Abstract
Soybean (Glycine max L.), a crucial crop that provides essential nutrition, is experiencing increasing demand to meet protein and oil requirements. However, the menace of soybean cyst nematode (SCN) disease, caused by Heterodera glycines, poses a substantial threat globally, resulting in significant annual economic losses. While cultivating resistant varieties is an eco-friendly approach to control SCN, the excessive use of a single variety triggers ongoing evolution of SCN races, jeopardizing the soybean industry's stability. Leveraging advanced technologies, research on soybean SCN resistance mechanisms has progressed significantly across genetics, transcriptomics, and protein functions. This review consolidates insights into major resistance loci (rhg1 and Rhg4), elucidating their connections with vesicle transport and plant hormone signaling pathways. It also discusses the role of key functional proteins in soybean resistance and addresses potential research issues. This study explores superior soybean resistance genes, laying a foundation for creating new SCN-resistant germplasms, thereby ensuring the sustainable growth of the global soybean industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated for approximately 5000 years in China, soybean (Glycine max L.) stands as a vital leguminous crop, offering renewable vegetable protein and oil. Its cultivation has spread globally, encompassing diverse regions [1]. Beyond human consumption, soybeans are extensively used, particularly as animal feed [2]. In 2022, China emerged as the fourth-largest global soybean producer, yielding 20.28 million metric tons. However, an array of biotic and abiotic threats imperil soybean production, including pathogens such as viruses, bacteria, fungi, and nematodes [3]. Among these reported soybean pathogens, the soybean cyst nematode (SCN) stands out as a global menace, causing substantial yield losses. Its impact is profound, and effectively preventing and controlling SCN infection remains challenging [4].
SCN is a soil-borne, sedentary, and specialized parasitic nematode that has caused economic losses exceeding $32 billion in the United States alone between 1996 and 2016, averaging over $1.5 billion annually. SCN-induced losses exhibit substantial variability, ranging from 30–40% and even reaching as high as 100% in severely infested fields [5]. While there are excellent comprehensive recent reviews that cover SCN resistance in general [4,5,6,7], our focus in this review is specifically on the molecular-level aspects of SCN resistance. Generally, SCN is believed to originate in China or Japan [8]. Its first identification was reported in China in 1954. Since then, ten races of SCN have been documented in China. However, a recent discovery identified a new race (X12) in China capable of infecting all tested soybean sources and exhibiting higher virulence than the previously considered most virulent race, race 4 [9, 10]. As SCN has spread to many countries, such as the USA, Brazil and China, it has emerged as a significant problem, primarily due to the widespread areas of cultivated soybeans. The challenge in eradicating SCN, coupled with significant economic losses in soybean, further accentuate its impact. Common symptoms of SCN infestation include stunted growth, yellowing of plants, reduced seed production, and leaf loss. Infected rootlets also exhibit a decrease in bacterial nodules. Initially, the disease appears in patchy formations, and it takes approximately two years to fully infect a field.
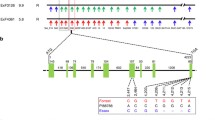
The life cycle of the SCN can be divided into four stages, including juvenile stages and one adult stage (Fig. 1). The development begins with the eggs and progresses through molting. Second-stage juveniles penetrate the host roots and reach the vascular cylinder. By continuously feeding and secreting effectors, the second-stage juveniles (J2) of the nematode establish permanent feeding sites called syncytia. The nematodes transition to a sedentary lifestyle [6]. These feeding sites are formed in the vascular cylinder of the roots [11]. The interaction between the nematode and host can be either compatible or incompatible. In a compatible interaction, the nematode successfully completes its entire life cycle within the host, which typically takes approximately 24–30 days under optimal conditions.
Life Cycle of Soybean Cyst Nematode (SCN). Schematic representation illustrating the life cycle of the soybean cyst nematode (SCN) in the root system of soybean plants. The diagram highlights the stages of nematode development and depicts the damaging effects on the plant, including nutrient depletion leading to yellowing of leaves. The cycle encompasses the various phases J0-J4 of nematode reproduction and infection, shedding light on the crucial interactions between SCN and soybean roots
Crop rotation and the implementation of resistant varieties are recognized as the most eco-friendly and economic measures among the diverse approaches to manage SCN. The utilization of resistant varieties, in particular, plays a pivotal role in achieving environmentally sustainable control strategies [6]. The application of resistant varieties, including rhg1-b derived from the resistant soybean variety PI 88788 and rhg1-a and Rhg4 derived from PI 548402/Peking, along with other minor-effect quantitative trait loci (QTLs), such as cqSCN-006 and cqSCN-007 from PI 468916 and Chr10-QTL from PI 567516C, plays a crucial role in green control strategies. Over 300 SCN resistance-related QTLs have been identified through genome-wide association studies (GWAS) and population segregation analysis. These QTLs span all 20 chromosomes in soybean (Table S1) (https://www.soybase.org).
As an obligate parasitic nematode, SCN exhibits distinct physiological differentiation, and based on its varying pathogenicity on different host resistance backgrounds, it can be further divided into different physiological races [12]. With the continuous field application of single resistance loci, both domestically and internationally, SCN populations of different physiological races have evolved under the selection pressure of resistance, resulting in a reduction in the effectiveness of existing natural resistance genes [13,14,15,16]. With the limited and declining natural resistance in the face of potential resistance failure, it is crucial to conduct comprehensive research on the resistance mechanism of present resistance loci and explore high-quality targets for resistance genes in transgenic soybean varieties. This has become a challenging yet vital focus of environmentally sustainable control of SCN globally. In recent years, significant breakthroughs have been made in the exploration of SCN resistance-related genes in soybean, shedding light on their physiological and biochemical functions and enhancing our understanding of resistance mechanisms. This article provides an overview of the recent advancements in functional research on soybean resistance-related genes against SCN, while the prospects and future directions for further exploration in this field are also discussed.
Research on the gene functions of soybean's QTL loci against SCN
Due to the unique characteristics of plant-parasitic nematodes, their interaction mechanisms differ significantly from currently known mechanisms of other pathogens, including fungi, bacteria, and viruses. One impressive example is the absence of traditional pathogen-associated molecular pattern (PAMP) receptors or intracellular nucleotide-binding-leucine-rich-repeat (NLR) receptors encoded by known SCN resistance loci. Further studies indicate that LRR-RKL kinases located near significant quantitative trait loci (QTLs) do not confer resistance to SCN [17, 18]. These findings demonstrate that SCN may exhibit more vigor and proactivity than other pathogens due to its unique properties, and the recognition mechanism of SCN differs from that of other conventional plant‒microbe immune activation pathways.
In recent years, with the application of technologies such as soybean gene chips, next-generation transcriptome sequencing, and laser microdissection, an increasing number of potential SCN resistance-related genes have been identified, along with their functions [19,20,21,22,23,24,25,26,27]. In particular, by the use of a nematode-adapted single-cell RNA-seq approach, novel virulence gene candidates have been uncovered within SCNs [28, 29]. Stable T2 generation mutant lines are obtained through ethylmethanesulfonate (EMS) mutagenesis of susceptible or resistant varieties, followed by traditional targeting-induced local lesions in genomes (TILLING) or TILLING combined with second-generation sequencing technology, which is an effective method for screening and discovering potential resistance-related genes [30,31,32]. Currently, as the study of known SCN resistance genes deepens, the mechanism of interaction between SCN and soybean is gradually being elucidated.
