Abstract
Pancreatic cystic lesions (PCLs) include a broad spectrum of entities, which greatly differ in their malignant potential and may provide a diagnostic challenge. PCLs can be categorised into: pseudocysts; common cystic neoplasms; uncommon cystic neoplasms and tumours with cystic degeneration. Large lesions are usually possible to differentiate, but small lesions’ characterisation is often not possible. This means that many pancreatic cysts remain undetermined and guidelines are needed for follow-up and management. It is important to make distinction between mucinous cystic neoplasms (MCN) and intraductal pancreatic mucinous neoplasms (IPMN), because all MCN should be resected whereas there is time for observation in specific cases of side branch IPMN. With regards to IPMN, guidelines have established features associated with increased risk of malignancy: the “worrisome” features and high-risk stigmata. It should be considered that the presence of an IPMN may portend an increased risk of invasive pancreatic cancer in a different site of the pancreas. The assessment of PCLs should provide imaging features such as cyst morphology or presence of duct communication, and clinical findings including cystic fluid analysis to improve diagnostic accuracy. Radiomics represents an emerging field of interest that could add some information in the future for the differential diagnosis and follow-up of these lesions. The aim of this study was to define a diagnostic approach to PCLs according to the latest guidelines; a review of the latest developments in radiomics regarding PCLs was also carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction to pancreatic cystic lesions
Pancreatic cystic lesions (PCLs) are a large group of pancreatic tumours that differ from each other in terms of demographical, histological, morphological characteristics and biological behaviour. The incidence of PCLs has been steadily growing, actually their identification rate is approximately of 2.5%, and their prevalence increases with age [1]. This trend is primarily caused by the widespread use of advanced cross-sectional imaging: PCLs are found in up to 3% of computed tomography (CT) scans and in 20–44% of abdominal magnetic resonance imaging (MRI) images [2]. PCLs range from benign neoplasms to potentially malignant neoplasms, and they all can have different prognosis and management, hence the urgent need for them to be typified. Currently, according to the WHO classification, PCLs are subdivided in epithelial and non-epithelial and then further classified into neoplastic and non-neoplastic categories. Alternatively, it is possible to classify PCLs into: (1) pseudocysts; (2) common cystic neoplasms, which include intraductal pancreatic papillary neoplasm (IPMN), serous cystic neoplasm (SCN), mucinous cystic neoplasm (MCN); (3) uncommon cystic neoplasms, which include solid pseudopapillary epithelial neoplasm (SPEN); (4) malignant tumours with cystic degeneration. [3, 4]. The radiological evaluation includes size, number of cysts, possible presence of internal septa or nodules and enhancing cyst walls (Table 1); the key feature to characterise a PCL, though, is to define whether the lesion is connected or not to the main pancreatic duct (MPD) [2]. Radiomics is a field of artificial intelligence that is not clinically applicable yet. PCLs are not always distinguishable, and radiomics could add quantitative information to improve differential diagnosis and follow-up of these lesions. The aim of our study was, therefore, to perform a literature review about the diagnostic approach of PCLs according to the latest guidelines and on the latest developments about the radiomics of PCLs.
Insight into PCLs
Serous cystic neoplasms
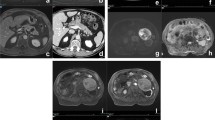
SCN are quite common mainly benign lesions, that account for 10–15% of PCLs and mostly affect women in their 50–60s: this is the reason why they also are defined as “Grandma”-like tumours [5]. Radiological images may show different patterns in relation to the pathological anatomy: the most common is the microcystic one (45%), followed by macrocystic pattern (32%), mixed pattern that is macrocystic and microcystic (18%) and honeycombing/solid pattern (5%); all these forms do not communicate with the MPD [5]. The microcystic type often has lobulated margins and is made of numerous serous fluid-filled tiny cysts whose diameter must be less than 2 cm (Figure1); a stellate scar is often seen in the central zone of the lesion: this is the area where the various septa that divide the multiple cysts converge, and it may be calcified. The coexistence of both central scar, lobulated margins and multiple microcysts, is pathognomonic for SCN [6]. The central scar is not seen in the macrocystic pattern, where few cysts are organised to make a lesion that is not very likely distinguishable from MCN and pseudocyst due to morphological similarities [7]. The honeycombing type is characterised by multiple tiny cysts stick together: in this case, the lesion may seem solid on CT due to the enhancement of the very tight cyst walls, misleading to an incorrect diagnosis of neuroendocrine tumour [8]. MRI can be helpful in the diagnosis of these lesions: on T1w images, the fluid is hypointense compared to the dense tissue; on the other hand, on T2w images, the fluid shines very brightly [9]. Although SCNs are benign and mostly asymptomatic lesions, they tend to grow slowly, possibly causing pain and obstructive chronic pancreatitis-related symptoms, whenever the lesion obstructs the MPD. These are the only times surgical resection may be needed: in fact, patients who show no symptoms do not require any kind of treatment [10]. After the diagnosis of SCN, the asymptomatic patient should be followed up for 1 year; after that, no more checks are required [4].
