 2.9-, 3.4-, and 4.6-fold higher in neonates, respectively (Mann–Whitney test, all P<0.001). The AUC0–500 min of acetaminophen glucuronide was 18.5-fold lower in neonates compared with adults (Mann–Whitney test, P<0.05). The boxes indicate the interquartile ranges, and the whiskers indicate the minimum and maximum ranges. Significance of Mann–Whitney test was indicated with *P<0.05; **P<0.01; ***P<0.001. # Indicates one AUC value of 96,468 μmol·l/min, which is the maximum value.
Despite the strengths of this study, such as the relatively large scale, the stratification into two gestational age groups, and randomization to three different dosages, the fact that only a single dose was evaluated may be a limitation. Furthermore, the last sample was drawn at 9 or 12 h after dosing. Hence, for the AUC calculation we interpolated our data to 500 min after dosing. The number of blood samples per newborn was limited for ethical reasons. Sampling at later time points would have provided more insight in the exposure to the metabolites on a longer term. More data on both pharmacokinetics and safety are needed on prolonged acetaminophen administration, particularly with respect to the CYP2E1-mediated metabolites. In addition, pharmacokinetics studies of consecutive doses above 10 mg/kg have not been performed in very-preterm neonates, with even higher dosages gaining more interest with expansion of indications. However, the question is whether doses higher than 10 mg/kg are actually needed when acetaminophen is used for analgesia. To date, in the absence of more evidence, the optimal approach for providing analgesia in preterm neonates seems to aim for similar acetaminophen concentrations as in older children and adults. Using this approach, Wang et al. (22) proposed for preterm neonates weighing up to 1,500 g a 12 mg/kg loading dose, with which the target concentration of 9 mg/l is immediately reached, followed by a maintenance dose of around 6 mg/kg administered four times daily. Their estimated acetaminophen clearance of 0.25 l/h/kg was recently confirmed with a reported 0.15 l/h/kg by Cook et al. (20). Furthermore, it is important to realize that the concentrations of metabolites are not only determined by the fraction of the dose that is transformed into each metabolite but also by the metabolites’ volume of distribution and rate of elimination. Differences in exposure between preterm infants and adults may therefore not only be caused by an increased formation but also by differences in volume of distribution or reduced elimination of the metabolite in question. Another issue is the quantification of acetaminophen–glutathione, and more specifically the instability of acetaminophen–glutathione after sample collection (27). Hydrolysis of acetaminophen–glutathione quickly transforms it to acetaminophen–cysteine, presumably by gamma-glutamyl transpeptidase and dipeptidases. This might lead to an underestimation of the concentration of glutathione at the time of sample collection, and may lead to an increased acetaminophen–cysteine concentration. For future research, addition of peptidase inhibitors during sample collection could prevent or reduce this degradation.
Conclusions
In this study, we found that acetaminophen glucuronidation is low in very-preterm infants and increases with gestational age, already detected from 24 to 32 weeks of gestation. Exposure to acetaminophen-sulfate was high, but did not show saturation, not even after administration of 20 mg acetaminophen per kg bodyweight, which is a relevant and comforting finding for clinical practice. Compared with adults, a more than threefold increase in exposure to sulfate, cysteine, and mercaptopurine metabolites was found, which calls for future investigation on the complete maturation of acetaminophen metabolism during infancy. Furthermore, further research is required on the efficacy and safety of acetaminophen in the smallest newborns, as well as in older infants, and with respect to prolonged acetaminophen dosing.
References
Roofthooft DW, Simons SH, Anand KJ et al, Eight years later are we still hurting newborn infants? Neonatology 2014;105:218–26.
Carbajal R, Rousset A, Danan C et al, Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300:60–70.
Menon G, McIntosh N. How should we manage pain in ventilated neonates? Neonatology 2008;93:316–23.
Carbajal R, Eriksson M, Courtois E et al, Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir Med 2015;3:796–812.
Autret E, Dutertre JP, Breteau M et al, Pharmacokinetics of paracetamol in the neonate and infant after administration of propacetamol chlorhydrate. Dev Pharmacol Ther 1993;20:129–34.
Allegaert K, Anderson BJ, Naulaers G et al, Intravenous paracetamol (propacetamol) pharmacokinetics in term and preterm neonates. Eur J Clin Pharmacol 2004;60:191–7.
Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 1980;10 (Suppl 2):291S–8SS.
Kim DW, Tan EY, Jin Y et al, Effects of imatinib mesylate on the pharmacokinetics of paracetamol (acetaminophen) in Korean patients with chronic myelogenous leukaemia. Br J Clin Pharmacol 2011;71:199–206.
Arana A, Morton NS, Hansen TG. Treatment with paracetamol in infants. Acta Anaesthesiol Scand 2001;45:20–9.
Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol 2002;40:3–20.
Park JM, Lin YS, Calamia JC et al, Transiently altered acetaminophen metabolism after liver transplantation. Clin Pharmacol Ther 2003;73:545–53.