Among the QTLs linked to SCN resistance, rhg1 and Rhg4 are of paramount importance. In the following sections, we provide a detailed introduction to the functions of the genes encoded by these two major-effect resistance loci, their gene structure characteristics, and their collaborative mechanism.
rhg1
Rhg1 (Resistance to Heterodera glycines 1) is the earliest identified major resistance locus derived from the classical cultivar PI 88788, located on chromosome 18. According to statistics, more than 95% of the SCN-resistant cultivars in the United States carry the rhg1 locus from the PI 88788 line, indicating the stability and practicality of this locus in providing resistance [33].
Cook et al. (2012) identified rhg1 as a 31.2 kb gene segment using fluorescence in situ hybridization (FISH), and the multicopy nature of rhg1 is directly associated with SCN resistance. The study unveiled that susceptible cultivars (e.g., Williams 82) possess a single rhg1 copy, termed the rhg1-c type. Resistant cultivars (e.g., PI548402/Peking) display 2–3 tandem copies, identified as the rhg1-a type. Notably, the representative resistant cultivar PI 88788 features 7–10 tandem copies recognized as the rhg1-b type [34]. Recently, a new rhg1 haplotype, called rhg1-ds, was discovered in six accessions of wild-type soybean, Glycine soja Siebold & Zucc, using SoySNP50K data and assessing the presence of the NSFRAN07 allele [35]. With the rapid development of pangenome sequencing technology, multiple copies of genes in soybean have been found to be widespread and are thought to be indispensable for soybean evolution in nature [36]. Transcriptional analysis of the rhg1-encoded resistance genes in soybean samples with different copy numbers showed that under noninfected conditions, transcript abundance for these genes scales with rhg1 copy number. In addition, quite a few genetic structural variations, including single-nucleotide polymorphisms (SNPs), DNA fragment insertions, and deletions, are observed in these genes encoded by different types of rhg1 in soybean (Cook et al., 2014). The effects of genetic structural variations of genes encoded by rhg1 on SCN resistance are still under investigation.
Three out of the four genes encoded by the rhg1 locus have been demonstrated to engage in the resistance response of rhg1 high-copy cultivars against SCN [34]. These three genes are as follows: Glyma.18G022400 (Wm82.a1, Glyma18g02580), which encodes a putative amino acid transporter protein called GmAAT; Glyma.18G022500 (Wm82.a1, Glyma18g02590), which encodes a functionally conserved protein α-SNAP (α-soluble NSF attachment protein, also known as SNAP18) involved in vesicular transport processes; and Glyma.18G022700 (Wm82.a1, Glyma18g02610), which encodes a protein WI12Rhg1 (WI12-induced protein) containing a WI12 injury-induced structural domain. When these rhg1 genes from soybean sources were transferred into Arabidopsis or Solanaceae (potato), enhanced resistance against sugar beet cyst nematode (Heterodera schachtii), potato cyst nematode (Globodera rostochiensis), and potato pale cyst nematode (G. pallida) was observed [37].
The functional mechanism of α-SNAP encoded by the rhg1 locus against SCN is well characterized [38,39,40]. α-SNAP is a conserved protein involved in vesicular transport processes and forms a complex with N-ethylmaleimide-sensitive factor (NSF) to participate in the recycling of soluble NSF attachment protein receptors (SNAREs). α-SNAP specifically accumulates in the feeding sites called syncytia of SCN during nematode infection [38, 41]. Different soybean varieties with varying copy numbers of rhg1 encode α-SNAP with amino acid polymorphisms, particularly in the C-terminal region, which affects its interaction with NSF and indirectly influences intracellular vesicular transport mechanisms. Overexpression of α-SNAP encoded by high-copy rhg1 can lead to plant cell death, while α-SNAP from susceptible varieties does not cause such effects [38]. However, α-SNAP itself is an essential gene for cellular processes, and mutations occurring in resistant types of α-SNAP pose a destructive threat to plant viability. To balance the toxicity of the α-SNAP mutant in resistant varieties, almost all known soybean varieties with resistant α-SNAP have coevolved with a stronger binding partner called NSFRAN07, which ensures that normal cellular functions are not compromised [39]. Different mutations in α-SNAP result in distinct physiological functions, indicating that rhg1 affects resistance not only through copy number variation but also through intrinsic genetic sequence variation [40, 42]. The homologous gene of α-SNAP, SNAP11 (Glyma.11G234500), on chromosome 11 has been identified as a minor-effect gene involved in SCN resistance and contributes to the resistance of Peking-type soybean hosts against SCN [43, 44]. While not yet conclusively proven, the significance of α-SNAP in cyst nematode resistance suggests its vulnerability to nematode effectors that could potentially dampen host defense against cyst nematodes through α-SNAP-mediated mechanisms. An SCN gene encodes a bacterial-like protein, HgSLP-1, containing a putative SNARE domain, which physically interacts with α-SNAP in vitro [45]. Recently, an effector named HsSNARE1, containing a t-SNARE domain, was identified from beet cyst nematode (BCN). HsSNARE1 and its highly homologous counterpart HgSNARE1 from the SCN can both interact with AtSNAP2, which is highly homologous to GmSNAP18. Overexpressing HgSNARE1 suppresses BCN infection, while HsSNARE1 overexpression promotes it in Arabidopsis thaliana [46]. Further exploration is required to identify additional nematode effectors that directly target GmSNAP18/α-SNAP.
The mechanism underlying another resistance gene, GmAAT, encoded by the rhg1 locus, remains inadequately comprehended to date. There are no amino acid sequence differences among different rhg1 haplotypes for GmAAT, suggesting that the resistance mechanism of GmAAT is quite distinct from that of the α-SNAP gene. Silencing GmAAT leads to compromised soybean resistance against SCN [34]. GmAAT contains a conserved amino acid transport domain, but there is no direct evidence showing that this protein directly transports amino acids in soybean. Overexpression of GmAAT increases soybean tolerance to excess glutamate, indicating its involvement in glutamate tolerance-related functions [47]. Furthermore, overexpression of GmAAT can activate the jasmonic acid signaling pathway, indicating that GmAAT potentially operates by stimulating plant hormone pathways [47].
Using immunoelectron microscopy with a GmAAT-specific antibody, specific expression and localization patterns of GmAAT in response to SCN infection were observed. During the early stages of SCN invasion in the root system, GmAAT protein specifically accumulates in cells penetrated by SCN and forms resistance vesicles with GmAAT localization in cells directly contacted by the nematode. The copy number of rhg1 is positively correlated with the enrichment of GmAAT in these structures, and the expression level of GmAAT and the extent of vesicle aggregation are both associated with rhg1-mediated resistance [48]. The physiological and biochemical properties of resistance vesicles formed by GmAAT and the molecular mechanisms of GmAAT's involvement in other plant hormone signaling pathways, such as JA and ethylene, still require further investigation. Collectively, current research suggests that while GmAAT and α-SNAP both belong to the rhg1 locus, their resistance mechanisms significantly diverge in terms of protein localization and physiological function.