Mucinous cystic neoplasms
MCN are typically seen in female in their 40–50 s: as well as the SCN are defined Grandmas tumours, it is easy to define MCN as “Mother”-like tumours [11]. MCN is a mucin-producing pre-malignant neoplasm that may degenerate into cancerous lesion, the cystadenocarcinoma. An interesting finding is the underlying ovarian-type dense stroma these lesions have, which may explain their almost-exclusive appearance in women [8]. MCNs usually grow in the body tail of the pancreas, and they can reach large diameters: despite this, MPD dilatation is a finding we do not see when dealing with MCN [7]. MCN occurs as single round mucin-filled cyst, with possible thick internal septa resulting in a unilocular or multilocular mass; calcifications may also be seen in 25%, which are in the periphery of the lesion. Once again, it is not possible to find any communication with the main pancreatic duct [9]. On CT, they appear as great cysts with high contrast-enhanced septa and possible peripheral calcifications. On MRI, the T2w images show high signal intensity related to the cystic part; instead the T1w images best show the dense component of walls and septa: they also can show high signal intensity of the cystic component, due to the presence of mucin or haemorrhage [12]. Endoscopic ultrasound (EUS) is also very helpful to define the nature of the lesion, as it may verify the non-communication between the lesion and the MPD, thus allowing to distinguish between MCN and IPMN. Patients may have symptoms such as abdominal pain and discomfort, as well as dyspepsia, weight loss, fatigue, jaundice if the lesion turns malignant. Due to their malignant potential, Fukuoka guidelines suggest all MCNs should be surgical resected [13]. In unfit patients, however, radiological follow-up may be an option if the lesion is < 40 mm and it does not show any signs associated with malignancy (papillary vegetations, nodules, wall thickness > 3 mm, hyperintensity of cystic content on T1w images) [14]. Radiological follow-up involves surveillance with MRI, EUS or a combination of both every 6 months in the first year; after that, checks can be executed every 12 months [15].
Intraductal papillary mucinous neoplasms
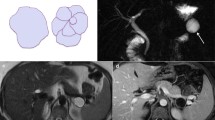
Intraductal papillary mucinous neoplasms (IPMN) are a heterogeneous group of mucinous pancreatic cystic lesions originating from the cells forming the lining of the MPD, when they organise themselves into a papillary-type mucin-secreting epithelium. IPMNs are classified as main duct type (MD-IPMN), branch duct type (BD-IPMN) and mixed type, depending on morphological features. MD-IPMN is characterised by focal or diffuse dilatation (> 5 mm) of MPD, with no other causes of obstruction, as well as filling defect due to mucin production; to differentiate between diffuse MD-IPMN and chronic pancreatitis may be tricky: in the first one, though, the margins of dilated MPD are smoother and regular [16]. BD-IPMN appears as a pancreatic cyst (> 5 mm) which communicates with the MPD, this is the most important characteristic that has to be demonstrated to make diagnosis of BD-IPMN. The cystic lesion may be unilocular or multilocular, often assembled in bunches divided by thin contrast-enhanced septa (Fig. 2). Mixed-type IPMN has both MD and BD-IPMN characteristics, appearing as focal or diffuse dilatation of MPD and secondary ducts [17]. Imaging is capable of first detecting IPMNs and then distinguishing their various types. IPMNs are often suspected on the basis of CT findings, but only MRI is able to assess communication with the MPD thanks to magnetic resonance cholangiopancreatography (MRCP) sequences. [18]. IPMNs are often detected incidentally, as patients usually are asymptomatic. However, some patients may complain nonspecific or pancreatitis-like symptoms; also, sometimes they may cause acute pancreatitis. Jaundice, abdominal pain, weight loss are symptoms more often linked to malignant transformation [8]. In fact, IPMNs range from pre-malignant lesions to frankly malignant ones, with a strong inclination to turn into an invasive carcinoma. This is the reason why it is so much important for us to distinguish each IPMN type from the other: they all have a different behaviour and tendency to become malignant pancreatic ductal adenocarcinoma (PDAC). Malignancy is generally associated with MD-IPMN and mixed type, with a mean frequency of 61.6%; on the other hand, the frequency of malignant transformation of BD-IPMNs is around 25.5% [19]. According to the 2017 Fukuoka guidelines, two main criteria are valid to predict malignancy: (1) the “worrisome features” (WF): a group of imaging findings such as cyst diameter > 3 cm, a solid enhanced nodule < 5 mm, thickened enhanced cyst wall, a sudden change in MPD calibre associated with pancreatic atrophy and a cyst growth rate > 5 mm per 2 years. These findings lead to a further in-depth study with EUS; (2) the “high risk stigmata” of malignancy: imaging findings including obstructive jaundice, an enhancing mural nodule > 5 mm, and a MPD diameter > 10 mm without any other cause of obstruction (Fig. 3). The presence of these is actually an indication for surgical resection [20]. If any high-risk stigmata of malignancy are detected, surgery is consequently requested; if there are no high-risk stigmata of malignancy, but any WF are found, EUS will be performed. Finally, patients with no high-risk stigmata nor WF should be followed up 6 months for the first year and then yearly. Patients unfit for surgery or with short life expectancy should be strictly followed up with clinical evaluation, laboratory tests and radiological imaging (MRI and /or EUS) every 6 months [15].
Solid pseudopapillary epithelial neoplasms
SPEN are rare exocrine neoplasms of the pancreas that almost exclusively affect female and young (20–30 years old) patients. They can have both a solid and a cystic component with evidence of intralesional haemorrhage. The CT scan shows a mixed density lesion, with a solid component in the peripheral position and a cystic component in the central position. The larger tumours have a clear demarcation from the normal pancreas whose capsule shows enhancement. The lesion has an MRI variable signal in T1w, T2w in relation to its components and haemorrhage [21].
