Abstract
Demand for freshwater is increasing rapid due to population growth and climate change. A potential solution to this problem is the use of biodesalination, which involves the removal of salt from seawater and brackish water using biological agents. In this study, we investigated the feasibility of using Chlorella vulgaris, a green microalga, to remove salt from seawater to produce fresh water. The effects of salinity, light intensity, and nutrient concentration on the growth and salt removal efficiency of C.vulgaris were examined. Our results showed that C. vulgaris was able to grow and remove salt from seawater under salinities of approximately 24ppt and nutrient concentrations of 50%. The highest salt removal efficiency was achieved at a desalination setup involving C. vulgaris and seawater concentration of 1:5 ratio. We also evaluated the economic feasibility of biodesalination using C. vulgaris by estimating the production costs and comparing them with those of conventional desalination technologies. Our results showed that biodesalination using C.vulgaris is potentially a cost-effective and sustainable alternative to conventional desalination technologies. In conclusion, our study demonstrated the potential of C. vulgaris for the biodesalination of seawater, which could contribute to meeting the growing demand for fresh water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water scarcity is one of the major global challenges today, especially in arid and semi-arid regions. Desalination is a promising technology to obtain fresh water from saline sources such as seawater and brackish water. One of the emerging desalination techniques is the biodesalination technique. Biodesalination is a novel technology that uses biological processes to remove salt and other impurities from water sources, such as seawater or brackish water. Biodesalination has several advantages over conventional methods, such as lower energy consumption, environmental friendliness, and potential for value-added products (Amezaga et al. 2014). A promising approach for biodesalination is the use of microalgae, which are photosynthetic microorganisms that can grow in various aquatic environments and produce oxygen and biomass (Jaroo et al. 2019). Among these microorganisms, microalgae have several advantages, such as high photosynthetic efficiency, high growth rate, ability to adapt to different salinities, and potential for biomass production (Hassan et al. 2023). One such organism that has shown potential in this field is Chlorella vulgaris (C. vulgaris). C. vulgaris is a type of green microalgae that has several advantageous properties for use in the process of biodesalination. These properties include its wide distribution in both freshwater and seawater, its short growth cycle, and its ability to directly observe toxicity at the cellular level. C. vulgaris plays a crucial role in biodesalination by aiding in the conversion of seawater into freshwater. The propagation of C. vulgaris is carried out using sterile artificial saline water, which has a comparable salinity to seawater. Additionally, C. vulgaris can increase its growth in the presence of certain concentrations of chromium, indicating its potential for phytoremediation and bioenergy synthesis (Gaur et al. 2021). Moayedi et al. (2021), reported that using microalgae such as Dunaliella salina, C. vulgaris, Nannochloropsis oculate, and Scenedesmus quadricauda can be an effective way of reducing salinity. Furthermore, they suggested that green algae can be used for reducing salinity. Two pathways can be used to explain how microalgae remove salt from seawater or metal from industrial wastewater (Moghazy et al. 2022). Among these are metabolism-dependent and non-metabolism-dependent processes. The process of bioaccumulation involves incorporating salts into algal cells metabolism. Using algal biological systems with biomass byproducts that can be reused in a variety of ways, the findings of this study provide promising data that may be used to develop energy-efficient, eco-friendly alternatives to desalination (Ghobashy et al. 2022). One of the key advantages of using C. vulgaris in biodesalination is its ability to thrive in full-strength seawater, making it suitable for cultivation in saline environments.
Microalgae can desalinate water through osmosis, ion exchange, or biosorption, and can also be used for wastewater treatment, biofuel production, and aquaculture (Chisti, 2007). The effect of total dissolved solids (TDS) on the performance of single-pot microbial desalination, which is a novel system that integrates microbial desalination cells (MDCs) and microalgae in one reactor investigated. MDCs are bioelectrochemical systems that use bacteria to generate electricity and desalinate water simultaneously (Ahmed et al. 2022). In this study, we investigated the potential of C. vulgaris, a widely used microalga in aquaculture and wastewater treatment, for the biodesalination of seawater and brackish water. We collected samples of C. vulgaris from varoius locations and optimized their growth conditions under laboratory settings. We used gas chromatography-mass spectrometry (GC–MS) to analyze the chemical composition of the microalgal biomass and the desalinated water. We aimed to assess the feasibility and efficiency of C. vulgaris biodesalination and its potential applications.
