Abstract
Background
The aim of this study was to describe propacetamol pharmacokinetics in term and preterm neonates to suggest dosing regimens.
Methods
A population pharmacokinetic analysis of paracetamol (acetaminophen) time–concentration profiles in 48 neonates was undertaken using non-linear mixed-effects models. Neonates were given either single (n=30) or multiple doses (n=18) of propacetamol infusion over 15 min. Neonates had a median postnatal age of 1 day (range, 1–76 days). Median post-conceptual age (PCA) was 35 weeks (range, 27–42 weeks), and median weight was 2.4 kg (range, 0.51–4 kg).
Results
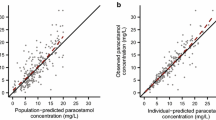
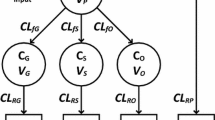
The population volume of distribution estimate and between-subject variability (%) for a one-compartment model with zero-order input and first-order elimination was 70.4 l (30.7%)/70 kg. Clearance increased from 2.85 l/70 kg, CV 40.7% at 27 weeks PCA to reach 7.05 l/h per 70 kg by 42 weeks PCA (standardised to a 70-kg person using allometric “1/4-power” models). Between-occasion variability for volume of distribution and clearance were 17.4% and 26%, respectively.
Conclusions
A mean paracetamol steady-state target concentration above 10 mg/l at trough can be achieved using a loading dose of 40 mg/kg and maintenance doses of 20 mg/kg 6 h in 28-week PCA neonates, 25 mg/kg 6 h at 32 weeks, 30 mg/kg 6 h at 36 weeks and 20 mg/kg 4 h at term (propacetamol doses). Since the role of the oxidative enzyme CYP2E1 and production of the hepatotoxic metabolite N-acetyl-p-benzoquinone-imine still is unknown in premature neonates, lower doses scaled by age-related clearance and centred on a daily dose of 60 mg/kg per day in a child of 6–8 years with a clearance of 0.25 l/h per kg (12.5 l/h per 70 kg) may be more appropriate.





Similar content being viewed by others
References
Barr J, Brenner-Zada G, Heiman E et al (2002) Unlicensed and off-label medication use in a neonatal intensive care unit: a prospective study. Am J Perinatol 19:67–72
Allegaert K, Tibboel D, Naulaers G et al (2003) Systematic evaluation of pain in neonates: effect on the number of intravenous analgesics prescribed. Eur J Clin Pharmacol 59:87–90
Autret E, Dutertre JP, Breteau M, Jonville AP, Furet Y, Laugier J (1993) Pharmacokinetics of paracetamol in the neonate and infant after administration of propacetamol chlorhydrate. Dev Pharmacol Ther 20:129–134
Allegaert K, van der Marel CD, Debeer A et al (2004) Pharmacokinetics of single dose intravenous propacetamol in neonates: effect of gestational age. Arch Dis Child Fetal Neonat Ed 89:F25–F28
Depre M, van Hecken A, Verbesselt R, Tjandra-Maga TB, Gerin M, de Schepper PJ (1992) Tolerance and pharmacokinetics of propacetamol, a paracetamol formulation for intravenous use. Fundam Clin Pharmacol 6:259–262
Ganry JC, Rod B, Boccard E, Hermann P, Gendron A, Saint-Maurice C (1992) Pharmacokinetics and antipyretic effects on an injectable pro-drug of paracetamol (propacetamol) in children. Pediatr Anaesth 2:291–295
Anderson BJ, van Lingen RA, Hansen TA, Lin Y-C, Holford NHG (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants. Anesthesiology 96:1336–1345
Van Lingen RA, Simons SH, Anderson BJ, Tibboel D (2002) The effects of analgesia in the vulnerable infant during the perinatal period. Clin Perinatol 29:511–34
Van Der Marel CD, Anderson BJ, Van Lingen RA et al (2003) Paracetamol and metabolite pharmacokinetics in infants. Eur J Clin Pharmacol 59:243–251
Ameer B, Greenblatt DJ, Divoll M, Abernethy DR, Shargel L (1981) High-performance liquid chromatographic determination of acetaminophen in plasma: single-dose pharmacokinetic studies. J Chromatogr 226:224–230
Buskin JN, Upton RA, Williams RL (1982) Improved acetaminophen assay sensitivity by modification of a high-performance liquid chromatography technique. J Chromatogr 230:443–447
Beal SL, Sheiner LB, Boeckmann A (1999) Nonmember user’s guide. Division of Pharmacology, University of California, San Francisco
Holford NHG (1996) A size standard for pharmacokinetics. Clin Pharmacokinet 30:329–332
Anderson BJ, Meakin GH (2002) Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth 12:205–219
Peters HP (1983) Physiological correlates of size. In: Beck E, Birks HJB, Conner EF (eds) The ecological implications of body size. Cambridge University Press, Cambridge, pp 48–53
Prothero JW (1980) Scaling of blood parameters in animals. Comp Biochem Physiol 67:649–657
Gabrielsson J, Weiner D (1994) Interspecies scaling. In: Gabrielsson J, Weiner D (eds) Pharmacokinetic and pharmacodynamic data analysis. Swedish Pharmaceutical Press Ltd, Stockholm, pp 153–171
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Anderson BJ, Woollard GA, Holford NH (2001) Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol 57:559–569
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284:1677–1679
Holford NHG (1999) Target concentration intervention: beyond Y2K. Br J Clin Pharmacol 48:9–13
Krumbiegel P, Domke S, Morseburg B, Boehm G, Braun W (1997) Maturation of hepatosomal mono-oxygenation and glucuronidation activities in pre- and full-term infants as studied using the [15 N]methacetin urine test. Acta Paediatr 86:1236–1240
Anderson BJ, Woollard GA, Holford NH (2000) A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol 50:125–134
Nielsen JC, Bjerring P, Arendt Nielsen L, Petterson KJ (1992) Analgesic efficacy of immediate and sustained release paracetamol and plasma concentration of paracetamol. Double blind, placebo-controlled evaluation using painful laser stimulation. Eur J Clin Pharmacol 42:261–264
Heubi JE, Bien JP (1997) Acetaminophen use in children: more is not better. J Pediatr 130:175–177
Acknowledgements
We greatly acknowledge the assistance of Marien A.L. Pluim, PharmD, Department of Pharmacy, Erasmus Medical Centre, Rotterdam, The Netherlands and of S. Demarsin, laboratory technician, Center for Clinical Pharmacology, Leuven, Belgium for analysing the plasma samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research of Gunnar Naulaers is supported by the Fund for Scientific Research—Flanders (Belgium), Clinical Doctoral Grant A6/5—CM. D 11.354. Warning: all doses in this paper are reported as prodrug; 1 g of propacetamol equals 0.5 g of paracetamol.
Rights and permissions
About this article
Cite this article
Allegaert, K., Anderson, B.J., Naulaers, G. et al. Intravenous paracetamol (propacetamol) pharmacokinetics in term and preterm neonates. Eur J Clin Pharmacol 60, 191–197 (2004). https://doi.org/10.1007/s00228-004-0756-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0756-x