Pseudocyst
Pancreatic pseudocysts are the result of previous episodes of pancreatitis, and account for about 80% of PCLs. The term pseudocyst refers to the absence of an epithelial lining, the fluid is delimited by granulation tissue. They mainly develop in adult men, but sometimes a diagnosis is not always possible because of an unclear clinical manifestation of pancreatitis; in doubtful cases, it is necessary to aspirate the cystic fluid which contains amylase. Ultrasound and CT may show a round lesion filled with fluid with walls of varying thickness (Fig. 4). Pseudocysts are most commonly unilocular, but fibrotic filaments within the cavity can lead to the formations of septa. Walled-off necrosis (WON), on the other hand, is a cyst which necrotic material inside that occurs ≥ 4 weeks after the onset of necrotising pancreatitis, MRI can better characterise the heterogeneity of the content [8].
Cystic pancreatic neuroendocrine tumour (PNET)
Cystic PNETs are often the result of degeneration of a solid lesion, but smaller subtypes of cystic PNETs with their own anatomical pathology are also known and occur most commonly in the body and tail of the pancreas. The symptomatology is related to the hormonal release typical of these tumours and are associated with multiple endocrine neoplasia type 1, neurofibromatosis or Von Hippel–Lindau. Non-functional PNETs are asymptomatic until they grow large enough to cause a mass effect. Cystic lesions tend to be large and mostly non-functional and demonstrate less aggressive behaviour with a lower rate of metastasis. Imaging is not always diriment as some features overlap with other PCL. Increased wall enhancement should, however, arise the suspect of a cystic PNET due to the abundant blood supply, whereas MRI usually demonstrates the presence of cystic components [2].
Artificial intelligence and radiomics
Differential diagnosis (DD) of PCLs is not straightforward and current research is geared towards identifying artificial intelligence (AI) as a problem solver. Its main subfields are Machine Learning (ML), Deep Learning (DL), Convolutional Neural Networks (CNN) and radiomics. These can potentially be used in conjunction with each other to improve diagnosis and prevention [22, 23]. ML is the ability of an algorithm to acquire and learn from data in order to recognise patterns, make inferences and predictions [24]. Artificial Neural Networks (ANNs) are ML algorithms consisting of artificial “neurons” arranged in more or less deep layers, interconnected and able to change according to information during the learning phase [25]. DL is a term related to the depth of the neural layers previously described, it is a subfield of ML based on ANNs. DL models operate on large-scale neuronal architectures, apply multiple non-linear transformations to the input images, allowing higher level information to be obtained. The most popular DL models are Autoencoders, CNNs, Recurrent Neural Networks (RNNs), and Generative Adversarial Networks (GANs) [22, 26]. CNNs use mathematical convolution in at least one of the neural layers; they are particularly suitable for image processing, widely used in medical image analysis for the detection, segmentation and classification of abnormalities or lesions [27, 28]. CNN are applied especially in areas where studies have to deal with large amount of data (Big Data), through complex algorithms, it is possible to extract useful information from this data to build predictive models [29, 30]. Radiological images contain non-perceptible quantitative features that can only be analysed by AI to provide a possible clinical outcome. The radiologist currently interprets the images, differentiating a pathological tissue from a healthy one by means of visible different grey scales and personal knowledge. Radiomics is a science based on the quantitative analysis of medical images by mathematically obtaining a series of values representing signal intensities or a variety of pixel interrelationship metric [31]. It has enabled the conversion of routine radiology images into high-throughput quantitative data to describe non-intuitive properties of the imaging phenotype and tissue micro-environment [32]. In general, a radiomics analysis workflow comprises four main steps: image segmentation, image pre-processing, radiomics extraction and ML modelling. All these steps have evolved over time to increase the robustness of the extracted quantitative data. In the first step (image segmentation), it is necessary to circumscribe the specific region of interest (ROI) within the image from which radiomics features are extracted. The most accurate segmentation methods involve fully automated AI methodologies with the use of DL to minimise intra- and inter-observer variability [33, 34]. The second phase (image pre-processing) is used for removing or alleviating noise and artefacts, a very important detail for the accuracy of the radiomic model. In this case, CNNs proved to be useful for improving image quality [35]. The next critical step is radiomic extraction, a complex process that can be based on mathematical equations to obtain handcrafted features (descriptors of shape, size and textural patterns) or on a totally automated complex DL architecture that uses non-linear image transformations to extract a huge amount of “deep features”. Although AI enables correct feature extraction, the weakness of radiomics studies mainly concerns certain sources of variability such as the patient himself or the different machine used. To standardise this pre-processing step and achieve greater reproducibility, image biomarker standardisation initiative (IBSI) guidelines were established [31, 36]. The last step involves the construction of a radiomic model which is now mostly DL-based and requires the training of AI algorithms that will subsequently make statistical inferences to make previsions [22, 26].
Radiogenomics
The combination of artificial intelligence and genomics may encourage a further evolution of personalised medicine [37], several studies have, therefore, also focussed on radiogenomics in pancreas oncology. Attiyeh et al. [38] identified radiomic features associated with PDAC genetic alterations and stromal content. In particular, their algorithm was able to distinguish between tumour with and without SMAD4 alterations. The studies by Yosuke Iwatate et al. [39] and Gao et al. [40] suggest that the construction of a radiomic model based, respectively, on CT and certain MRI sequences (T2W, ADC, DWI and CE T1W) can identify the TP53 mutation status in PDAC. Li qu.et al. [41] recently developed a radiomic normogram able to evaluate preoperatively the proliferation status of KI-67 in PDAC.
Cancer or not cancer: how radiomics helps us?