WI12Rhg1 is the third resistance gene encoded by the rhg1 locus, and it has not garnered significant research attention thus far. In addition, there is a lack of studies on homologous proteins of WI12Rhg1 in other species. A recent study showed that WI12Rhg1 directly interacted with DELLA18 (Glyma.18G040000) in yeast and plants. Double knockout of DELLA18 and its homeolog DELLA11 (Glyma.11G216500) leads to a significant reduction in SCN resistance and causes changes in root morphology [49]. Due to its brief protein sequence, uncovering valuable functional domains within WI12Rhg1 proves to be challenging. The specific physiological function of WI12Rhg1, which contains a putative damage-inducible domain, also needs to be confirmed. However, one potential resistance mechanism of WI12Rhg1 likely involves damage-associated disease progression molecular patterns (wound-inducible DAMPs) through interactions with other proteins, including those encoded by the rhg1 locus. This mechanism may contribute to the early response of SCN infection during plant-nematode interactions.
Rhg4
Rhg4 is located on chromosome 8 and is one of the major QTLs derived from Peking and PI 437654 [50,51,52]. Liu et al. identified the serine hydroxymethyltransferase (SHMT or SHMT08) gene located at the Rhg4 locus using TILLING technology, a commonly employed reverse genetics method. shmt mutations can impact the resistance of Peking-type resistant cultivars [32]. Similar to α-SNAP, SHMT is a housekeeping protein and is involved in the folate synthesis signaling pathway. The sequence of SHMT genes exhibits only five variations between wild-type and Peking-type resistant varieties; however, these variations profoundly impact SCN resistance. The underlying mechanisms linking the mutation sites to the phenotypes still require further research. It is worth noting that, similar to α-SNAP, SHMT is specifically expressed in the syncytial cells formed during SCN infection [32]. Genetic screening has provided further validation of the structure and resistance function of SHMT [53]. Through protein‒protein interaction screening, the identification of a 70-kDa heat shock protein (HgHSP70) in H. glycines revealed its interaction with GmSHMT08. These studies have provided insights into the interaction mechanism between soybean and SCN [54].
Similar to rhg1, a recent whole-genome sequencing study discovered that Rhg4 also undergoes multicopy amplification [55]. A tandem repeat sequence of approximately 35.7 kb exists within Rhg4, and high-level amplification of Rhg4 can lead to increased transcription levels of the genes encoded by Rhg4. This segment of the Rhg4 locus contains three genes: Glyma.08g108800 (Wm82.a1, Glyma08g11480), Glyma.08g108900 (SHMT, Wm82.a1, Glyma08g11490), and Glyma.08g109000 (Wm82.a1, Glyma08g11500). Additionally, Rhg4 also exhibits allelic variation and is primarily categorized into Rhg4-b (single copy) represented by the wild type and Rhg4-a (1–4.3 copies) represented by the resistant Peking-type [55]. The promoter sequence of SHMT08 in resistant varieties such as Peking and PI 88788 differs from that in susceptible varieties and is closely associated with a broader spectrum of SCN resistance [55].
In resistant varieties such as PI548655 (Forrest, a Peking-type resistant variety) and PI 88788, the transcription levels of SHMT08 are approximately twofold higher during early SCN infection compared to that in wild type [56]. This indicates that the resistance-type promoter may play a role in early infection regulation at the transcriptional level. The genomic studies of rhg1 and Rhg4, combined with the functional genomics of SCN resistance phenotypes, once again demonstrate that soybeans generally adapt to the invasion of external pathogens by multicopy amplification and sequence structural variations at specific gene loci. The association between gene changes and SCN resistance, particularly the expression regulatory mechanism, still requires further research.
Diverse mechanisms and associations of rhg1 and Rhg4
Although rhg1 and Rhg4 are both important resistance loci, their resistance mechanisms are quite different. They share significant associations and similarities, mainly manifested in the following aspects. First, the functional proteins SHMT08 and α-SNAP encoded by these two loci interact with each other and coexist in a protein complex. [57]. Overexpressing soybean GmSNAP18 in Arabidopsis increased its susceptibility to BCN. Transcriptome analysis showed that GmSNAP18 overexpression in Arabidopsis led to the suppression of AtSHMT4, a homolog of GmSHMT08, post BCN infection. This reveals the adverse negative modulation of AtSHMT4 in BCN susceptibility by GmSNAP18 overexpression [58]. However, the detailed function and mechanism of their interaction are still unknown. Second, when the copy number of rhg1 is less than 5.6, it cannot provide sufficient broad-spectrum SCN resistance independently and requires cooperation with Rhg4. The combination of low-copy rhg1 and resistance-associated Rhg4 is collectively referred to as Peking-type resistance (rhg1-a + Rhg4-a/Rhg4-c) [55, 59]. The specific mechanism of this cooperation needs further investigation. Third, as evolution progresses, both rhg1 and Rhg4 in resistant varieties have multiple homologous genes in different resistance types. This is evident in the abundance of nonsynonymous single nucleotide polymorphisms (SNPs) causing amino acid sequence variations. The structural and functional changes resulting from these variations require further study. Fourth, both loci exert additional resistance functions through multicopy amplification. Higher copy numbers of Rhg4 provide broader resistance against various virulent races of SCN, while increased copy numbers of rhg1 independently provide sufficient resistance. Fifth, based on whole-genome resequencing of 106 soybean lines, specific mutations in the promoter and gene sequences of rhg1 and Rhg4, as well as differences in copy numbers, collectively determine resistance to different physiological races of SCN [55]. The coevolutionary relationship between these resistance genes and the pathogen deserves further investigation.
Vesicle transport and autophagy in SCN resistance
Vesicle transport in SCN resistance
Vesicle transport is a specific and dynamic process involving the translocation of cargo vesicles from the donor membrane to the target membrane. This process includes complex events such as endocytosis and exocytosis, which require a range of conserved proteins [60]. Vesicle transport plays a significant role in plant immunity. Resistance proteins in the extracellular space are secreted through this process to exert their functions. Additionally, the endocytosis of resistance molecule receptors on the plant's surface can significantly activate downstream signaling pathways. Proteins involved in vesicle transport pathways are often targeted by pathogens to interfere with plant resistance [61].
The exocyst, acting as a receptor protein, plays a pivotal role in signal transduction. The exocyst serves as the "bridge" between incoming vesicles and receptor proteins, leading to the aggregation of SNARE protein complexes and membrane fusion. The exocyst complex is an octameric structure, and a study in yeast identified eight protein components: Sec3p (EXOC1), Sec5p (EXOC2), Sec6p (EXOC3), Sec8p (EXOC4), Sec10p (EXOC5), Sec15p (EXOC6), Exo70p (EXOC7), and Exo84p (EXOC8). Each component of the exocyst complex has its own molecular mechanism involved in plant resistance. The exocyst protein PR-1 (Glyma.15g062400) has been demonstrated to directly participate in SCN resistance in soybean, indicating the involvement of the exocyst pathway in SCN resistance [62]. Sec4p physically interacts with Sec15p (EXOC6), which can regulate the assembly of exocyst vesicles. Overexpression of Sec4 can also enhance SCN resistance in soybean [63]. All homologous genes of each component of the exocyst complex have been identified in soybean. Additionally, the expression levels of each component gene were significantly upregulated in the syncytia, indicating that exocyst vesicles contribute to SCN resistance in soybean. Overexpression of exocyst component genes in the susceptible cultivar Williams 82 enhances resistance, while silencing of the related genes in the resistant cultivar Peking renders the plants more susceptible [64].
