Abstract
Objectives
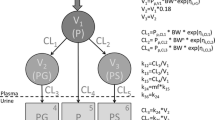
This study aimed to model the population pharmacokinetics of intravenous paracetamol and its major metabolites in neonates and to identify influential patient characteristics, especially those affecting the formation clearance (CLformation) of oxidative pathway metabolites.
Methods
Neonates with a clinical indication for intravenous analgesia received five 15-mg/kg doses of paracetamol at 12-h intervals (<28 weeks’ gestation) or seven 15-mg/kg doses at 8-h intervals (≥28 weeks’ gestation). Plasma and urine were sampled throughout the 72-h study period. Concentration–time data for paracetamol, paracetamol-glucuronide, paracetamol-sulfate, and the combined oxidative pathway metabolites (paracetamol-cysteine and paracetamol-N-acetylcysteine) were simultaneously modeled in NONMEM 7.2.
Results
The model incorporated 259 plasma and 350 urine samples from 35 neonates with a mean gestational age of 33.6 weeks (standard deviation 6.6). CLformation for all metabolites increased with weight; CLformation for glucuronidation and oxidation also increased with postnatal age. At the mean weight (2.3 kg) and postnatal age (7.5 days), CLformation estimates (bootstrap 95% confidence interval; between-subject variability) were 0.049 L/h (0.038–0.062; 62 %) for glucuronidation, 0.21 L/h (0.17–0.24; 33 %) for sulfation, and 0.058 L/h (0.044–0.078; 72 %) for oxidation. Expression of individual oxidation CLformation as a fraction of total individual paracetamol clearance showed that, on average, fractional oxidation CLformation increased <15 % when plotted against weight or postnatal age.
Conclusions
The parent–metabolite model successfully characterized the pharmacokinetics of intravenous paracetamol and its metabolites in neonates. Maturational changes in the fraction of paracetamol undergoing oxidation were small relative to between-subject variability.






Similar content being viewed by others
References
Allegaert K, Tibboel D, van den Anker J. Pharmacological treatment of neonatal pain: in search of a new equipoise. Semin Fetal Neonatal Med. 2013;18(1):42–7.
Pacifici GM, Allegaert K. Clinical pharmacology of paracetamol in neonates: a review. Curr Ther Res Clin Exp. 2015;77:24–30.
Duggan ST, Scott LJ. Intravenous paracetamol (acetaminophen). Drugs. 2009;69(1):101–13.
Allegaert K, Palmer GM, Anderson BJ. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch Dis Child. 2011;96(6):575–80.
Zuppa AF, Hammer GB, Barrett JS, Kenney BF, Kassir N, Mouksassi S, et al. Safety and population pharmacokinetic analysis of intravenous acetaminophen in neonates, infants, children, and adolescents with pain or fever. J Pediatr Pharmacol Ther. 2011;16(4):246–61.
van Ganzewinkel C, Derijks L, Anand KJ, van Lingen RA, Neef C, Kramer BW, et al. Multiple intravenous doses of paracetamol result in a predictable pharmacokinetic profile in very preterm infants. Acta Paediatr. 2014;103(6):612–7.
Cook SF, Roberts JK, Samiee-Zafarghandy S, Stockmann C, King AD, Deutsch N, et al. Population pharmacokinetics of intravenous paracetamol (acetaminophen) in preterm and term neonates: model development and external evaluation. Clin Pharmacokinet. 2016;55(1):107–19.
Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology. 2002;96(6):1336–45.
Allegaert K, Vanhaesebrouck S, Verbesselt R, van den Anker JN. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31(4):411–5.
McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30(9):2174–87.
James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–506.
Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44(1):88–106.
Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405.
Levy G, Khanna NN, Soda DM, Tsuzuki O, Stern L. Pharmacokinetics of acetaminophen in the human neonate: formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and d-glucaric acid excretion. Pediatrics. 1975;55(6):818–25.