Radiomics has the potential to solve some diagnostic doubts related to the presence or absence of a tumour. Pancreatitis can occur with a mass-forming appearance (MFP), and a DD with pancreatic cancer (PC) may become difficult. Ren et al. [42] successfully developed a predictive radiomic model based on non-enhanced CT images (NECT) that can perform DD between PDAC and MFP. Ma et al. [43] instead built a combined clinical and radiomic model based on contrast-enhanced (CE) venous phase CT able to help in diagnosis. Deng et al. [44] pointed out the low specificity and low predictive value of the radiologist in distinguishing MFP that may lead to overtreatment. They, therefore, developed and validated a multi-parametric MRI-based radiomic method that can differentiate PC from MFP before surgery. Ye et al. [45] developed a combined CT-based radiomic and clinical model (which included: main pancreatic duct dilatation, tumour enhancement, vascular wrapping, CA19-9) that proved to be superior to the individual clinical and radiomic models by achieving a better predictive performance. Other studies have focussed more specifically on the distinction between PC and autoimmune pancreatitis, always obtaining promising results [46,47,48]. PC is often not easily visible in its early stages although certain morphological changes (parenchyma inhomogeneity, loss of fatty marbling and dilatation of the main pancreatic duct) become more visible over time and help in diagnosis [49]. A quantitative analysis of these factors was the aim of the study by Chu et al. [50]: they manually segmented the venous phase of PC patients and compared it with the pancreas of healthy subjects. They demonstrated high sensitivity (100%) and specificity (98.5%) and accuracy (99.2%) of radiomic features in differentiating PC cases from normal control cases. The same author [51] was able to distinguish between normal and abnormal tissue utilising both a commercially available radiomics research prototype and in-house radiomics software. In his study, despite differences and variations in the radiomics features employed by the software (854 features in commercial programme vs. 478 features in in-house programme), they did not seem to impact the overall diagnostic performance of the constellation of radiomics features. That also mean that commercially available radiomics software may be a viable alternative to in-house computer science expertise.
Malignant degeneration of IPMN or not?
As explained above, there are a number of clinical and imaging features that can point to malignant degeneration of cystic lesions of the pancreas. Online calculators based on clinical data which demonstrated excellent performance have been proposed to improve prediction [52]. However, an addition of radiomic features obtained by different diagnostic images could further help in defining lesions capable of degenerating. Chakraborty et al. [53] demonstrated that features extracted from pre-treatment CECT images can predict the risk of IPMNs degeneration. Hanania et al. [54] built an high sensibility and specificity radiomic model able to differentiate in a more accurate way high-grade IPMNs than low-grade IPMNs compared with a false positive rate of 36% using the clinical Fukuoka criteria alone. Flammia et al. [55] manually segmented CE MRI images and identified radiomic features which correlate significantly with WF in patients with BD-IPMNs. Their MRI-based radiomic model can also predict the occurrence of WF. The study by Li et al. [56] instead proposed a computer aided diagnosis (CAD) system based on radiomics to differentiate the malignant and benign form of IPMN and MCN. Schulz et al. [57] developed a deep learning-based method able to distinguish between IPMN with low-grade dysplasia and IPMN with high-grade dysplasia/invasive carcinoma using EUS images.
What kind of tumour?
Several studies focussed on a DD between PCL analysing radiomic features in different imaging method. Dmitriev et al. [58] in a CT0based study tried to correctly differentiate pancreatic cysts histopathologically using a probabilistic random forest (RF) classifier, which analyses manually selected quantitative features, and a CNN trained to discover high-level features for better differentiation. Wei et al. [59] successfully differentiated pancreatic serous cystic neoplasms from other pancreatic cystic neoplasm with a combined clinical and radiomic model. Yang et al. [60] and Wang et al. [61] were able to differentiate between MCN and serous cystadenomas analysing textural features derived from CECT and MRI images, respectively. A similar purpose was reached by Chen et al. [62] who developed a combined clinical and radiomic nomogram to differentiate SCNs from mucin-producing PCNs. Shen et al. [63] developed a CT-based combined radiomic model after the extraction of five significant radiomic features and four clinical factors (CA 19–9, gender, age and carcinoembryonic antigen) for the differentiation between SCA, MCN and IPMN. A CECT-based radiomics signature was able to differentiate between low-grade from high-grade PDAC in the multicentre study by Chang et al. [64].
Management: surveillance or surgical resection?
Surgical treatment is mandatory in PDAC, which sometimes requires neoadjuvant chemo(radio)therapy, especially in resectable and borderline resectable disease, resulting in longer survival compared to patients treated with non-surgical treatment alone. It also leads to conversion in initially unresectable patients. Not all tumours show the same response to treatment, and proper assessment of response to chemo-radiotherapy is essential to optimise results. In addition to the well-known radiological, serum and EUS sampling methods, radiomic models have been proposed. The study by Chen et al. [65] showed changes in radiomic features during chemo-radiotherapy in 20 patients, parameters that could be used to intensify or not intensify therapy. Borhani et al. [66] investigated the correlation between CT-derived texture features and progression disease (PR); they assessed that CT-derived tumour textural features may have a relationship with tumour response to neoadjuvant chemotherapy. Nasief et al. [67] demonstrated that serum biomarkers such as CA19-9 and radiomic features in combination have a higher predictive power of response to therapy than when used individually. The study by Bian et al. [68] demonstrated a correlation between CECT and lymph nodes metastasis in patients with PDAC. Ikuta et al. [69] used post-chemotherapy CT image to build a radiomics-based ML model able to predict PR in PDAC undergoing preoperative chemotherapy. The study by Wang et al. [70] aimed to use radiomics to aid in the potential choice of neoadjuvant treatment in patients with resectable PC by establishing an early recurrence risk model with a cut-off at 12 months. They differed from previous studies [71, 72] with a focus on obtaining significant characteristics from preoperative CECT by excluding pathological or postoperative variables not applicable to the preoperative context.