SNARE proteins mediate vesicle fusion, which is a crucial step in vesicle transport. After membrane fusion, the dissociation and recycling of SNAREs require NSF and α-SNAP proteins, which together with SNAREs form a supercomplex called the 20S complex. This complex is involved in the recycling process of vesicle transport components [65]. Transcriptional analysis of SCN-infected root cells reveals that the expression of genes encoding the 20S complex is induced [62]. As mentioned above, α-SNAP is a key SCN resistance gene encoded by the rhg1 locus. Once more, these findings underscore that SCN can potentially target vesicle fusion components to facilitate susceptibility.
Synaptic fusion protein (syntaxin, SYN) is a part of the SNARE protein complex, which means that syntaxin interacts physically with α-SNAP protein. In soybean, overexpression of α-SNAP induces the expression of SYN31, and the upregulation of SYN31 predominantly occurs within cytoplasmic cells upon SCN infection. Further study shows that overexpression of SYN31 can confer SCN resistance in susceptible soybean roots [66]. Overexpression of SYN121, the homologous gene in soybean of Arabidopsis PEN1, can also enhance SCN resistance in soybean. The transcription levels of SYN121 and other genes encoding the SNARE complex are upregulated in SCN-infected soybean cells [67]. Recent research has demonstrated that α-SNAP interacts with two syntaxin proteins (SYN12, Glyma.12g194800, and SYN16, Glyma.16g154200) belonging to the t-SNARE family. Both of these syntaxin proteins are reported to be located within SCN resistance QTLs. Knocking out two syntaxin genes using CRISPR technology in the Peking resistant soybean variety compromises resistance [68]. Another protein complex closely associated with SNARE in the 20S complex, COG, is involved in regulating membrane fusion and has been reported to directly influence the SCN resistance response. Overexpression of the COG gene significantly inhibits SCN parasitism, and silencing the COG gene in the Peking resistant variety significantly affects resistance [69]. In conclusion, vesicle transport plays a crucial role in SCN pathogenicity and host resistance, but more direct evidence and the detailed mechanism of vesicle transport still need to be explored.
Autophagy in SCN resistance
Plant autophagy, a conserved intracellular degradation system, is gaining increasing recognition as a crucial player in plant‒pathogen interactions, yet its involvement in nematode parasitism remains largely unexplored [70]. It was demonstrated that disrupting autophagy in Arabidopsis mutants led to reduced susceptibility to Heterodera schachtii infection, highlighting the pivotal role of autophagy in plant-nematode interactions [71]. Recently, research conducted by Zou et al. underscores the significance of autophagy in bolstering jasmonate-driven defenses in plants against nematode challenges [72]. As elaborated above, vesicle transport is pivotal in SCN pathogenicity and host resistance. Additionally, vesicle transport contributes to autophagy by facilitating autophagosome formation. It is plausible to suggest that autophagy could contribute to defense against SCN, with the nematode potentially disrupting host vesicle trafficking to aid infection. However, direct evidence is still lacking.
Signaling molecules in SCN resistance
The resistance mechanisms of plant hosts against various pathogens differ, and these mechanisms typically involve the transduction of signals related to plant disease resistance. Cellular signaling is the process by which cells receive initial stimulus signals that are subsequently transmitted and amplified. Plant hormones represent a significant category of signaling molecules that play a crucial role in plant immunity. These include auxins, ethylene, cytokinins, gibberellins, abscisic acid, jasmonates, salicylic acid, brassinosteroids, and strigolactones. Other nonhormonal signaling molecules produced by plant cells function as defense regulators, primarily encompassing small peptides, reactive oxygen species (ROS), calcium ions (Ca2+) and more. Transcriptomic analyses have demonstrated that numerous genes encoding plant immune-related signaling molecules are influenced by nematode infection. The involvement of plant signaling molecules in nematode resistance has been a prominent subject within this research domain.
Plant hormone molecules in SCN resistance
Ethylene in SCN resistance
The ethylene signaling pathway is crucial for plant root development, but its involvement in SCN resistance is largely unknown. Hu et al. reported that the ethylene signaling pathway is involved in regulating root attraction to SCN, and soybean roots treated with ethylene inhibitors are more attracted to SCN [73]. Additionally, the expression levels of genes encoding the ethylene precursor ACC synthase were upregulated upon SCN infection [74]. Transcription factors downstream of the ethylene signaling pathway have been demonstrated to undergo upregulation following SCN infection. As an example, the expression of GmEREBP (Glyma.18G252300), an ethylene-responsive transcription factor that binds to the GCC-box motif, is downregulated in susceptible soybean varieties and upregulated in resistant soybean varieties [75]. Subsequent research suggests that the ethylene pathway may regulate ethylene-responsive genes, such as PR3 and PDF1.2, through these transcription factors upon SCN infection [76]. Therefore, the interplay between the genes encoding resistance (such as rhg1 and Rhg4) and the ethylene signaling pathway in susceptible or resistant soybean varieties remains to be explored.
Auxin in SCN resistance
Currently, investigations into the role of the auxin signaling pathway in SCN resistance remain limited. The expression of the auxin-responsive gene ADR12 was found to be downregulated in soybean roots upon SCN infection [77]. Transcriptomic profiling of SCN-infected root cells in soybean confirmed that the expression of the genes related to auxin signaling pathways, such as IAA and ARF, was significantly regulated [20]. While it is acknowledged that sedentary plant-parasitic nematodes, such as root-knot nematodes and sugar beet cyst nematodes, utilize diverse strategies to alter auxin homeostasis [78,79,80,81], concrete evidence for the involvement of auxin signaling in SCN resistance has yet to be established.
Salicylic acid in SCN resistance
Salicylic acid (SA) plays a pivotal role and becomes activated during the initial phase of pathogen invasion. Soybean salicylic acid methyltransferase (GmSAMT1) has been identified as a contributor to SCN resistance [82]. Overexpression of GmSAMT1 in susceptible varieties significantly inhibits nematode development and enhances SCN resistance. In soybean roots that overexpress GmSAMT1, the expression of SA signaling pathway-related genes (GmNPR1, GmICS1) is also significantly induced [82]. Therefore, it is predicted that the accumulation of the biosynthetic enzyme GmSAMT1 is faster in resistant varieties, which can activate the SA signaling pathway and contribute to SCN resistance.
Nonhormonal signaling molecules in SCN resistance
The CLAVATA receptor complex, which plays a crucial role in shoot apical and root apical meristem development, is regulated by a small peptide called CLV3, which consists of a 12-amino acid peptide and belongs to the CLE (Clavat Like Elements) family. Plant-parasitic nematodes secrete CLE-like effectors to manipulate plant root development and facilitate their infection. In soybean, it has been demonstrated that the CLV3 receptor protein can specifically bind to CLE-like effectors secreted by SCN, and silencing this receptor leads to increased resistance against SCN [83]. Structural and functional analysis of SCN CLE-like effectors has identified a specific domain responsible for transporting these effectors from the endoplasmic reticulum to the extracellular space. Nematode CLE effectors can hijack the plant cell secretory system to export their signaling proteins outside the cell [84]. In addition, plant elicitor peptides (PEPs) have been shown to participate in regulating SCN resistance. Treatment of soybean seeds with PEP1, PEP2, and PEP3 significantly inhibits SCN proliferation. Further experiments have demonstrated that PEP treatment can induce the production of reactive oxygen species (ROS) in soybean, leading to SCN resistance [85]. Exploring the precise molecular mechanisms of plant elicitor peptide-induced resistance holds significant potential.
