Abstract
Adventitious root (AR) formation is critical for cutting survival and nutrient absorption re-establishment. This complex genetic trait involves the interplay of nitrogen, endogenous hormones, and several key genes. In this study, we treated GL-3 apple (Malus domestica) in vitro shoots with nitrate and ammonium to determine their impact on AR formation, hormonal content, and gene expression patterns. Nitrate treatment significantly promotes adventitious rooting by increasing cell division, differentiation, and AR primordia formation compared to ammonium treatment. Elevated indole-3-acetic acid (IAA), reduced abscisic acid, and zeatin riboside concentrations were consistently observed with nitrate, likely crucial for promoting ARs over ammonium. Furthermore, Malus domestica auxin resistance1 (MdAUX1) expression was induced, increasing IAA levels. MdIAA23 was upregulated. Further results indicate that the higher expression levels of Malus domestica WUSCHEL-related Homeobox gene 11 (MdWOX11), Malus domestica lateral organ boundaries domain gene 16 (MdLBD16), and MdLBD29, and increased cell cycle-related gene expressions, contribute to auxin-stimulated adventitious rooting under nitrate conditions. In conclusion, this study establishes that auxin content and associated genes related to root development and cell cycle contribute to superior ARs in response to nitrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asexual propagation through stem cuttings is a common method for the vegetative multiplication of numerous crops due to its fidelity to the mother plant, short life span, and efficiency. However, the induction of adventitious roots (ARs) can impede the ability of detached stem cuttings to re-establish resource absorption (Druege et al. 2019). Root architecture is crucial for determining how plants acquire water and nutrients. ARs can develop from any non-root tissue. Anatomical studies on apple stem cuttings show that adventitious rooting begins with an induction phase at 3 d. At this stage, cambial cells undergo critical divisions, contributing to cellular reprogramming. After root founder cell establishment, the AR initiation phase (8 d) involves fundamental changes in cell architecture, followed by cell division and differentiation of emergent cell sets, resulting in the formation of a dome-shaped root primordium. The final phase, starting after 8 d, begins with primordium differentiation into a new root with distinct vascular bundles connected to the stem’s vascular cylinder, ultimately leading to AR occurrence (Bai et al. 2020; Patial et al. 2021; Tahir et al. 2022d). These observations suggest that adventitious rooting is a three-phase process: induction (0–3 d), initiation (3–8 d), and emergence (8–16 d). While the underlying mechanisms of adventitious rooting are documented in some model crops, further exploration is required specifically for apples.
Numerous external and endogenous factors influence adventitious rooting in stem cuttings. In particular, nitrogen regulates adventitious rooting in apples and other plant species. Inorganic nitrogen in higher plants is primarily sourced from ammonium and nitrate (Masclaux-Daubresse et al. 2010). Researchers have primarily focused on these nitrogen sources, demonstrating complex physiological and molecular responses in plants. Nitrate is an important nitrogen source that also acts as a signaling molecule, regulating blossom, lateral root (LR), and AR de velopment and stimulating the transcription of auxin signaling genes (Tahir et al. 2021c, 2021d, 2021b; O'Brien et al. 2016). Similarly, ammonium is essential for rooting in apple plants and other crops (Sriskandarajah et al. 1990; Bhat 1983; Hilo et al. 2017). Plant endogenous hormones, critical for regulating adventitious rooting in stem cuttings, are primary elements that control excision-induced adventitious rooting by establishing a signaling system to regulate cell fate and specialization (Lei et al. 2018; Tahir et al. 2022c, 2021a). Auxin, specifically indole-3-acetic acid (IAA), plays a crucial role in inducing adventitious rooting (da Costa et al. 2013; Bellini et al. 2014); however, cytokinin (CK) impedes this process (Mao et al. 2019). High endogenous IAA content stimulates AR primordium induction by inducing the differentiation and proliferation of phloem parenchyma cells adjacent to the stem’s vascular bundles (Lund et al. 1996; De Klerk et al. 1999). Auxin resistant/auxin (AUX/IAA) protein breakdown activates auxin response factors (ARFs), triggering the transcription of auxin-responsive genes in auxin signaling pathways (Gray et al. 2001; Orman-Ligeza et al. 2013). In apples, WUSCHEL-related Homeobox gene 11 (WOX11) interacts with lateral organ boundaries domain gene 29 (LBD29), promoting AR formation (Mao et al. 2023). Furthermore, abscisic acid (ABA) accumulation in apples inhibits adventitious rooting (Sriskandarajah 1984).