2 Materials and method
2.1 Study area
The Adhirampattinam and Muthupettai coasts, are located in the southern part of India along the Bay of Bengal. They are situated in the Thanjavur and Nagapattinam Districts of the Indian state of Tamil Nadu (Fig. 1). It lies on the eastern coast of the Indian subcontinent, bordering the Bay of Bengal. The region encompasses a stretch of approximately 40 km along the coast. During the winter (November to February), SST in this region typically ranges from 25℃ to 28℃, while during the summer months (March to June), SST can rise to 30℃ or higher.
2.2 Sample collection
The seawater samples were collected from the Muthupettai coast (10.46°N 79.49°E) and Adirampattinam coast (10.20°N 79.24°E), which are located on the coast of the Bay of Bengal (Fig. 1). The samples were collected in 500-mL sterile polypropylene bottles. The water sample was transported to the laboratory for various physiochemical, microalgae culture techniques, and microbial desalination techniques. The physicochemical characteristics of the water sample were measured using standard methods (APHA, 1998, Jenkins & Medsken, 1964, Strickland & Persons, 1972). The sample was stored under refrigeration to minimize changes in its characteristics.
The microalga C. vulgaris was cultured in a special air conditioning room. The stock culture was kept in culture flasks. The collected water sample was serially diluted to concentrations of 10–1 and 10–2 before it was sterilized using an autoclave. After it cooled, the seawater was transferred to the culture flask. BG11 medium was used for indoor algal culture (Shah et al. 2003). The strain was preserved in BG11 medium containing the following chemicals: NaNO3: 1.5 g/L, K2HPO4.3H2O: 0.04 g/L, MgSO4.7H2O: 0.075 g/L, CaCL2.2H2O: 0.036 g/L, Na2CO3: 0.02 g/L, Citric acid: 0.006 g/L, Ferric ammonium citrate: 0.006 g/L, EDTA: 0.001 g/L, A5 + Co solution (1 ml/1) that consists of: H3BO3: 2.86 g/L, MnCl2.H2O: 1.81 g/L, ZnSO4.7H2O: 0.222 g/L, CuSO4.5H2O: 0.079 g/L, Na2MoO4.2H2O: 0.390 g/L, Co(NO3)2.6H2O: 0.049 g/L and additional nutrient were added into the BG11 medium like vitamin C and Vitamin E.
Approximately 1 mL of inoculums in the growing phase were transferred to the culture flasks and culture was provided with 12:12 h light and dark cycle with 5000- lux from two tube lights. The temperature was maintained in the range of 23 °C to 25 °C for the entire culture period. The culture was provided with continuous aeration. To isolate single microalgal species from a water sample, standard plating methods were followed using the BG11 media (Safi et al. 2014; Shah et al. 2003). The field sample was first acclimatized in media for further isolation process. Inoculated plates were placed in a temperature-controlled incubator maintained at 25 °C–30 °C, where the algae were allowed to grow initially for about approximately 5–7 days. A drop of distilled water mixed with a small amount of culture was placed on a microscopic slide. The culture was properly teased to separate the cells/filaments. The drop was carefully covered with a coverslip without trapping any air bubbles. The cells were observed at 10x, 40x, and 100x. Microalgal culture growth rates were monitored using a UV spectrophotometer at a wavelength of 688 nm.
2.3 Desalination setup
The desalination method was performed by treating with C.vulgaris, in this desalination method, different concentrations of the sample and the test solution were used in comparative analysis of the desalination method (Nadersha et al. 2022). This type of comparative technique is useful for finding the most effective ratio for biodesalination.
The first desalination setup contained, seawater and C.vulgaris culture in a reaction mixture of 1:1, 1:2, 1:3, 1:4 & 1:5, which were obtained by mixing 5 mL of seawater and 45 mL of C.vulgaris culture, 10 mL seawater and 40 mL C.vulgaris culture, 15 mL seawater and 35 mL C. vulgaris culture, 20 mL seawater and 30 mL C.vulgaris culture and 25 mL seawater and 25 mL C.vulgaris culture respectively.