Extensive research has delved into the systemic response of plants to pathogen infection and wounding, revealing a network of compounds and signals involved [86]. Nematodes can induce physical damage during their early infection stages, yet the extent to which ROS and Ca2+ signaling mechanisms play a role in orchestrating this response remains largely unexplored. ROS are frequently generated in response to biotic or abiotic stress in plants, serving as essential components in various signaling pathways. RBOHC2, a key factor in plant ROS production, physically interacts with GmAAT encoded by rhg1 in vesicles. Coexpression of RBOHC2 and GmAAT induces the production of superoxide anions (O2−), which serve as ROS precursors in plant cells. The O2− generated around the nematode-containing vesicles can be further converted into hydrogen peroxide (H2O2) outside the plant cells, directly affecting the nematode or acting as a secondary signaling molecule to induce stronger downstream resistance responses [48]. Chen et al. also observed that the rate and extent of ROS accumulation varied greatly among different types of resistant varieties, and ROS production is directly related to host resistance against SCN [87]. Overexpression of miR408 in soybean can decrease soybean resistance to SCN by suppressing ROS accumulation [88]. Ca2+ and ROS signaling interplay significantly in plant immunity. Ca2+ plays a pivotal role in signaling events during the invasion of the potato cyst nematode Globodera rostochiensis [89]. The MeTCTP effector from Meloidogyne enterolobii impedes immunity by suppressing cytoplasmic free Ca2+ through secretion [90]. No reports have indicated direct engagement of Ca2+ signaling pathways in SCN resistance; further attention is required to understand the relationship between ROS and Ca2+ signaling pathways and SCN resistance.
Other mechanisms
Host cell wall degradation is prominent during syncytium formation. Many nematode secreted effectors are believed to have cell wall-degrading enzyme activity. However, the involvement of the host’s cell wall synthesis pathway in SCN resistance has been less studied. Recently, the biochemical enzyme GmXTH43, which is involved in the regulation of cell wall synthesis and the elongation of glycan side chains, was found to be specifically expressed in syncytial cells. Overexpression of GmXTH43 in susceptible soybean varieties can enhance resistance, suggesting that it may function in syncytia by affecting cell wall formation [91]. This is another example of a fundamental biochemical enzyme involved in the response to SCN resistance, but the underlying regulatory mechanism remains unclear.
In addition, recent research has reported that overexpression of a plasma membrane-localized broad-spectrum resistance gene, disease resistance 1 (GmDR1, Glyma.10g094800), can enhance plant resistance to SCN, aphids, and fungal diseases. However, the specific regulatory mechanism is still unknown [92].
In the context of amino acid metabolism and the SCN resistance mechanism mediated by rhg1 and Rhg4, several intriguing questions arise. Rhg4, which encodes a serine hydroxymethyltransferase (SHMT08) involved in cellular one-carbon metabolism, plays a crucial role in interconverting serine and glycine. On the other hand, it has been suggested that rhg1, encoding the GmAAT transporter, may function as an amino acid transporter. However, the specific amino acid(s) transported by GmAAT and its potential connection to the resistance loci remain unclear. It is hypothesized that GmAAT transports glycine, which can serve as a substrate for SHMT08 encoded by Rhg4. Moreover, the relationship between the amino acid pool and the plant hormone response signaling pathway upon SCN infection needs to be explored. A recent study found that the addition of specific free amino acids from an external source had a significant ability to attract second-stage juveniles (J2) of SCN in chemotaxis assays. Interestingly, it was observed that nine particular amino acids showed strong attraction to SCN J2 [93]. One possible explanation for this phenomenon is that the upregulation of GmAAT expression upon SCN infection may subsequently impact the balance of free amino acids within the apoplast of SCN-infected cells. This mechanism may serve as a defense strategy to deter the attraction of newly hatched J2s to soybean roots. Enzymes encoded by the GH3 family can conjugate jasmonates, auxins, and benzoates to amino acids. This conjugation process has diverse effects, including the activation, inactivation, or degradation of these hormone molecules. By conjugating amino acids to plant hormones, plants have evolved a sophisticated immune system in the face of SCN infection and other environmental stimuli. Thus, through the examination of the interplay between amino acid homeostasis and the modulation of plant hormone responses, a new strategy for enhancing resistance in soybean varieties may potentially be uncovered. Further investigation into the relationship between these two resistance loci, rhg1 together with Rhg4, and the amino acid pool and exploration of the impact of conjugated plant hormones on SCN infection holds great potential for gaining a deeper understanding of the complex mechanisms underlying SCN resistance.
In contrast to other plant microbiome pathogens, plant parasitic nematodes exhibit characteristics similar to animals and often rely on vitamins to support their parasitic activities. On the other hand, the ability of the plant host to regulate vitamin supply during the plant-nematode interaction may play a crucial role in facing cyst nematode infection. SHMT08, encoded by Rhg4, is a widely conserved enzyme regulating one-carbon folate metabolism across different kingdoms. It is speculated that folate (a natural form of vitamin B9) deficiency resulting from disrupted folate homeostasis can lead to significant resistance derived from Rhg4. A recent study conducted on sugar beet cyst nematode, H. schachtii, revealed an atypical completion of vitamin B5 biosynthesis by the parasitic animal [94]. The specific investigation of the resistance mechanisms associated with the balance of vitamin supply during SCN infection, as well as the study of plant-related genes involved in the modulation of disease resistance and susceptibility in this process, represents a promising avenue for future research in this field.
Summary and outlook
Significant progress has been made in the functional study of soybean resistance genes against SCN, but there are still several limitations. Currently, transcriptomics and functional genomics analyses have identified a range of resistance genes, while research on expression and epigenetic regulation is lacking. Additionally, potential resistance genes are often essential for plant life processes, and silencing them can lead to defects in root development and growth, indirectly affecting resistance phenotypes. Discovering new nematode resistance genes involves utilizing modern techniques such as comparative genomics, expression quantitative trait locus (eQTL) mapping, association studies, gene editing, and omics technologies to identify candidate genes linked to nematode resistance. Functional validation and network analysis further validate their roles and interactions, enabling the development of resistant soybean varieties.
The detailed relationship between known plant immunity and SCN resistance genes has not been elucidated. Further research is needed to explore the differences and connections between PAMP-triggered immunity (PTI) and the wounding defense response induced by SCN. Additionally, it is important to investigate whether nematode effectors secreted into host cells can trigger effector-triggered immunity (ETI). Screening for interacting proteins using known important resistance-associated proteins as baits in the soybean system can help answer these questions and provide a better understanding of the interaction pathways between SCN and soybean hosts.
China, the birthplace of soybean, boasts extensive germplasm resources. Wild soybeans (Glycine soja) can serve as a valuable gene pool that likely contains abundant resistance genes different from those present in current cultivated varieties. Further exploration of resistance genes using wild soybeans is needed. Additionally, with the rapid advancement of single-cell sequencing technology, transcriptional profiling analysis can pinpoint key developmental nodes at the single-cell level. Moreover, it is essential to construct more intricate profiles through single-cell sequencing in both compatible or incompatible interactions between SCN and soybean. This approach can enhance our understanding of SCN pathogenicity and the host's resistance defense response.
Last, research on pathogenicity centered around nematodes is equally important, especially in the identification and functional verification of nematode effectors. The classification and identification of SCN-secreted biochemical signaling molecules such as ascarosides, as well as the development and application of gene manipulation techniques targeting plant-parasitic nematodes, hold great potential for development and application in plant breeding and scientific research.