What to improve: future prospective
It has been pointed out that radiomics cannot be applied clinically yet due to known problems related to standardisation and generalisation of radiomic results, data quality control, repeatability, reproducibility, database matching and model overfitting problems. The mentioned studies present different methods of image segmentation, feature extraction and model construction. Some of them lack external validation and have an unbalanced dataset. To obtain standardised models, it is mandatory to follow the IBSI guidelines and a common radiomic feature extraction tool. Important steps to bring radiomics into clinical practice are the establishment of a single acquisition protocol and the conduction of multicentre prospective studies [73].
Data availability
No new data were created or analysed in this study.
References
Gardner TB, Glass LM, Smith KD et al (2013) Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Off J Am Coll Gastroenterol | ACG 108(October):1546–1550. https://doi.org/10.1038/ajg.2013.103
Miller FH, Vendrami CL, Recht HS, Wood CG, Mittal P, Keswani RN, Gabriel H, Borhani AA, Nikolaidis P, Hammond NA (2022) Pancreatic cystic lesions and malignancy: assessment, guidelines, and the field defect. Radiographics 42(1):87–105
Hecht EM, Khatri G, Morgan D et al (2020) Intraductal papillary mucinous neoplasm ( IPMN ) of the pancreas : recommendations for Standardized Imaging and reporting from the society of abdominal radiology IPMN disease focused panel. Abdom Radiol. https://doi.org/10.1007/s00261-020-02853-4
Del Chiaro M, Besselink MG, Scholten L et al (2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67(5):789–804. https://doi.org/10.1136/gutjnl-2018-316027
Jais B, Rebours V, Malleo G et al (2016) Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International association of pancreatology and European pancreatic club (European Study group on cystic tumors of the pan- creas). Gut 65:305–312
Choi J-Y, Kim M-J, Lee JY et al (2009) Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol 193:136–142
Osman H (2020) Pancreatic cysts cystic neoplasm pancreas. Surg Clin NA. https://doi.org/10.1016/j.suc.2020.02.006
Morana G, Ciet P, Venturini S (2021) Cystic pancreatic lesions: MR imaging findings and management. Insights Imaging 12(1):115. https://doi.org/10.1186/s13244-021-01060-z. (PMID:34374885;PMCID:PMC8355307)
Brugge WR (2015) Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol 6(4):375–388. https://doi.org/10.3978/j.issn.2078-6891.2015.057. (PMID:26261724;PMCID:PMC4502158)
Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG (2011) Primary pancreatic cystic neoplasms revisited. Part I: serous cystic neoplasms. Surg Oncol 20(2):e84-92. https://doi.org/10.1016/j.suronc.2010.12.002. (Epub 2011 Jan 14. PMID: 21237638)
Scourtas A, Dudley JC, Brugge WR, Kadayifci A, Mino-Kenudson M, Pitman MB (2017) Preoperative characteristics and cytological features of 136 histologically confirmed pancreatic mucinous cystic neoplasms. Cancer Cytopathol 125(3):169–177. https://doi.org/10.1002/cncy.21806
Buetow PC, Rao P, Thompson LD (1998) From the archives of the AFIP. Mucinous cystic neoplasms of the pancreas: radiologic-pathologic correlation. Radiographics 18(2):433–449. https://doi.org/10.1148/radiographics.18.2.9536488. (PMID: 9536488)
Kaimakliotis P, Riff B, Pourmand K et al (2015) Sendai and Fukuoka consensus guide lines identify advanced neoplasia in patients with suspected mucinous cystic neo plasms of the pancreas. Clin Gastroenterol Hepatol 13(10):180
Al Efishat M, Allen PJ (2016) Therapeutic approach to cystic neo- plasms of the pancreas. Surg Oncol Clin N Am 25:351–361. https://doi.org/10.1016/j.soc.2015.11.006
European Study Group on Cystic Tumours of the Pancreas (2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67(5):789–804. https://doi.org/10.1136/gutjnl-2018-316027. (Epub 2018 Mar 24. PMID: 29574408; PMCID: PMC5890653)
Kim JH, Hong SS, Kim YJ, Kim JK, Eun HW (2012) Intraductal papillary mucinous neoplasm of the pancreas: differentiate from chronic pancreatits by MR imaging. Eur J Radiol 81(4):671–676. https://doi.org/10.1016/j.ejrad.2011.01.066. (Epub 2011 Feb 15 PMID: 21324627)
Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C, Pistolesi GF (1999) Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics 19(6):1447–1463. https://doi.org/10.1148/radiographics.19.6.g99no011447. (PMID: 10555668)
Machado NO, Al Qadhi H, Al WK (2015) Intraductal papillary mucinous neoplasm of pancreas. N Am J Med Sci 7(5):160–175. https://doi.org/10.4103/1947-2714.157477. (PMID:26110127;PMCID:PMC4462811)
Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K (2012) International association of pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197. https://doi.org/10.1016/j.pan.2012.04.004. (Epub 2012 Apr 16. PMID: 22687371)
Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL (2017) Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17(5):738–753. https://doi.org/10.1016/j.pan.2017.07.007. (Epub 2017 Jul 13. PMID: 28735806)
Sunkara S, Williams TR, Myers DT, Kryvenko ON (2012) Solid pseudopapillary tumours of the pancreas: spectrum of imaging findings with histopathological correlation. Br J Radiol 85(1019):e1140–e1144. https://doi.org/10.1259/bjr/20695686. (Epub 2012 Apr 18. PMID: 22514105; PMCID: PMC3500814)
Hinton G (2018) deep learning-a technology with the potential to transform health care. JAMA 320(11):1101–1102. https://doi.org/10.1001/jama.2018.11100. (PMID: 30178065)