Apple cultivation is widespread in China; however, its production per hectare lags significantly behind developed countries. Unfortunately, most apple orchards in the region’s Loess Plateau suffer from poor-quality soil and low organic matter (below 1%), adversely affecting AR formation and fruit production. This restricts the overall growth of the apple industry. Therefore, scientific studies on AR formation in apples are essential for developmental biology. Despite comprehending the AR developmental mechanism and facilitating the asexual reproduction of excellent varieties, which greatly benefits the apple industry, poor soil quality remains challenging. This study aimed to determine the impact of nitrate and ammonium on adventitious rooting in GL-3 apple in vitro shoots. Specifically, we sought to understand whether nitrate or ammonium inhibits or promotes AR formation. To gain insights, we observed the AR phenotype and stem basal part anatomy, measured endogenous hormone concentrations, and examined the gene expression of key genes intricately involved in adventitious rooting.
Materials and methods
Plant material and treatments
GL-3 in vitro shoots, obtained from Northwest A&F University, People's Republic of China, were used as test material. In the preliminary experiment, 162 four-week-old morphologically homogeneous in vitro shoots were cultivated on 1/2 MS medium, 30 g L−1 sugar, 1.2 mg L−1 indole-3-butyric acid (IBA), and 7.5 g L−1 agar, and pH was adjusted to 5.8 using two nitrogen sources: potassium nitrate (KNO3) and ammonium chloride (NH4Cl). Three concentrations (T1, 9.4 mM; T2, 18.8 mM; T3, and 37.6 mM) of both were assessed to determine the optimal nitrogen concentration for AR formation and development. The shoots were kept under a 24 h cycle: 16 h of light at 25 ± 1°C, followed by 8 h of darkness at 15 ± 1°C. Furthermore, relative humidity was retained at 70–80%. After 30 d of treatments, root numbers were counted manually, and root lengths were measured using an Epson Expression 10000XL scanner. The images were analyzed using WinRHIZO software. Based on phenotypic and measured parameters, we selected T1 (9.4 mM) from both sources for further experiments.
Subsequently, 630 in vitro shoots were treated with potassium nitrate (T1, 9.4 mM) and ammonium chloride (T1, 9.4 mM) to determine their role at different AR formation stages (0, 3, 8, and 16 d). The rooting zone is the basal section of the stem cutting (0.5 cm), which was harvested at 0, 3, 8, and 16 d from both sources (Li et al. 2022). Ninety GL-3 apple in vitro shoots were harvested from each source at each time point. Three biological replications were conducted, with harvesting at 0, 3, 8, and 16 d. The harvested stem basal parts were immediately dipped in liquid nitrogen and stored at –80°C for subsequent experiments.
Anatomical analysis
Stem basal parts of approximately 0.5 cm were collected at various periods (0, 3, and 8 d) from both sources (T1) for anatomical observations. Samples were fixed, paraffin-embedded, and sectioned as described previously (Naija et al. 2008).
Determination of endogenous hormone levels
Stem basal sections from both sources at 0, 3, 8, and 16 d were harvested and used for determining IAA, ABA, jasmonic acid (JA), gibberellic acid 3 (GA3), zeatin riboside (ZR), and brassinosteroids (BRs) using the enzyme‐linked immunosorbent assay (ELISA). Three independent replications were used at each time point. An in-depth explanation is available in a previous article (Wang et al. 2020).
RNA extraction and cDNA synthesis
Total RNA was extracted from both sources at 0, 3, 8, and 16 d using Plant RNA Purification Reagent (Invitrogen, Waltham, MA, USA). Genomic DNA was eliminated using rDNase I, RNase free (Takara Bio, San Jose, CA, USA), and RNA integrity was validated by running samples on an agarose gel. The cDNA was prepared using the PrimeScript RT Reagent Kit (Takara Bio).
Gene expression and promoter analysis
RT-qPCR was used to assess the transcript levels of genes related to nitrate, ammonium, auxin, root development, and cell cycle. The primers were created using Primer 6.0 (Table S1). The RT-qPCR expression analysis was conducted as previously described (Fan et al. 2017). Actin was used as a reference gene (Tahir et al. 2021c, 2022b, 2023). Three biological and three technical repetitions were prepared and calculated using a previously described procedure (Livak and Schmittgen 2001). The 2000-bp sequences upstream of several key gene coding sequences (CDSs) were extracted as promoter sequences. The cis-acting elements were identified using Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html), and the results were visualized using TBtools (Chen et al. 2023).