The study of SCN resistance mechanisms contributes to understanding the interaction between plant-parasitic nematodes and hosts, providing references for traditional breeding and valuable targets for the construction of resistant transgenic cultivars. This study has significant implications for China's independent intellectual property rights in related breeding and scientific research fields. With the development of biotechnology and continuous investment in science and technology in China, significant breakthroughs are expected in the comprehensive and environmentally friendly control of soybean cyst nematodes in theory, research, and practical applications. Emerging theories, concepts, and practices will have a vital role in safeguarding the security of global soybean production and ensuring the safety and sustainability of the soybean industry worldwide.
Availability of data and materials
All data used in this study are included in this article.
References
Liu S, Zhang M, Feng F, Tian Z. Toward a “Green Revolution” for soybean. Mol Plant. 2020;13(5):688–97.
Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiol. 2003;131(3):872–7.
Nazarov PA, Baleev DN, Ivanova MI, Sokolova LM, Karakozova MV. Infectious plant diseases: etiology, current status, problems and prospects in plant protection. Acta Naturae. 2020;12(3):46–59.
Bent AF. Exploring soybean resistance to soybean cyst nematode. Annu Rev Phytopathol. 2022;60:379–409.
Peng DL, Jiang R, Peng H, Liu SM. Soybean cyst nematodes: a destructive threat to soybean production in China. Phytopathol Res. 2021;3(1):1–16.
Niblack TL, Lambert KN, Tylka GL. A model plant pathogen from the kingdom animalia: Heterodera glycines, the soybean cyst nematode. Annu Rev Phytopathol. 2006;44(1):283–303.
Mitchum MG. Soybean resistance to the soybean cyst nematode Heterodera glycines: an update. Phytopathol. 2016;106(12):1444–50.
Riggs RD, Schmitt DP. Complete characterization of the race scheme for Heterodera glycines. J Nematol. 1988;20(3):392–5.
Lian Y, Guo J, Li H, Wu Y, Wei H, Wang J, et al. A new race (X12) of soybean cyst nematode in China. J Nematol. 2017;49(3):321–6.
Lian Y, Wei H, Wang J, Lei C, Li H, Li J, et al. Chromosome-level reference genome of X12, a highly virulent race of the soybean cyst nematode Heterodera glycines. Mol Ecol Resour. 2019;19(6):1637–46.
Endo BY. Pathogenesis of nematode-infected plants. Annu Rev Phytopathol. 1975;13(1):213–38.
Tylka GL. Understanding soybean cyst nematode HG types and races. Plant Health Progress. 2016;17(2):149–51.
Hua C, Li C, Hu Y, Mao Y, You J, Wang M, et al. Identification of HG types of soybean cyst nematode Heterodera glycines and resistance screening on soybean genotypes in Northeast China. J Nematol. 2018;50(1):41–50.
Chen J, Zhou Y, Wang Y, Fan H, Liu X, Wang D, et al. Characterization of virulence phenotypes of Heterodera glycines in Heilongjiang, Northeast China. Plant Dis. 2021;105(8):2056–60.
Chowdhury IA, Yan G, Plaisance A, Markell S. Characterization of virulence phenotypes of soybean cyst nematode (Heterodera glycines) populations in North Dakota. Phytopathol. 2021;111(11):2100–9.
Meinhardt C, Howland A, Ellersieck M, Scaboo A, Diers B, Mitchum MG. Resistance gene pyramiding and rotation to combat widespread soybean cyst nematode virulence. Plant Dis. 2021;105(10):3238–43.
Melito S, Heuberger AL, Cook D, Diers BW, MacGuidwin AE, Bent AF. A nematode demographics assay in transgenic roots reveals no significant impacts of the Rhg1 locus LRR-Kinase on soybean cyst nematode resistance. BMC Plant Biol. 2010;10(2):104.
Liu X, Liu S, Jamai A, Bendahmane A, Lightfoot DA, Mitchum MG, Meksem K. Soybean cyst nematode resistance in soybean is independent of the Rhg4 locus LRR-RLK gene. Funct Integr Genomics. 2011;11(4):539–49.
Shi X, Chen Q, Liu S, Wang J, Peng D, Kong L. Combining targeted metabolite analyses and transcriptomics to reveal the specific chemical composition and associated genes in the incompatible soybean variety PI 437654 infected with soybean cyst nematode HG1.2.3.5.7. BMC Plant Biol. 2021;21(1):217.
Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact. 2007;20(5):510–25.
Klink VP, Overall CC, Alkharouf NW, MacDonald MH, Matthews BF. Laser capture microdissection (LCM) and comparative microarray expression analysis of syncytial cells isolated from incompatible and compatible soybean (Glycine max) roots infected by the soybean cyst nematode (Heterodera glycines). Planta. 2007;226(6):1389–409.
Klink VP, Overall CC, Alkharouf NW, MacDonald MH, Matthews BF. A time-course comparative microarray analysis of an incompatible and compatible response by Glycine max (soybean) to Heterodera glycines (soybean cyst nematode) infection. Planta. 2007;226(6):1423–47.
Kandoth PK, Ithal N, Recknor J, Maier T, Nettleton D, Baum TJ, et al. The soybean Rhg1 locus for resistance to the soybean cyst nematode Heterodera glycines regulates the expression of a large number of stress- and defense-related genes in degenerating feeding cells. Plant Physiol. 2011;155(4):1960–75.
Matsye PD, Kumar R, Hosseini P, Jones CM, Tremblay A, Alkharouf NW, et al. Mapping cell fate decisions that occur during soybean defense responses. Plant Mol Biol. 2011;77(4–5):513–28.
Tian B, Wang S, Todd TC, Johnson CD, Tang G, Trick HN. Genome-wide identification of soybean microRNA responsive to soybean cyst nematodes infection by deep sequencing. BMC Genom. 2017;18(1):572.
Jiang HP, Tian LZ, Bu FS, Sun QX, Zhao X, Han YP. RNA-seq-based identification of potential resistance genes against the soybean cyst nematode (Heterodera glycines) HG Type 1.2.3.5.7 in “Dongnong L-10.” Physiol and Mol Plant Pathol. 2021;114:101627.
Huang M, Jiang Y, Qin R, Jiang D, Chang D, Tian Z, et al. Full-Length transcriptional analysis of the same soybean genotype with compatible and incompatible reactions to Heterodera glycines reveals nematode infection activating plant defense response. Front Plant Sci. 2022;13:866322.
Ste-Croix DT, St-Marseille AG, Lord E, Belanger RR, Brodeur J, Mimee B. Genomic profiling of virulence in the soybean cyst nematode using single-nematode sequencing. Phytopathol. 2021;111(1):137–48.
Ste-Croix DT, Bélanger RR, Mimee B. Single nematode transcriptomic analysis, using long-read technology, reveals two novel virulence gene candidates in the soybean cyst nematode, Heterodera glycines. Int J Mol Sci. 2023;24(11):9440.
Liu S, Ge F, Huang W, Lightfoot DA, Peng D. Effective identification of soybean candidate genes involved in resistance to soybean cyst nematode via direct whole genome resequencing of two segregating mutants. Theor Appl Genet. 2019;132(9):2677–87.
Ge FY, Zheng N, Zhang LP, Huang WK, Peng DL, Liu SM. Chemical mutagenesis and soybean mutants potential for identification of novel genes conferring resistance to soybean cyst nematode. J Int Agri. 2018;17(12):2734–44.
Liu S, Kandoth PK, Warren SD, Yeckel G, Heinz R, Alden J, et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature. 2012;492(7428):256–60.