Stead WW (2018) Clinical implications and challenges of artificial intelligence and deep learning. JAMA 320(11):1107–1108. https://doi.org/10.1001/jama.2018.11029. (PMID: 30178025)
Samuel AL (1959) Some studies in machine learning. IBM J Res Dev 3:210–229
Han SH, Kim KW, Kim S, Youn YC (2018) Artificial neural network understanding the basic concepts without mathematics. Dement Neurocogn Disord. 17(3):83–89. https://doi.org/10.12779/dnd.2018.17.3.83. (Epub 2018 Dec 13. PMID: 30906397; PMCID: PMC6428006)
Puttagunta M, Ravi S (2021) Medical image analysis based on deep learning approach. Multimed Tools Appl 80(16):24365–24398
Frid-Adar M, Diamant I, Klang E, Amitai M, Goldberger J, Greenspan H (2018) GAN-based synthetic medical image augmentation for increased CNN performance in liver lesion classification. Neurocomputing 321(321–31):83
Kamnitsas K, Ledig C, Newcombe V, Simpson J, Kane A, Menon D, Rueckert D, Glocker B (2017) Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med Image Anal 36:61–78
Hinton GE, Salakhutdinov RR (2006) Reducing the dimensionality of data with neural networks. Science 313(5786):504–507. https://doi.org/10.1126/science.1127647. (PMID: 16873662)
Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP (2020) Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol 9(2):14. https://doi.org/10.1167/tvst.9.2.14. (PMID:32704420;PMCID:PMC7347027)
van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B (2020) Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 11:91
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, De Jong EEC, Van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762
Stamoulou E, Spanakis C, Manikis GC, Karanasiou G, Grigoriadis G, Foukakis T, Tsiknakis M, Fotiadis DI, Marias K (2022) Harmonization strategies in multicenter MRI-based radiomics. J Imaging 8(11):303. https://doi.org/10.3390/jimaging8110303. (PMID:36354876;PMCID:PMC9695920)
Hesamian MH, Jia W, He X, Kennedy P (2019) Deep learning techniques for medical image segmentation: achievements and challenges. J Digit Imaging 32(4):582–596. https://doi.org/10.1007/s10278-019-00227-x. (PMID:31144149;PMCID:PMC6646484)
Chen Z, Pawar K, Ekanayake M, Pain C, Zhong S, Egan GF (2023) Deep learning for image enhancement and correction in magnetic resonance imaging-state-of-the-art and challenges. J Digit Imaging 36(1):204–230. https://doi.org/10.1007/s10278-022-00721-9. (Epub 2022 Nov 2. PMID: 36323914; PMCID: PMC9984670)
Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative; arxiv 2016. Preprint. arXiv:1612.07003).
Katabathina VS, Marji H, Khanna L, Ramani N, Yedururi S, Dasyam A, Menias CO, Prasad SR (2020) Decoding genes: current update on radiogenomics of select abdominal malignancies. Radiographics 40(6):1600–1626. https://doi.org/10.1148/rg.2020200042. (PMID: 33001791)
Attiyeh MA, Chakraborty J, McIntyre CA, Kappagantula R, Chou Y, Askan G, Seier K, Gonen M, Basturk O, Balachandran VP, Kingham TP, D’Angelica MI, Drebin JA, Jarnagin WR, Allen PJ, Iacobuzio-Donahue CA, Simpson AL, Do RK (2019) CT radiomics associations with genotype and stromal content in pancreatic ductal adenocarcinoma. Abdom Radiol (NY) 44(9):3148–3157. https://doi.org/10.1007/s00261-019-02112-1. (PMID:31243486;PMCID:PMC6692205)
Iwatate Y, Hoshino I, Yokota H, Ishige F, Itami M, Mori Y, Chiba S, Arimitsu H, Yanagibashi H, Nagase H, Takayama W (2020) Radiogenomics for predicting p53 status, PD-L1 expression, and prognosis with machine learning in pancreatic cancer. Br J Cancer 123(8):1253–1261. https://doi.org/10.1038/s41416-020-0997-1. (Epub 2020 Jul 21. PMID: 32690867; PMCID: PMC7555500)
Gao J, Chen X, Li X, Miao F, Fang W, Li B, Qian X, Lin X (2021) Differentiating TP53 mutation status in pancreatic ductal adenocarcinoma using multiparametric MRI-derived radiomics. Front Oncol 17(11):632130. https://doi.org/10.3389/fonc.2021.632130. (PMID:34079753;PMCID:PMC8165316)
Li Q, Song Z, Li X, Zhang D, Yu J, Li Z, Huang J, Su K, Liu Q, Zhang X, Tang Z (2023) Development of a CT radiomics nomogram for preoperative prediction of Ki-67 index in pancreatic ductal adenocarcinoma: a two-center retrospective study. Eur Radiol. https://doi.org/10.1007/s00330-023-10393-w. (Epub ahead of print. PMID: 37938382)
Ren S, Zhao R, Zhang J, Guo K, Gu X, Duan S, Wang Z, Chen R (2020) Diagnostic accuracy of unenhanced CT texture analysis to differentiate mass-forming pancreatitis from pancreatic ductal adenocarcinoma. Abdom Radiol (NY) 45(5):1524–1533. https://doi.org/10.1007/s00261-020-02506-6. (PMID: 32279101)
Ma X, Wang YR, Zhuo LY, Yin XP, Ren JL, Li CY, Xing LH, Zheng TT (2022) Retrospective analysis of the value of enhanced CT radiomics analysis in the differential diagnosis between pancreatic cancer and chronic pancreatitis. Int J Gen Med 6(15):233–241. https://doi.org/10.2147/IJGM.S337455. (PMID:35023961;PMCID:PMC8747707)
Deng Y, Ming B, Zhou T, Wu JL, Chen Y, Liu P, Zhang J, Zhang SY, Chen TW, Zhang XM (2021) Radiomics model based on mr images to discriminate pancreatic ductal adenocarcinoma and mass-forming chronic pancreatitis lesions. Front Oncol 11:620981. https://doi.org/10.3389/fonc.2021.620981. (PMID: 33842325; PMCID: PMC8025779)
Ye Y, Zhang J, Song P, Qin P, Hu Y, An P, Li X, Lin Y, Wang J, Feng G (2023) Clinical features and computed tomography radiomics-based model for predicting pancreatic ductal adenocarcinoma and focal mass-forming pancreatitis. Technol Cancer Res Treat 22:15330338231180792. https://doi.org/10.1177/15330338231180792. (PMID: 37287274; PMCID: PMC10272634.)