The second desalination setup contained artificial seawater and C.vulgaris culture in the reaction mixture of 1:1, 1:2, 1:3, 1:4, and 1:5, which were obtained by mixing 5 mL of artificial seawater and 45 mL of C.vulgaris culture, 10 mL artificial seawater and 40 mL C.vulgaris culture, 15 mL artificial seawater and 35 mL C.vulgaris culture, 20 mL artificial seawater and 30 mL C.vulgaris culture and 25 mL artificial seawater and 25 mL C.vulgaris culture respectively.
After the desalinated sample was vacuum filtered with Whatman no.1 glass filter paper (Greenwell et al. 2010), it was analysed using GCMS. The identification and quantification of basil chemical constituents were evaluated by Gas chromatography coupled with Mass spectrometry QP2010 Plus, Shimadzu, Japan equipped with RTX-5 MS GC capillary column (5% diphenyl / 95% dimethyl polysiloxane) of 0.5 μ media and 30 m length. The following GC working conditions were used: the temperature was kept between 40℃ – 290℃ with a gradual increase of 8º C/min. Column oven and injection temperatures were set at 100ºC and 270ºC respectively. Injection mode was set as the split with a ratio of 20; Helium was used as carrier gas (mobile phase) with a flow rate of 1 mL/min. MS working conditions included the following: ion source and interface temperatures were set at 200 ℃ and 260 ℃. The solvent cut-off time was set as 4 min, and the detector voltage was set at 0.1 kV. Injection conditions included the following: 1μL injection volume; 10 μL injection syringe; injection temperature at 240ºC; mass ranged from 20–300 m/z. The analytes were matched with the NIST and Wiley library for similar hits of the basil chemical compositions.
3 Result
3.1 Growth assessment of algae
We monitored the growth of C. vulgaris for 15 days and measured the optical density of the culture every five days. We found that the optical density increased from 0.10 to 0.98 nm over the first 15 days.
3.2 Characteristics of untreated seawater
Physiochemical and nutritional tests were conducted on untreated seawater, and the results are shown in Table 1. The reflectometer and TDS meter were used to measure salinity and TDS, respectively. Seawater had a high salinity in our study location salinity ranging from 33 ppt, and it contained TDS ranging from 900 mg/L. The average temperature of seawater is about 30℃. This range is consistent with other studies from the Bay of Bengal salinity range of 33 to 34 ppt.
3.3 Biodesalination
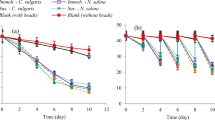
In this study, we used C.vulgaris culture as a biodesalination agent and observed its growth and salt removal efficiency for 60 days. We performed three different biodesalination setups, each using a different ratio of culture and seawater/artificial seawater. We monitored seawater parameters, including TDS, EC, salinity, and pH levels, every three days over a span of 45 days. In the first desalination setup, we used C. vulgaris and seawater. In this setup, TDS decreased from 900 to 500 mg/L (Fig. 2), EC decreased from 12.9 to 0.1 mS/cm (Fig. 3), pH decreased from 8 to 7.8, and salinity decreased from 33 to 25 ppt during this period (Fig. 4). Notably, the biodesalination setup with a 1:5 ratio had the highest salt reduction capacity among the five ratios.
In the second desalination setup, which used artificial seawater and C. vulgaris, we observed gradual reductions in salinity (from 33 to 20 ppt), TDS (from 800 to 545 mg/L, as shown in Fig. 2), and EC (from 14 to 0.1 mS/cm, as depicted in Fig. 3). Additionally, the pH decreased from 8 to 6 during the incubation period. Notably, the removal of total dissolved solids (TDS) was more effective in this setup than in the first desalination setup. Specifically, the 1:5 TDS ratio had both higher treatment efficacy and shorter treatment duration. Interestingly, the first EC desalination setup exhibited greater efficiency. A before-and-after comparison in Table 1 illustrates the seawater treatment outcomes. Whereas vacuum filtration and centrifugation effectively removed up to 99% of algae, an integrated biodesalination approach is necessary to remove the remaining algal biomass.