Rincker K, Cary T, Diers BW. Impact of soybean cyst nematode resistance on soybean yield. Crop Sci. 2017;57(3):1373–82.
Cook DE, Lee TG, Guo X, Melito S, Wang K, Bayless AM, et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science. 2012;338(6111):1206–9.
Grunwald DJ, Zapotocny RW, Ozer S, Diers BW, Bent AF. Detection of rare nematode resistance Rhg1 haplotypes in Glycine soja and a novel Rhg1 α-SNAP. Plant Genom. 2022;15(1):e20152.
Liu Y, Du H, Li P, Shen Y, Peng H, Liu S, et al. Pangenome of wild and cultivated soybeans. Cell. 2020;182(1):162–76.
Butler KJ, Chen S, Smith JM, Wang X, Bent AF. Soybean resistance locus Rhg1 confers resistance to multiple cyst nematodes in diverse plant species. Phytopathol. 2019;109(12):2107–15.
Bayless AM, Smith JM, Song J, McMinn PH, Teillet A, August BK, et al. Disease resistance through impairment of alpha-SNAP-NSF interaction and vesicular trafficking by soybean Rhg1. PNAS. 2016;113(47):7375–82.
Bayless AM, Zapotocny RW, Grunwald DJ, Amundson KK, Diers BW, Bent AF. An atypical N-ethylmaleimide sensitive factor enables the viability of nematode-resistant Rhg1 soybeans. PNAS. 2018;115(19):4512–21.
Liu S, Kandoth PK, Lakhssassi N, Kang J, Colantonio V, Heinz R, et al. The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat Commun. 2017;8(1):14822.
Bayless AM, Zapotocny RW, Han S, Grunwald DJ, Amundson KK, Bent AF. The rhg1-a (Rhg1 low-copy) nematode resistance source harbors a copia-family retrotransposon within the Rhg1-encoded α-SNAP gene. Plant direct. 2019;3(8):e00164.
Lee TG, Kumar I, Diers BW, Hudson ME. Evolution and selection of Rhg1, a copy-number variant nematode-resistance locus. Mol Ecol. 2015;24(8):1774–91.
Li YH, Shi XH, Li HH, Reif JC, Wang JJ, Liu ZX, et al. Dissecting the genetic basis of resistance to soybean cyst nematode combining linkage and association mapping. Plant Genom. 2016;9(2):e1007.
Lakhssassi N, Liu S, Bekal S, Zhou Z, Colantonio V, Lambert K, et al. Characterization of the soluble NSF attachment protein-encoding gene family identifies two members involved in additive resistance to a plant pathogen. Sci Rep. 2017;7(1):45226.
Bekal S, Domier LL, Gonfa B, Lakhssassi N, Meksem K, Lambert KN. A SNARE-like protein and biotin are implicated in soybean cyst nematode virulence. PLoS ONE. 2015;10(12):e0145601.
Zhao J, Liu S. Beet cyst nematode HsSNARE1 interacts with both AtSNAP2 and AtPR1 and promotes disease in Arabidopsis. J Adv Res. 2023;47:27–40.
Guo W, Zhang F, Bao A, You Q, Li Z, Chen J, et al. The soybean Rhg1 amino acid transporter gene alters glutamate homeostasis and jasmonic acid-induced resistance to soybean cyst nematode. Mol Plant Pathol. 2019;20(2):270–86.
Han S, Smith JM, Du Y, Bent AF. Soybean transporter AAT Rhg1 abundance increases along the nematode migration path and impacts vesiculation and ROS. Plant Physiol. 2023;192(1):133–53.
Dong J, Hudson ME. WI12Rhg1 interacts with DELLAs and mediates soybean cyst nematode resistance through hormone pathways. Plant Biotechnol J. 2022;20(2):283–96.
Matson AL, Williams LF. Evidence of a fourt gene for resistance to the soybean cyst nematode. Crop Sci. 1965;5(5):477.
Webb DM, Baltazar BM, Rao-Arelli AP, Schupp J, Clayton K, Keim P, et al. Genetic mapping of soybean cyst nematode race-3 resistance loci in the soybean PI 437654. Theor Appl Genet. 1995;91(4):574–81.
Yu N, Lee TG, Rosa DP, Hudson M, Diers BW. Impact of Rhg1 copy number, type, and interaction with Rhg4 on resistance to Heterodera glycines in soybean. Theor Appl Genet. 2016;129(12):2403–12.
Kandoth PK, Liu S, Prenger E, Ludwig A, Lakhssassi N, Heinz R, et al. Systematic mutagenesis of serine hydroxymethyltransferase reveals an essential role in nematode resistance. Plant Physiol. 2017;175(3):1370–80.
Zhi L, Jie Z, Jian J, Huan P, Huang W, Kong L, et al. A fragment of a 70-kDa Heterodera glycines heat shock protein (HgHSP70) interacts with soybean cyst nematode-resistant GmSHMT08. J Integr Agr. 2022;21(10):2973–83.
Patil GB, Lakhssassi N, Wan J, Song L, Zhou Z, Klepadlo M, et al. Whole-genome resequencing reveals the impact of the interaction of copy number variants of the rhg1 and Rhg4 genes on broad-based resistance to soybean cyst nematode. Plant Biotechnol J. 2019;17(8):1595–611.
Lakhssassi N, Piya S, Knizia D, El Baze A, Cullen MA, Meksem J, et al. Mutations at the serine hydroxymethyltransferase impact its interaction with a soluble NSF attachment protein and a pathogenesis-related protein in soybean. Vaccines (Basel). 2020;8(3):349.
Lakhssassi N, Piya S, Bekal S, Liu S, Zhou Z, Bergounioux C, et al. A pathogenesis-related protein GmPR08-Bet VI promotes a molecular interaction between the GmSHMT08 and GmSNAP18 in resistance to Heterodera glycines. Plant Biotechnol J. 2020;18(8):1810–29.
Zhao J, Duan Y, Kong L, Huang W, Peng D, Liu S. Opposite beet cyst nematode infection phenotypes of transgenic Arabidopsis between overexpressing GmSNAP18 and AtSNAP2 and between overexpressing GmSHMT08 and AtSHMT4. Phytopathol. 2022;112(11):2383–90.
Meksem K, Pantazopoulos P, Njiti VN, Hyten LD, Arelli PR, Lightfoot DA. ’Forrest’ resistance to the soybean cyst nematode is bigenic: saturation mapping of the Rhg1 and Rhg4 loci. Theor Appl Genet. 2001;103(5):710–7.
Hawes CR, Brandizzi F, Andreeva AV. Endomembranes and vesicle trafficking. Curr Opin Plant Biol. 1999;2(6):454–61.
Gu Y, Zavaliev R, Dong X. Membrane trafficking in plant immunity. Mol Plant. 2017;10(8):1026–34.
McNeece BT, Sharma K, Lawrence GW, Lawrence KS, Klink VP. The mitogen activated protein kinase (MAPK) gene family functions as a cohort during the Glycine max defense response to Heterodera glycines. Plant Physiol Biochem. 2019;137:25–41.
Klink VP, Sharma K, Pant SR, McNeece B, Niraula P, Lawrence GW. Components of the SNARE-containing regulon are coregulated in root cells undergoing defense. Plant Signal Behav. 2017;12(2):e1274481.
