Abstract
Background
Major depressive disorder (MDD) affects many people in the world. However, around 40% of patients do not respond to any pharmacological drugs. An alternative is to use a combination of different pharmacological groups or the combination of a classical antidepressant with a substance that can potentiate its effect. Thus, this study aimed to investigate the synergistic interactions between different antidepressants, including fluoxetine, quetiapine and lamotrigine in combination with ketamine, a N-methyl-d-aspartate (NMDA) receptor antagonist.
Methods
Wistar rats were acutely treated with fluoxetine (1.25 mg/kg), quetiapine (5 mg/kg), and lamotrigine (5.0 mg/kg) alone or in combination with ketamine (5.0 mg/kg), and then subjected to behavioral tests. In addition, oxidative damage and antioxidant capacity were assessed in the rat brain, and pro-inflammatory cytokines levels were evaluated in the serum.
Results
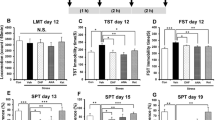
It was observed a synergistic effect of ketamine in combination with fluoxetine on the immobility time in the forced swimming test, indicating an antidepressant effect. Other antidepressant did not show effects when administrated alone or joint to ketamine. The combination of ketamine with other antidepressants, particularly quetiapine, in some brain regions induced an increase in damage to lipids and proteins. However, the combination of ketamine with fluoxetine increased the antioxidant activity of superoxide dismutase, and decreased oxidative damage, thus suggesting a neuroprotective effect of the combination of these drugs. The combination of ketamine with fluoxetine or lamotrigine reduced pro-inflammatory cytokines levels.
Conclusion
In conclusion, ketamine induced antioxidant or pro-antioxidant effects dependent of antidepressant classes or brain area.
Similar content being viewed by others
References
Pio de Almeida LS, Jansen K, Köhler CA, Pinheiro RT, da Silva RA, Bonini JS. Working and short-term memories are impaired in postpartum depression. J Affect Disord 2012;136:1238–42.
Ceretta LB, Réus GZ, Abelaira HM, Jornada LK, Schwalm MT, Hoepers NJ, et al. Increased prevalence of mood disorders and suicidal ideation in type 2 diabetic patients. Acta Diabetol 2012;1:227–34.
Güzel Özdemir P, Boysan M, Smolensky MH, Selvi Y, Aydin A, Yilmaz E. Comparison of venlafaxine alone versus venlafaxine plus bright light therapy combination for severe major depressive disorder. J Clin Psychiatry. 2015;76:645–54.
Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord 2016;203:204–12.
Salehi I, Hosseini SM, Haghighi M, Jahangard L, Bajoghli H, Gerber M, et al. Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. J Psychiatr Res 2016;76:1–8.
Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Arch Psychiat und Nervenkr 1982;232:299–304.
Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby Jr. CR, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1155–8.
Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 2007;62:1310–6.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:70–5.
Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, et al. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Arch Psychiatr Nervenkr 2014;231:685–93.
Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, et al. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci Lett 2015;584:83–7.
Zarate Jr. CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006;63:856–64.
Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 2014;231:3663–76.
Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:140–4.
Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:450–5.
Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, et al. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res 2011;221:166–71.
Réus GZ, Abelaira HM, dos Santos MA, Carlessi AS, Tomaz DB, Neotti MV, et al. Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res 2013;256:451–6.
Réus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, et al. MAPK signaling correlates with the antidepressant effects of ketamine. J Psychiatr Res 2014;55:15–21.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010;329:959–64.
Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci 2013;118:3–8.
Réus GZ, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, Carlessi AS, et al. Anxious phenotypes plus environmental stressors are related to brain DNA damage and changes in NMDA receptor subunits and glutamate uptake. Mut Res 2015;772:30–7.
Réus GZ, Abelaira HM, Maciel AL, Dos Santos MA, Carlessi AS, Steckert AV, et al. Minocycline protects against oxidative damage and alters energy metabolism parameters in the brain of rats subjected to chronic mild stress. Metab Brain Dis 2015;30:545–53.
Réus GZ, Carlessi AS, Titus SE, Abelaira HM, Ignácio ZM, da Luz JR, et al. A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Dev Neurobiol 2015;75:1268–81.
Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015;51:164–75.
Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 2009;54:358–62.
Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, et al. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatry Res 2009;43:864–9.
Arent CO, Réus GZ, Abelaira HM, Ribeiro KF, Steckert AV, Mina F, et al. Synergist effects of n-acetylcysteine and deferoxamine treatment on behavioral and toxidative parameters induced by chronic mild stress in rats. Neurochem Int 2012;61:1072–80.
Della FP, Abelaira HM, Réus GZ, Antunes AR, Dos Santos MA, Zappelinni G, et al. Tianeptine exerts neuroprotective effects in the brain tissue of rats exposed to the chronic stress model. Pharmacol Biochem Behav 2012;103:395–402.
Abelaira HM, Réus GZ, Ribeiro KF, Steckert AV, Mina F, Rosa DV, et al. Effects of lamotrigine on behavior, oxidative parameters and signaling cascades in rats exposed to the chronic mild stress model. Neurosci Res 2013;75:24–30.
Ortmann CF, Réus GZ, Ignácio ZM, Abelaira HM, Titus SE, de Carvalho P, et al. Enriched flavonoid fraction from Cecropia pachystachya Trécul leaves exerts antidepressant-like behavior and protects brain against oxidative stress in rats subjected to chronic mild stress. Neurotoxic Res 2016;29:469–83.
Kim Y, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:1044–53.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta- analysis of cytokines in major depression. Biol Psychiatry 2010;67:446–57.