Park S, Chu LC, Hruban RH, Vogelstein B, Kinzler KW, Yuille AL et al (2020) Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging 101:555–564
Liu Z, Li M, Zuo C, Yang Z, Yang X, Ren S, Peng Y, Sun G, Shen J, Cheng C, Yang X (2021) Radiomics model of dual-time 2-[18F]FDG PET/CT imaging to distinguish between pancreatic ductal adenocarcinoma and autoimmune pancreatitis. Eur Radiol 31(9):6983–6991. https://doi.org/10.1007/s00330-021-07778-0. (Epub 2021 Mar 6 PMID: 33677645)
Lu J, Jiang N, Zhang Y, Li D (2023) A CT based radiomics nomogram for differentiation between focal-type autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Front Oncol 1(13):979437. https://doi.org/10.3389/fonc.2023.979437. (PMID:36937433;PMCID:PMC10014827)
Gonoi W, Hayashi TY, Okuma H, Akahane M, Nakai Y, Mizuno S, Tateishi R, Isayama H, Koike K, Ohtomo K (2017) Development of pancreatic cancer is predictable well in advance using contrast-enhanced CT: a case-cohort study. Eur Radiol 27(12):4941–4950. https://doi.org/10.1007/s00330-017-4895-8. (Epub 2017 Jun 19 PMID: 28631079)
Chu LC, Park S, Kawamoto S, Fouladi DF, Shayesteh S, Zinreich ES, Graves JS, Horton KM, Hruban RH, Yuille AL, Kinzler KW, Vogelstein B, Fishman EK (2019) Utility of CT radiomics features in differentiation of pancreatic ductal adenocarcinoma from normal pancreatic tissue. AJR Am J Roentgenol 213(2):349–357. https://doi.org/10.2214/AJR.18.20901. (Epub 2019 Apr 23 PMID: 31012758)
Chu LC, Solmaz B, Park S, Kawamoto S, Yuille AL, Hruban RH, Fishman EK (2020) Diagnostic performance of commercially available vs. in-house radiomics software in classification of CT images from patients with pancreatic ductal adenocarcinoma vs. healthy controls. Abdom Radiol (NY). 45(8):2469–2475. https://doi.org/10.1007/s00261-020-02556-w. (PMID: 32372206)
Jiang D, Chen ZX, Ma FX, Gong YY, Pu T, Chen JM, Liu XQ, Zhao YJ, Xie K, Hou H, Wang C, Geng XP, Liu FB (2022) Online calculator for predicting the risk of malignancy in patients with pancreatic cystic neoplasms: a multicenter, retrospective study. World J Gastroenterol 28(37):5469–5482. https://doi.org/10.3748/wjg.v28.i37.5469. (PMID:36312834;PMCID:PMC9611704)
Chakraborty J, Midya A, Gazit L, Attiyeh M, Langdon-Embry L, Allen PJ, Do RKG, Simpson AL (2018) CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med Phys 45(11):5019–5029. https://doi.org/10.1002/mp.13159. (Epub 2018 Sep 27. PMID: 30176047; PMCID: PMC8050835)
Hanania AN, Bantis LE, Feng Z, Wang H, Tamm EP, Katz MH, Maitra A, Koay EJ (2016) Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget 7(52):85776–85784. https://doi.org/10.18632/oncotarget.11769. (PMID:27588410;PMCID:PMC5349873)
Flammia F, Innocenti T, Galluzzo A et al (2023) Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs): an MRI-based radiomic model to determine the malignant degeneration potential. Radiol med 128:383–392. https://doi.org/10.1007/s11547-023-01609-6
Li C, Wei R, Mao Y, Guo Y, Li J, Wang Y (2021) Computer-aided differentiates benign from malignant IPMN and MCN with a novel feature selection algorithm. Math Biosci Eng 18(4):4743–4760. https://doi.org/10.3934/mbe.2021241. (PMID: 34198463)
Schulz D, Heilmaier M, Phillip V, Treiber M, Mayr U, Lahmer T, Mueller J, Demir IE, Friess H, Reichert M, Schmid RM, Abdelhafez M (2023) Accurate prediction of histological grading of intraductal papillary mucinous neoplasia using deep learning. Endoscopy 55(5):415–422. https://doi.org/10.1055/a-1971-1274. (Epub 2022 Nov 2 PMID: 36323331)
Dmitriev K, Kaufman AE, Javed AA, Hruban RH, Fishman EK, Lennon AM, Saltz JH (2017) Classification of pancreatic cysts in computed tomography images using a random forest and convolutional neural network ensemble. Med Image Comput Comput Assist Interv. 10435:150–158. https://doi.org/10.1007/978-3-319-66179-7_18. (Epub 2017 Sep 4. PMID: 29881827; PMCID: -PMC5987215))
Wei R, Lin K, Yan W, Guo Y, Wang Y, Li J et al (2019) Computer-aided diagnosis of pancreas serous cystic neoplasms: a radiomics method on preoperative MDCT images. Technol Cancer Res Treat 18:1533033818824339
Yang J, Guo X, Ou X, Zhang W, Ma X (2019) Discrimination of pancreatic serous cystadenomas from mucinous cystadenomas with CT textural features: based on machine learning. Front Oncol 12(9):494. https://doi.org/10.3389/fonc.2019.00494. (PMID:31245294;PMCID:PMC6581751)