Miller RP, Roberts RJ, Fischer LJ. Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther. 1976;19(3):284–94.
van Lingen RA, Deinum JT, Quak JM, Kuizenga AJ, van Dam JG, Anand KJ, et al. Pharmacokinetics and metabolism of rectally administered paracetamol in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80(1):F59–63.
Allegaert K, de Hoon J, Verbesselt R, Vanhole C, Devlieger H, Tibboel D. Intra- and interindividual variability of glucuronidation of paracetamol during repeated administration of propacetamol in neonates. Acta Paediatr. 2005;94(9):1273–9.
Krekels EH, van Ham S, Allegaert K, de Hoon J, Tibboel D, Danhof M, et al. Developmental changes rather than repeated administration drive paracetamol glucuronidation in neonates and infants. Eur J Clin Pharmacol. 2015;71(9):1075–82.
Cook SF, King AD, van den Anker JN, Wilkins DG. Simultaneous quantification of acetaminophen and five acetaminophen metabolites in human plasma and urine by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: method validation and application to a neonatal pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1007:30–42.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Boeckmann AJ, Sheiner LB, Beal SL. NONMEM users guide, part v: introductory guide. San Francisco: NONMEM Project Group, University of California at San Francisco; 2011.
Bauer RJ. NONMEM users guide: introduction to NONMEM 7.2.0. Ellicott City: ICON Development Solutions; 2011.
Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike information criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22(5):431–45.
Owens KH, Murphy PG, Medlicott NJ, Kennedy J, Zacharias M, Curran N, et al. Population pharmacokinetics of intravenous acetaminophen and its metabolites in major surgical patients. J Pharmacokinet Pharmacodyn. 2014;41(3):211–21.
Kulo A, Peeters MY, Allegaert K, Smits A, de Hoon J, Verbesselt R, et al. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. Br J Clin Pharmacol. 2013;75(3):850–60.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development, part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2(4):e38.
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37.
Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev. 2011;43(1):41–52.
Krasniak AE, Knipp GT, Svensson CK, Liu W. Pharmacogenomics of acetaminophen in pediatric populations: a moving target. Front Genet. 2014;5:314.
Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT. Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther. 2007;81(6):840–8.
Owens KH, Medlicott NJ, Zacharias M, Curran N, Chary S, Thompson-Fawcett M, et al. The pharmacokinetic profile of intravenous paracetamol in adult patients undergoing major abdominal surgery. Ther Drug Monit. 2012;34(6):713–21.
Allegaert K, Verbesselt R, Rayyan M, Debeer A, de Hoon J. Urinary metabolites to assess in vivo ontogeny of hepatic drug metabolism in early neonatal life. Methods Find Exp Clin Pharmacol. 2007;29(4):251–6.
van der Marel CD, Anderson BJ, van Lingen RA, Holford NH, Pluim MA, Jansman FG, et al. Paracetamol and metabolite pharmacokinetics in infants. Eur J Clin Pharmacol. 2003;59(3):243–51.
Owen JS, Fiedler-Kelly J. Introduction to population pharmacokinetic/pharmacodynamic analysis with nonlinear mixed effects models. London: Wiley; 2014.
Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486–95.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:e50.
Brendel K, Comets E, Laffont C, Mentre F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010;37(1):49–65.
Holford NHG, Gobburu JVS, Mould DR, editors. Implications of including and excluding correlation of random effects in hierarchical mixed effects pharmacokinetic models. Verona: Population Approach Group in Europe; 2003.
Salinger DH, Blough DK, Vicini P, Anasetti C, O’Donnell PV, Sandmaier BM, et al. A limited sampling schedule to estimate individual pharmacokinetic parameters of fludarabine in hematopoietic cell transplant patients. Clin Cancer Res. 2009;15(16):5280–7.
Lowenthal DT, Oie S, Van Stone JC, Briggs WA, Levy G. Pharmacokinetics of acetaminophen elimination by anephric patients. J Pharmacol Exp Ther. 1976;196(3):570–8.