3.4 GCMS analysis of C. vulgaris
The RTX-5 MS GC capillary column separated and identified the compounds based on their order of elution (Fig. 5) and measured the retention time, molecular formula, and amount of each compound. The main compound extract from C.vulgaris was saline, trimethyl [5- methyl-2-(1- methylethyl)phenoxy] with a peak area 8.30%. This compound is used for organic synthesis and as a derivatizing agent. Trimethyl [4–1,1,3,3,- tetramethylbutyl)phenoxy]silane had a peak area of 39.22% (Table 2). This compound is used as a derivatizing agent and plays an important role in these industries. This study used cyclotrisiloxane, and hexamethyl to identify and compare the volatile compounds in the algal extract by GCMS. The bioactivity and isolated pure compounds and their bioactivity are shown in Table 2.
4 Discussion
Our investigation on the biodesalination of seawater using microalgae (C.vulgaris), is similar to the work Wei et al. (2020) on brackish water and Sahle-Demessie et al. (2019) on seawater. Limited large-scale testing and implementation of algae-based desalination have been conducted to date (Rawat & Kumar, 2022). Wei et al. (2020) explored the effect of various operating conditions on salt removal by S.obliquus, both dry and fresh, and the mechanisms of this process. They reported that the NaCl adsorption was completed within a short time for both types of algae, which agreed with the findings of Sahle-Demessie et al. (2019) who reported a pseudo-first-order kinetics of salt uptake by the halophyte algae S. sp. and C. vulgaris. These algae were selected for pilot-scale studies as a promising method for desalinating brackish water. A maximum TDS removal efficiency of more than 90% after 21 days, was reported by Nagy et al. (2017). However, in this study, the TDS reduction process took 45 days due to our unique desalination setup involving algae and seawater. Figler et al. (2019) also studied how microalgae species can adapt to salt-rich waste waters and improve water quality during bioremediation processes. Kokabian et al. (2018) compared and evaluated the performance and efficiency of the photosynthetic microbial desalination cells (PMDCs) in terms of wastewater treatment, water deionization, electricity generation, nutrient removal, and biomass production. C. vulgaris can potentially be used as an alternative to traditional water treatment methods for the extraction of metals from seawater, wastewater, or drinking water (Pascucci, 1999). Table 3 provides information on different algae species and their associated desalination technologies, as well as the salinity range, desalination efficiency, biomass production, and energy consumption for each case study. Sahle-Demessie et al. (2019) designed a 60L pilot photobioreactor to investigate the biodesalination performance of C. vulgaris and Scenedesmus sp.
Our results shows that TDS decreased from 900 to 500 ppm, indicating that C.vulgaris has high salt removal efficiency. According to El Sergany et al. (2014), a biological desalination system using microalgae can effectively reduce TDS in saline water. They indicate that the system can achieve high removal efficiency for TDS and that the water quality after the third basin meets the permissible limits for drinking water. Moayedi et al. (2021) suggest that this method is an affordable and efficient way to reduce salinity, which enhances the ecological health of aquatic ecosystems. Biological desalination also removes water pollutants (such as nitrogen compounds, heavy metals, and phosphorus compounds) at the same time. Generally, algae biomass production is important for making biofuels, cosmetics, drugs, and protein substances. Hence GCMS can be used to assist in the differentiation of water-based residues for investigative purposes. These results reveal the potential use of growing C. vulgaris in seawater, which would help to reduce salinity in the seawater during the whole desalination process. Silane, trimethyl[5-methyl-2-(1-methylethyl) phenoxy] derivate or thymol trimethylsilyl ether (kubicin), in terms of its physicochemical properties is weak, and its sensory properties have not been described to date (Kubinec et al. 2020). Bis(trimethylsilyl)benzene derivatives have been the subject of extensive study due to their unique chemical properties and potential applications in various fields (Kamanna, 2019). Research in this area has demonstrated the potential for these derivatives to exhibit antimicrobial and anticancer properties, making them candidates for drug development.
C. vulgaris biomass is influenced by different nutrient-stress conditions, such as nitrogen, phosphorus, and iron limitation (Ratomcki & Hawrot-Paw 2021). These conditions can affect the growth rate, biomass productivity, lipid content, and chlorophyll content of the microalgae. For example, one study reported that the optimal dose of aquaculture wastewater (AWW) for C. vulgaris cultivation was 80%, which resulted in the highest biomass concentration of 727 ± 19.64 mg/L and productivity of 225 g/L/ day. Another study reported that nitrogen limitation increased the lipid content of C. vulgaris from 5.75% to 11.81%, whereas phosphorus limitation decreased the chl-a content from 206 ± 11.33 mg/m3 to 78 ± 4.47 mg/m3 (Anyanwu et al. 2022). Furthermore, iron limitation reduced the growth rate of C. vulgaris by 50% and increased lipid content by 20% (Perumalsamy, 2022).