Wang BT, He L, Liu G, Liu MQ, Chen ZY (2018) Value of magnetic resonance imaging texture feature analysis in the differential diagnosis between pancreatic serous cystadenoma and mucinous cystadenoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 40(2):187–193. https://doi.org/10.3881/j.issn.1000-503X.2018.02.008. (PMID: 29724308)
Chen S, Ren S, Guo K, Daniels MJ, Wang Z, Chen R (2021) Preoperative differentiation of serous cystic neoplasms from mucin-producing pancreatic cystic neoplasms using a CT-based radiomics nomogram. Abdom Radiol (NY) 46(6):2637–2646. https://doi.org/10.1007/s00261-021-02954-8. (Epub 2021 Feb 8 PMID: 33558952)
Shen X, Yang F, Yang P, Yang M, Xu L, Zhuo J, Wang J, Lu D, Liu Z, Zheng SS, Niu T, Xu X (2020) A contrast-enhanced computed tomography based radiomics approach for preoperative differentiation of pancreatic cystic neoplasm subtypes: a feasibility study. Front Oncol 28(10):248. https://doi.org/10.3389/fonc.2020.00248. (PMID:32185129;PMCID:PMC7058789)
Chang N, Cui L, Luo Y, Chang Z, Yu B, Liu Z (2020) Development and multicenter validation of a CT-based radiomics signature for discriminating histological grades of pancreatic ductal adenocarcinoma. Quant Imaging Med Surg 10(3):692–702. https://doi.org/10.21037/qims.2020.02.21. (PMID:32269929;PMCID:PMC7136722)
Chen X, Oshima K, Schott D, Wu H, Hall W, Song Y, Tao Y, Li D, Zheng C, Knechtges P, Erickson B, Li XA (2017) Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: an exploratory study. PLoS ONE 12(6):e0178961. https://doi.org/10.1371/journal.pone.0178961. (PMID:28575105;PMCID:PMC5456365)
Borhani AA, Dewan R, Furlan A, Seiser N, Zureikat AH, Singhi AD, Boone B, Bahary N, Hogg ME, Lotze M, Zeh HJ III, Tublin ME (2020) Assessment of Response to neoadjuvant therapy using CT Texture analysis in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma. AJR Am J Roentgenol 214(2):362–369. https://doi.org/10.2214/AJR.19.21152. (Epub 2019 Dec 4. PMID: 31799875; PMCID: PMC7457395.)
Nasief H, Hall W, Zheng C, Tsai S, Wang L, Erickson B, Li XA (2020) Improving treatment response prediction for chemoradiation therapy of pancreatic cancer using a combination of delta-radiomics and the clinical biomarker CA19-9. Front Oncol 8(9):1464. https://doi.org/10.3389/fonc.2019.01464. (PMID:31970088;PMCID:PMC6960122)
Bian Y, Guo S, Jiang H, Gao S, Shao C, Cao K, Fang X, Li J, Wang L, Ma C, Zheng J, Jin G, Lu J (2022) Radiomics nomogram for the preoperative prediction of lymph node metastasis in pancreatic ductal adenocarcinoma. Cancer Imaging 22(1):4. https://doi.org/10.1186/s40644-021-00443-1. (PMID:34991733;PMCID:PMC8734356)
Ikuta S, Aihara T, Nakajima T, Yamanaka N (2024) Predicting pathological response to preoperative chemotherapy in pancreatic ductal adenocarcinoma using post-chemotherapy computed tomography radiomics. Cureus 16(1):e52193. https://doi.org/10.7759/cureus.52193. (PMID:38348011;PMCID:PMC10859726)
Wang G, Lei W, Duan S, Cao A, Shi H (2024) Preoperative evaluating early recurrence in resectable pancreatic ductal adenocarcinoma by using CT radiomics. Abdom Radiol (NY). 49(2):484–491. https://doi.org/10.1007/s00261-023-04074-x
He M, Chen X, Wels M et al (2023) Computed tomography-based radiomics evaluation of postoperative local recurrence of pancreatic ductal adenocarcinoma. Acad Radiol 30:680–688
Adamu M, Nitschke P, Petrov P et al (2018) Validation of prognostic risk scores for patients undergoing resection for pancreatic cancer. Pancreatology 18:585–591
Fusco R, Granata V, Simonetti I, Setola SV, Iasevoli MAD, Tovecci F, Lamanna CMP, Izzo F, Pecori B, Petrillo A (2024) An informative review of radiomics studies on cancer imaging: the main findings, challenges and limitations of the methodologies. Curr Oncol 31(1):403–424. https://doi.org/10.3390/curroncol31010027. (PMID:38248112;PMCID:PMC10814313)
Funding
No.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No interests to disclose.
Ethical approval
Not subjected.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galluzzo, A., Bogani, S., Fedeli, F. et al. Cystic pancreatic neoplasms: what we need to know and new perspectives. J Med Imaging Intervent Radiol 11, 22 (2024). https://doi.org/10.1007/s44326-024-00022-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44326-024-00022-1