Sharma K, Niraula PM, Troell HA, Adhikari M, Alshehri HA, Alkharouf NW, et al. Exocyst components promote an incompatible interaction between Glycine max (soybean) and Heterodera glycines (the soybean cyst nematode). Sci Rep. 2020;10(1):15003.
Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–43.
Pant SR, Matsye PD, McNeece BT, Sharma K, Krishnavajhala A, Lawrence GW, et al. Syntaxin 31 functions in Glycine max resistance to the plant parasitic nematode Heterodera glycines. Plant Mol Biol. 2014;85(1–2):107–21.
Sharma K, Pant SR, McNeece BT, Lawrence GW, Klink VP. Coregulation of the glycine max soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE)-containing regulon occurs during defense to a root pathogen. J Plant Interact. 2016;11(1):74–93.
Dong J, Zielinski RE, Hudson ME. t-SNAREs bind the Rhg1 alpha-SNAP and mediate soybean cyst nematode resistance. Plant J. 2020;104(2):318–31.
Lawaju BR, Niraula P, Lawrence GW, Lawrence KS, Klink VP. The Glycine max conserved oligomeric golgi (COG) complex functions during a defense response to Heterodera glycines. Front Plant Sci. 2020;11:564495.
Yang M, Ismayil A, Liu Y. Autophagy in Plant-Virus Interactions. Annu Rev Virol. 2020;7(1):403–19.
Chen J, Chen S, Xu C, Yang H, Achom M, Wang X. A key virulence effector from cyst nematodes targets host autophagy to promote nematode parasitism. New Phytol. 2023;237(4):1374–90.
Zou J, Chen X, Liu C, Guo M, Kanwar MK, Qi Z, et al. Autophagy promotes jasmonate-mediated defense against nematodes. Nat Commun. 2023;14(1):4769.
Hu Y, You J, Li C, Williamson VM, Wang C. Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci Rep. 2017;7(1):41282.
Tucker ML, Xue P, Yang R. 1-Aminocyclopropane-1-carboxylic acid (ACC) concentration and ACC synthase expression in soybean roots, root tips, and soybean cyst nematode (Heterodera glycines)-infected roots. J Exp Bot. 2010;61(2):463–72.
Mazarei M, Puthoff DP, Hart JK, Rodermel SR, Baum TJ. Identification and characterization of a soybean ethylene-responsive element-binding protein-encoding gene whose mRNA expression changes during soybean cyst nematode infection. Mol Plant Microbe Interact. 2002;15(6):577–86.
Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ. GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis. Mol Plant Microbe Interact. 2007;20(2):107–19.
Hermsmeier D, Mazarei M, Baum TJ. Differential display analysis of the early compatible interaction between soybean and the soybean cyst nematode. Mol Plant Microbe Interact. 1998;11(12):1258–63.
Lee C, Chronis D, Kenning C, Peret B, Hewezi T, Davis EL, et al. The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol. 2011;155(2):866–80.
Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J. Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant Microbe Interact. 2000;13(10):1121–9.
Grunewald W, Cannoot B, Friml J, Gheysen G. Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog. 2009;5(1):e1000266.
Oosterbeek M, Lozano-Torres JL, Bakker J, Goverse A. Sedentary plant-parasitic nematodes alter auxin homeostasis via multiple strategies. Front Plant Sci. 2021;12:668548.
Lin J, Mazarei M, Zhao N, Zhu JJ, Zhuang X, Liu W, et al. Overexpression of a soybean salicylic acid methyltransferase gene confers resistance to soybean cyst nematode. Plant Biotechnol J. 2013;11(9):1135–45.
Guo X, Chronis D, De La Torre CM, Smeda J, Wang X, et al. Enhanced resistance to soybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol J. 2015;13(6):801–10.
Wang J, Dhroso A, Liu X, Baum TJ, Hussey RS, Davis EL, et al. Phytonematode peptide effectors exploit a host posttranslational trafficking mechanism to the ER using a novel translocation signal. New Phytol. 2021;229(1):563–74.
Lee MW, Huffaker A, Crippen D, Robbins RT, Goggin FL. Plant elicitor peptides promote plant defences against nematodes in soybean. Mol Plant Pathol. 2018;19(4):858–69.
Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, et al. ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol. 2016;171(3):1606–15.
Chen X, Li S, Zhao X, Zhu X, Wang Y, Xuan Y, et al. Modulation of (homo)glutathione metabolism and H2O2 accumulation during soybean cyst nematode infections in susceptible and resistant soybean cultivars. Int J Mol Sci. 2020;21(2):388.
Feng Y, Qi N, Lei P, Wang Y, Xuan Y, Liu X, et al. Gma-miR408 enhances soybean cyst nematode susceptibility by suppressing reactive oxygen species accumulation. Int J Mol Sci. 2022;23(22):688.
Sheridan JP, Miller AJ, Perry RN. Early responses of resistant and susceptible potato roots during invasion by the potato cyst nematode Globodera rostochiensis. J Exp Bot. 2004;55(397):751–60.
Guo B, Lin B, Huang Q, Li Z, Zhuo K, Liao J. A nematode effector inhibits plant immunity by preventing cytosolic free Ca2+ rise. Plant, Cell & Environ. 2022;45(10):3070–85.
Niraula PM, Zhang X, Jeremic D, Lawrence KS, Klink VP. Xyloglucan endotransglycosylase/hydrolase increases tightly bound xyloglucan and chain number but decreases chain length contributing to the defense response that Glycine max has to Heterodera glycines. PLoS ONE. 2021;16(1):e0244305.
Ngaki MN, Sahoo DK, Wang B, Bhattacharyya MK. Overexpression of a plasma membrane protein generated broad-spectrum immunity in soybean. Plant Biotechnol J. 2021;19(3):502–16.
Jiang Y, Huang M, Li C, Hua C, Qin R, Chang D, et al. Responses of infective juveniles of the soybean cyst nematode (Heterodera glycines) and the root-knot nematodes (Meloidogyne hapla, M. incognita) to amino acids. Nematology. 2022;24(9):1049–62.
Siddique S, Radakovic ZS, Hiltl C, Pellegrin C, Baum TJ, Beasley H, et al. The genome and lifestage-specific transcriptomes of a plant-parasitic nematode and its host reveal susceptibility genes involved in trans-kingdom synthesis of vitamin B5. Nat Commun. 2022;13(1):6190.
Acknowledgements
The authors would like to acknowledge the valuable contributions of Yixiao Cai and Liang Wang from ZJU's Plant Nematology Laboratory in revising the manuscript. Our expertise and the length of this review paper impose limitations on the breadth of coverage, and it is important to acknowledge that not all advancements may be fully represented.
Funding
This work was supported by the National Natural Science Foundation of China (32272478, 32102146), Natural Science Foundation of Zhejiang Province (LTGN23C130003) (to S.H.), Postdoctoral Science Foundation of China (2022M710128) and Zhejiang Lab Open Research Project (K2022PE0AB03).
Author information
Authors and Affiliations
Contributions
L.H. and N.N.U.G. completed the manuscript. L.C. assisted in completing Fig. 1. Q.L. primarily revised the entire manuscript. S.H. reviewed and finalized the manuscript. All authors thoroughly read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
All the authors hereby declare that there are no conflicts of interest.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
SCN-related QTLs in soybean.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, L., Ghani, N.N.U., Chen, L. et al. Research progress on the functional study of host resistance-related genes against Heterodera glycines. Crop Health 1, 8 (2023). https://doi.org/10.1007/s44297-023-00008-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44297-023-00008-7