Manyike PT, Kharasch ED, Kalhorn TF et al, Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther 2000;67:275–82.
Chun LJ, Tong MJ, Busuttil RW et al, Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol 2009;43:342–9.
Clements JA, Critchley JA, Prescott LF . The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br J Clin Pharmacol 1984;18:481–5.
Critchley JA, Nimmo GR, Gregson CA et al, Inter-subject and ethnic differences in paracetamol metabolism. Br J Clin Pharmacol 1986;22:649–57.
Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet 1982;7:93–107.
Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 2010: 369–405.
Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 2012;44:88–106.
Larson AM, Polson J, Fontana RJ et al, Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364–72.
Cook SF, Roberts JK, Samiee-Zafarghandy S et al, Population Pharmacokinetics of intravenous paracetamol (acetaminophen) in preterm and term neonates: model development and external evaluation. Clin Pharmacokinet 2016;55:107–19.
Cook SF, Stockmann C, Samiee-Zafarghandy S et al, Neonatal maturation of paracetamol (acetaminophen) glucuronidation, sulfation, and oxidation based on a parent-metabolite population pharmacokinetic model. Clin Pharmacokinet 2016;55:1395–411.
Wang C, Allegaert K, Tibboel D et al, Population pharmacokinetics of paracetamol across the human age-range from (pre)term neonates, infants, children to adults. J Clin Pharmacol 2014;54:619–29.
McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 2013;30:2174–87.
Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 2008;118:250–267.
Allegaert K, de Hoon J, Verbesselt R et al, Intra- and interindividual variability of glucuronidation of paracetamol during repeated administration of propacetamol in neonates. Acta Paediatr 2005;94:1273–9.
Knibbe CA, Krekels EH, van den Anker JN et al, Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet 2009;48:371–85.
Cook SF, King AD, van den Anker JN et al, Simultaneous quantification of acetaminophen and five acetaminophen metabolites in human plasma and urine by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: method validation and application to a neonatal pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2015;1007:30–42.
van Lingen RA, Deinum JT, Quak JM et al, Pharmacokinetics and metabolism of rectally administered paracetamol in preterm neonates. Arch Dis Child Fetal Neonatal Ed 1999;80:F59–63.
Krekels EH, Danhof M, Tibboel D et al, Ontogeny of hepatic glucuronidation; methods and results. Curr Drug Metab 2012;13:728–43.
Krekels EH, van Ham S, Allegaert K et al, Developmental changes rather than repeated administration drive paracetamol glucuronidation in neonates and infants. Eur J Clin Pharmacol 2015;71:1075–82.
van Rongen A, Valitalo PA, Peeters MY et al, Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet 2016;55:833–847.
Allegaert K, Rayyan M, De Rijdt T et al, Hepatic tolerance of repeated intravenous paracetamol administration in neonates. Paediatr Anaesth 2008;18:388–92.
van Ganzewinkel C, Derijks L, Anand KJ et al, Multiple intravenous doses of paracetamol result in a predictable pharmacokinetic profile in very preterm infants. Acta Paediatr 2014;103:612–7.
Allegaert K, Palmer GM, Anderson BJ. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch Dis Child 2011;96:575–80.
Roofthooft DW, van Beynum IM, de Klerk JC et al, Limited effects of intravenous paracetamol on patent ductus arteriosus in very low birth weight infants with contraindications for ibuprofen or after ibuprofen failure. Eur J Pediatr 2015;174:1433–40.
Allegaert K, Anderson B, Simons S et al, Paracetamol to induce ductus arteriosus closure: is it valid? Arch Dis Child 2013;98:462–6.
Hammerman C, Bin-Nun A, Markovitch E et al, Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics 2011;128:e1618–21.
Terrin G, Conte F, Oncel MY et al, Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2016;101:F127–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
This study was enabled by funding from the Netherlands Organisation for Health Research and Development ZonMw (Grant number: 80-83600-98-10190).
Rights and permissions
About this article
Cite this article
Flint, R., Roofthooft, D., van Rongen, A. et al. Exposure to acetaminophen and all its metabolites upon 10, 15, and 20 mg/kg intravenous acetaminophen in very-preterm infants. Pediatr Res 82, 678–684 (2017). https://doi.org/10.1038/pr.2017.129
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.129
- Springer Nature America, Inc.
This article is cited by
-
Is postnatal acetaminophen therapy problematic for preterm infants?
Pediatric Research (2024)
-
Postnatal acetaminophen exposure and neurodevelopmental outcomes at 18–21 months corrected gestational age in preterm infants <29 weeks gestation: a retrospective cohort study
Pediatric Research (2023)
-
Acetaminophen for the patent ductus arteriosus: has safety been adequately demonstrated?
Journal of Perinatology (2023)
-
Maturation of Paracetamol Elimination Routes in Preterm Neonates Born Below 32 Weeks of Gestation
Pharmaceutical Research (2023)
-
Unraveling the effect of intra- and intercellular processes on acetaminophen-induced liver injury
npj Systems Biology and Applications (2022)