Johnsrud EK, Koukouritaki SB, Divakaran K, Brunengraber LL, Hines RN, McCarver DG. Human hepatic CYP2E1 expression during development. J Pharmacol Exp Ther. 2003;307(1):402–7.
Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver: hypermethylation control of gene expression during the neonatal period. Eur J Biochem. 1996;238(2):476–83.
Krekels EH, Danhof M, Tibboel D, Knibbe CA. Ontogeny of hepatic glucuronidation; methods and results. Curr Drug Metab. 2012;13(6):728–43.
Xie Y, McGill MR, Cook SF, Sharpe MR, Winefield RD, Wilkins DG, et al. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica. 2015;45(10):921–9.
Allegaert K, Anderson BJ, Naulaers G, de Hoon J, Verbesselt R, Debeer A, et al. Intravenous paracetamol (propacetamol) pharmacokinetics in term and preterm neonates. Eur J Clin Pharmacol. 2004;60(3):191–7.
Palmer GM, Atkins M, Anderson BJ, Smith KR, Culnane TJ, McNally CM, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. Br J Anaesth. 2008;101(4):523–30.
Siegers CP, Loeser W, Gieselmann J, Oltmanns D. Biliary and renal excretion of paracetamol in man. Pharmacology. 1984;29(5):301–3.
Jayasinghe KS, Roberts CJ, Read AE. Is biliary excretion of paracetamol significant in man? Br J Clin Pharmacol. 1986;22(3):363–6.
Allegaert K, Naulaers G, Vanhaesebrouck S, Anderson BJ. The paracetamol concentration-effect relation in neonates. Paediatr Anaesth. 2013;23(1):45–50.
Acknowledgments
The authors thank Dr. Syamala Mankala of the Division of Clinical Pharmacology at Children’s National Health System (Washington, DC, USA) for administrative support, Dr. David M. Reith of the Dunedin School of Medicine at the University of Otago (Dunedin, New Zealand) and Dr. Katie H. Owens of the Department of Pharmaceutics at the University of Washington (Seattle, WA, USA) for helpful advice during model development, and Dr. Jessica K. Roberts of the Department of Pharmaceutical Sciences at St. Jude Children’s Research Hospital (Memphis, TN, USA) for constructive feedback during manuscript preparation.
Author contributions
JNA designed the clinical study; JNA, SSZ, ND, and EFW performed the trial and acquired the clinical data; SFC, ADK, and DGW developed and validated the analytical methods and analyzed the study samples; SFC and CS developed the pharmacokinetic model; CMTS supervised the pharmacokinetic model development; SFC drafted the manuscript. All authors critically revised the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Institutes of Health grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD060543; to John van den Anker) and the National Center for Advancing Translational Sciences (UL1TR000075; to Children’s National Health System) and by a contract for analytical laboratory services from McNeil Consumer Healthcare (Division of McNEIL-PPC, Inc., Fort Washington, PA, USA; to Diana Wilkins). Sarah Cook received stipend support from the Howard Hughes Medical Institute (Med into Grad Initiative). Sarah Cook and Chris Stockmann were supported by pre-doctoral fellowships from the American Foundation for Pharmaceutical Education.
Conflicts of interest
Sarah Cook, Chris Stockmann, Samira Samiee-Zafarghandy, Amber King, Nina Deutsch, Elaine Williams, Diana Wilkins, Catherine Sherwin, and John van den Anker have no potential conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from a parent or legal guardian of all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cook, S.F., Stockmann, C., Samiee-Zafarghandy, S. et al. Neonatal Maturation of Paracetamol (Acetaminophen) Glucuronidation, Sulfation, and Oxidation Based on a Parent–Metabolite Population Pharmacokinetic Model. Clin Pharmacokinet 55, 1395–1411 (2016). https://doi.org/10.1007/s40262-016-0408-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0408-1