In this study, our desalination setup achieved a desalination efficiency of 50%–60% of salinity. Recently, several studies have explored the potential of algae-based desalination methods. Notably, Moayedi et al. (2021) investigated a combination of the algae species—Dunaliella salina, C. vulgaris, Nannochloropsis oculate, and Scenedesmus quadricauda—using thermal distillation. Their findings revealed that a 1:5 ratio resulted in moderate desalination efficiency (20% – 30%) and biomass production (0.5–1.0 g/L), with energy consumption ranging from 10 to 15 kWh/m3. Ahmed et al. (2022) focused on Nannochloropsis oculata and employed reverse osmosis, achieving slightly higher desalination efficiency (30%–40%) and biomass production (1.0–1.5 g/L) with lower energy consumption (5–10 kWh/m3) (Table 3). Sahle-Demessie et al. (2019) explored forward osmosis using Scenedesmus quadricauda, achieving better efficiency (40%–50%) and biomass production (1.5–2.0 g/L) at a similar salinity range. Aly et al. (2023) introduced microbial desalination cells with C. vulgaris, resulting in higher desalination efficiency (50–60%) and biomass production (2.0–2.5 g/L) while minimizing energy consumption (0.5–2 kWh/m3). Lastly, Ghobashy et al. (2022) used kinetic modeling with Scenedismus arcuatusa, C.vulgaris, and Spirulina maxima, achieving comparable results. These findings provide valuable insights for advancing algae-based desalination technologies and addressing water scarcity challenges. Followed by our desalination setup also gives 50–60% of salinity removal efficiency.
Nagy et al. (2017) concluded that the number of stages in desalination depends on both the degree of water salinity and the required level of desalination. Our findings were similar, in that we obtained a high salinity removal efficiency is 1:5. These results suggest that nutrient-stress conditions can be used to manipulate the biochemical composition of C. vulgaris biomass and enhance its suitability for different purposes. For instance, high lipid content is desirable for biodiesel production, whereas low chlorophyll content is conductive for wastewater treatment and water deionization. However, nutrient-stress conditions may also have some drawbacks, such as lower biomass yield, slower growth rate, and higher production costs. Therefore, it is important to optimize the nutrient supply and balance the trade-offs between biomass quantity and quality when using C. vulgaris as a source of renewable energy and for environmental remediation (Zhu et al. 2018). Our results showed that the algae-based biological desalination system can produce freshwater from saline water cheaply and effectively while also enhancing the ecological safety of aquatic ecosystems. Our findings indicated that the 1:5 ratio exhibits higher salinity removal than other ratios. Future research can focus on enhancing the efficiency of this ratio through biomass culture and large-scale approaches could lead to improved biodesalination capacity.
5 Conclusion
The use of microalgae to remediate aquatic environmental pollutants is an option to solve the crisis water depletion and water scarcity-related deaths in the near future. This study focused on the characterizing desalination process of C. vulgaris and its nutrient consumption in filtered seawater. The result showed an increase in nutrient consumption, biodesalination, and biomass production, accompanied by a reduction in salt and heavy metal concentration when an external C. vulgaris source was added. The control experiment exhibited a decrease in total dissolved solids (TDS) and pH relative to the other experimental setups. The high biodesalination ratio of 1:5 (C. vulgaris to seawater concentration) suggests that seawater could be used as a nutrient source in industrial microalgal production. Subsequent experiments demonstrated the feasibility of using C. vulgaris to biodesalination seawater, potentially transforming it into portable drinking water.
Availability of data and materials
The data are available on request.