Zellweger MJ, Osterwalder RH, Langewitz W, Pfisterer ME. Coronary artery disease and depression. Eur Heart J 2004;25:3–9.
Porsolt RD, Le Pichon M, Jalfre M. Animal model of depression. Nature 1977;266:730–2.
Réus GZ, Abelaira HM, Stringari RB, Fries GR, Kapczinski F, Quevedo J. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in theprefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis 2012;27:175–82.
Réus GZ, Abelaira HM, Agostinho FR, Ribeiro KF, Vitto MF, Luciano TF, et al. The administration of olanzapine and fluoxetine has synergistic effects on intracellular survival pathways in the rat brain. J Psychiatry Res 2012;46:1029–35.
Rosa PB, Ribeiro CM, Bettio LE, Colla A, Lieberknecht V, Moretti M, et al. Folic acid prevents depressive-like behavior induced by chronic corticosterone treatment in mice. Pharmacol Biochem Behav 2014;127:1–6.
Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005;52:90–110.
Paxinos G, Watson C. The rat brain: stereotaxic coordinates. second ed. Australia: Academic Press; 1986.
Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990;86:421–31.
Levine RL, Garland D, Oliver CN. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464–78.
Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121–6.
Bannister JV, Calabrese L. Assays for superoxide dismutase. Methods Biochem Anal 1987;32:279–312.
Maoz H. Failure of first SSRI for depression—what is the next step? Isr J Psychiatry Relat Sci 2007;44:327–9.
Carvalho AF, Cavalcante JL, Castelo MS, Lima MC. Augmentation strategies for treatment-resistant depression: a literature review. J Clin Pharm Ther 2007;32:415–28.
Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N-Desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist: as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology 2008;33:2303–12.
Björkholm C, Jardemark K, Schilström B, Svensson TH. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur Neuropsychopharmacol 2015;25:1842–7.
Maj J, Rogoz Z, Skuza G, Wedzony K. The synergistic effect of fluoxetine on the locomotor hyperactivity induced by MK-801, a non-competitive NMDA receptor antagonist. J Neural Transm 1996;103:131–46.
Yang C, Li WY, Yu HY, Gao ZQ, Liu XL, Zhou ZQ, et al. Tramadol pretreatment enhances ketamine-induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex. J Biomed Biotechnol 2012;2012:175619.
Yang C, Li X, Wang N, Xu S, Yang J, Zhou Z. Tramadol reinforces antidepressant effects of ketamine with increased levels of brain-derived neurotrophic factor and tropomyosin-related kinase B in rat hippocampus. Front Med 2012;6:411–5.
McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 2014;156:1–7.
Agostinho FR, Réus GZ, Stringari RB, Ribeiro KF, Pfaffenseller B, Stertz L, et al. Olanzapine plus fluoxetine treatment increases Nt-3 protein levels in the rat prefrontal cortex. Neurosci Lett 2011;497:99–103.
Rogóz Z, Skuza G, Maj J, Danysz W. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology 2002;42:1024–30.
Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats, a new model sensitive to antidepressant treatments. Eur J Pharmacol 1978;47:379–91.
Mjellem N, Lund A, Hole K. Reduction of NMDA-induced behaviour after acute and chronic desipramine in mice. Neuropharmacology 1993;32:591–5.
Fedorova M, Griesser E, Vemula V, Weber D, Ni Z, Hoffmann R. Protein and lipid carbonylation in cellular model of nitrosative stress: mass spectrometry, biochemistry and microscopy study. Free Radic Biol Med 2014;1:15.
Réus GZ, Abaleira HM, Titus SE, Arent CO, Michels M, da Luz JR, et al. Effects of ketamine administration on the phosphorylation levels of CREB and TrKB and on oxidative damage after infusion of MEK inhibitor. Pharmacol Rep 2016;68:177–84.
Abelaira HM, Réus GZ, Ignácio ZM, Dos Santos MA, de Moura AB, Matos D, et al. Ketamine exhibits different neuroanatomical profile after mammalian target of rapamycin inhibition in the prefrontal cortex: the role of inflammation and oxidative stress. Mol Neurobiol 2016 in press.
Zafir A, Ara A, Banu N. In vivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:220–8.
Gałecki P, Szemraj J, Bieńkiewicz M, Zboralski K, Gałecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol 2009;24:277–86.
Agostinho FR, Jornada LK, Schröder N, Roesler R, Dal-Pizzol F, Quevedo J. Effects of chronic haloperidol and/or clozapine on oxidative stress parameters in rat brain. Neurochem Res 2007;32:1343–50.
Kriisa K, Haring L, Vasar E, Koido K, Janno S, Vasar V, et al. Antipsychotic treatment reduces indices of oxidative stress in first-episode psychosis patients. Oxid Med Cell Longev 2016;2016:9616593.
Nathan C. Points of control in inflammation. Nature 2002;420:846–52.
Woodroofe MN. Cytokine production in the central nervous system. Neurology 1995;45:6–10.
Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 2013;103:853–9.
De Long NE, Hyslop JR, Raha S, Hardy DB, Holloway AC. Fluoxetine- induced pancreatic beta cell dysfunction: new insight into the benefits of folic acid in the treatment of depression. J Affect Disord 2014;166:6–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Réus, G.Z., Matias, B.I., Maciel, A.L. et al. Mechanism of synergistic action on behavior, oxidative stress and inflammation following co-treatment with ketamine and different antidepressant classes. Pharmacol. Rep 69, 1094–1102 (2017). https://doi.org/10.1016/j.pharep.2017.04.021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2017.04.021