References
Ahmed, M. E., Zafar, A. M., Hamouda, M. A., Aly Hassan, A., & Arimbrathodi, S. (2022). Biodesalination research trends: A bibliometric analysis and recent developments. Sustainability, 15 (1), 16. https://doi.org/10.3390/su15010016
Aly, S. M., ElBanna, N. I., & Fathi, M. (2023). Chlorella in aquaculture: Challenges, opportunities, and disease prevention for sustainable development. Aquaculture International, 1–28. https://doi.org/10.1007/s10499-023-01229-x
Amezaga, J. M., Amtmann, A., Biggs, C. A., Bond, T., Gandy, C. J., Honsbein, A., et al. (2014). Biodesalination: A case study for applications of photosynthetic bacteria in water treatment. Plant Physiology, 164 (4), 1661–1676. https://doi.org/10.1104%2Fpp.113.233973
Anyanwu, R. C., Rodriguez, C., Durrant, A., & Olabi, A. G. (2022). Evaluation of growth rate and biomass productivity of Scenedesmus quadricauda and Chlorella vulgaris under different LED wavelengths and photoperiods. Sustainability, 14 (10), 6108. https://doi.org/10.3390/su14106108
APHA. (1998). American public health association, standard methods: for the examination of water and wastewater. APHA, AWWA, WEF/1998, APHA publication.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25 (3), 294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
El Sergany, F. A. G. H., El Hosseiny, O. M., & El Nadi, M. H. A. (2014). Desalination using algae ponds under nature Egyptian conditions. Journal of Water Resources and Ocean Science, 3 (6), 69–73. https://doi.org/10.11648/j.wros.20140306.11
Figler, A., B-Béres, V., Dobronoki, D., Márton, K., Nagy, S. A., & Bácsi, I. (2019). Salt tolerance and desalination abilities of nine common green microalgae isolates. Water, 11 (12), 2527. https://doi.org/10.3390/w11122527
Gaur, V. K., Sharma, P., Gaur, P., Varjani, S., Ngo, H. H., Guo, W., et al. (2021). Sustainable mitigation of heavy metals from effluents: Toxicity and fate with recent technological advancements. Bioengineered, 12 (1), 7297–7313. https://doi.org/10.1080/21655979.2021.1978616
Ghobashy, M. O., Bahattab, O., Alatawi, A., Aljohani, M. M., & Helal, M. M. (2022). A novel approach for the biological desalination of major anions in seawater using three microalgal species: A kinetic study. Sustainability, 14(12), 7018. https://doi.org/10.3390/su14127018
Greenwell, H. C., Laurens, L. M. L., Shields, R. J., Lovitt, R. W., & Flynn, K. J. (2010). Placing microalgae on the biofuels priority list: A review of the technological challenges. Journal of the Royal Society Interface, 7 (46), 703–726. https://doi.org/10.1098/rsif.2009.0322
Hassan, M. K., AbouElkheir, W., Hashem, A. I., El Malky, M. G., Helal, A. M., & Abdelkader, S. A. (2023). Artificial seawater biodesalination and biodiesel production using some microalgal species. Egyptian Journal of Aquatic Biology & Fisheries, 27 (4), 1181. https://doi.org/10.21608/ejabf.2023.315215
Jaroo, S. S., Jumaah, G. F., & Abbas, T. R. (2019). Photosynthetic microbial desalination cell to treat oily wastewater using microalgae Chlorella vulgaris. Civil Engineering Journal, 5 (12), 2686–2699. https://doi.org/10.28991/cej-2019-03091441
Jenkins, D., & Medsken, L. (1964). A brucine method for the determination of nitrate in ocean, estuarine, and fresh waters. Analytical Chemistry., 36 (3), 610–612. https://doi.org/10.1021/ac60209a016
Kamanna, K. (2019). Synthesis and pharmacological profile of benzimidazoles. In U. K. London (Ed.), Chemistry and Applications of Benzimidazole and Its Derivatives (pp. 51–69). London, UK: IntechOpen. https://doi.org/10.5772/intechopen.85229
Kokabian, B., Ghimire, U., & Gude, V. G. (2018). Water deionization with renewable energy production in microalgae-microbial desalination process. Renewable Energy, 122, 354–361. https://doi.org/10.1016/j.renene.2018.01.061
Kubinec, R., Blaško, J., Galbavá, P., Jurdáková, H., Sadecká, J., Pangallo, D., et al. (2020). The antifungal activity of vapour phase of odourless thymol derivate. PeerJ, 8, e9601. https://doi.org/10.7717/peerj.9601
Moayedi, A., Yargholi, B., Pazira, E., & Babazadeh, H. (2021). Investigation of bio-desalination potential algae and their effect on water quality. Desalination Water Treat, 212, 78–86. https://doi.org/10.5004/dwt.2021.26638
Moghazy, R. M., Abdo, S. M., & Mahmoud, R. H. (2022). Algal biomass as a promising tool for CO2 sequestration and wastewater bioremediation: An integration of green technology for different aspects. Handbook of Algal Biofuels (pp. 149–166). Elsevier. https://doi.org/10.1016/B978-0-12-823764-9.00015-7
Nadersha, S., & Hassan, A. A. (2022). Biodesalination and treatment of raw hypersaline produced water samples using indigenous wastewater algal consortia. Desalination, 528, 115638. https://doi.org/10.1016/j.desal.2022.115638
Nagy, A. M., El Nadi, M. H., & El Hosseiny, O. M. (2017). Determination of the best retention time for desalination by algae ponds. Journal of Applied Science and Research, 5, 1–5.
Pascucci, P., & Kowalak, A. (1999). Metal distributions in complexes with Chiarella vulgaris in seawater and wastewater. Water Environment Research, 71 (6), 1165–1170. https://doi.org/10.2175/106143097X122040
Perumalsamy, M. (2022). Bioremediation of dairy industry wastewater and assessment of nutrient removal potential of Chlorella vulgaris. Biomass Conversion and Biorefinery, 1–12. https://doi.org/10.1007/s13399-022-03068-x
Ratomski, P., & Hawrot-Paw, M. (2021). Influence of nutrient-stress conditions on Chlorella vulgaris biomass production and lipid content. Catalysts, 11 (5), 573. https://doi.org/10.3390/catal11050573
Rawat, S., & Kumar, S. (2022). The Feasibility Study of Green Microalgae Assisted Coal Mine Effluent Desalination. https://scite.ai/reports/10.2991/978-94-6463-020-6_25. Accessed 13 Dec 2022
Safi, C., Zebib, B., Merah, O., Pontalier, P. Y., & Vaca-Garcia, C. (2014). Morphology, composition, production, processing, and applications of Chlorella vulgaris: A review. Renewable and Sustainable Energy Reviews, 35, 265–278. https://doi.org/10.1016/j.rser.2014.04.007
Sahle-Demessie, E., Hassan, A. A., & El Badawy, A. (2019). Bio-desalination of brackish and seawater using halophytic algae. Desalination, 465, 104–113. https://doi.org/10.1016/j.desal.2019.05.002
Shah, M. M. R., Alam, M. J., & Mia, M. Y. (2003). Chlorella sp.: Isolation, pure culture, and small-scale culture in brackish-water. Bangladesh Journal of Scientific and Industrial Research, 38 (3–4), 165–174.
Strickland, J. D. H., & Parsons, T. R. (1972). A Practical Handbook of the Seawater Analysis (2nd ed., p. 310). Ottawa: Bulletin Fisheries Research Board of Canada.
Wei, J., Gao, L., Shen, G., Yang, X., & Li, M. (2020). The role of adsorption in microalgae biological desalination: Salt removal from brackish water using Scenedesmus obliquus. Desalination, 493, 114616. https://doi.org/10.1016/j.desal.2020.114616
Zhu, L., Li, Z., & Hiltunen, E. (2018). Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnology for Biofuels, 11, 1–10. https://doi.org/10.1186/s13068-018-1183-z
Acknowledgements
The authors are thankful to Rashtriya Uchchattar Shiksha Abhiyan (RUSA 2.0.) for providing financial support (Project Grant no: 21-3/BDU/RUSA 2.0/TRP/BS/Date:08.10.2021).
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Monisha Balasubramaniyan and Dinesh Kasiraman conceptualized the presented idea. Monisha Balasubramaniyan and Amirtham S wrote the original manuscript. Monisha Balasubramaniyan and Dinesh Kasiraman were responsible for the mapping and materials and method. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare there is no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balasubramaniyan, M., Kasiraman, D. & Amirtham, S. Chlorella vulgaris in biodesalination: a sustainable future from seawater to freshwater. Mar Dev 2, 7 (2024). https://doi.org/10.1007/s44312-024-00019-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44312-024-00019-0