Molecular docking simulation of nitrate and IAA
The three-dimensional (3D) crystal structure of the proteins of interest, Pdb I'd:8GVH (KNO3), was obtained from the protein data bank, and compound CID:802 (IAA) was downloaded from the PubChem database and prepared by removing water molecules, adding hydrogen atoms, and assigning appropriate charges and force field parameters. The ligand structure was prepared using cheminformatics tools to ensure proper protonation and geometry optimization. Subsequently, we utilized PyRx to set up the molecular docking simulations.
Statistical analysis
GraphPad Prism 7 was used to prepare figures and analyze data. For Fig. 1, the analysis of variance (ANOVA) factorial design was followed by a multiplicative Sidak test at the 0.05% level using Statistics 8.1 software. Lowercase letters indicate statistical differences between in vitro shoots within each nitrate and ammonium treatment, whereas uppercase letters represent statistical differences between nitrate and ammonium-treated in vitro shoots at each treatment (P≤0.05). For other figures, ANOVA was followed by Sidak’s multiple comparison tests in GraphPad. P<0.05 was set as statistically significant. The results are shown as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
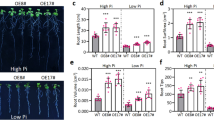
Effect of nitrate and ammonium treatments (T1, 9.4 mM; T2, 18.8 mM; and T3, 37.6 mM) on adventitious root (AR) formation in GL-3 apple in vitro shoots. (a) Phenotype of ARs after nitrate and ammonium treatments; the scale bar is 1 cm. (b) Root number and (c) root length (cm) of 27 in vitro shoots in each treatment were measured at 30 d. Error bars refer to the average value ± SD from three biological replicates. Lowercase letters indicate statistical differences between in vitro shoots within each nitrate and ammonium treatment, whereas uppercase letters show statistical differences between nitrate- and ammonium-treated in vitro shoots at each treatment (P≤0.05)
Results
Morphological and anatomical observations of AR formation
We aimed to determine the optimal nitrate and ammonium concentrations (T1, 9.4 mM; T2, 18.8 mM; T3, and 37.6 mM) for AR formation and development (Fig. 1a). In all treatments, root number and length were elevated in response to nitrate as compared to ammonium (Fig. 1b, c). Interestingly, the higher doses of both sources inhibited AR formation and development (Fig. 1). Therefore, we selected the optimum concentration of 9.4 mM to compare the effects of different nitrogen sources on different stages of adventitious rooting in GL-3 apple in vitro shoots. No morphological variations were observed between 0 and 8 d (Fig. 2a); however, at day 16, both in vitro shoots began AR formation, but nitrate-treated shoots developed more ARs than ammonium-treated shoots, suggesting that nitrate was more important for AR formation (Fig. 2b, c).
Effect of nitrate and ammonium (T1, 9.4 mM) treatments on the different stages (0, 3, 8, and 16 d) of adventitious root (AR) formation in GL-3 apple in vitro shoots. (a) Phenotype of ARs at different stages of AR formation; The scale bar is 1 cm. (b) Root number per in vitro shoot and c root length per in vitro shoot (cm) were measured at 16 d. Error bars refer to the average value ± SD from three biological replicates. Asterisks indicate significant differences at **P<0.01 and ***P<0.001
Anatomical analysis of the stem basal parts revealed component cells at 0 d. At 3 d, mitosis was observed in cambial cells, and cells differentiated in agminated, differentiated cells. Furthermore, at 8 d, AR primordia were formed in both materials; however, the number of primordia was higher in nitrate-treated shoots (Fig. 3) than in ammonium-treated shoots. These results show that nitrate elevates the number and formation of early primordia, resulting in increased AR number and length.
Measurement of endogenous hormonal content
Hormonal levels are directly related to rooting. To understand how different endogenous hormones adjust their levels in response to nitrate and ammonium treatments, regulating AR formation, we quantified the endogenous content of IAA, ZR, ABA, JA, GA3, and BR at different time points during AR formation (Fig. 4). At 3 and 8 d, the IAA content was higher in response to nitrate treatment than to ammonium treatment; however, at 16 d, the IAA content was significantly improved in ammonium-treated shoots (Fig. 4a). Endogenous ZR content was elevated at all time points in response to ammonium. Similarly, ABA exhibited a trend similar to ZR at 3 and 8 d but differed at 16 d (Fig. 4b, c). Furthermore, the JA and GA3 levels were increased in ammonium-treated shoots only at 8 d; other time points showed no significant difference (Fig. 4d, e). The BR content was higher in ammonium-treated shoots than in nitrate-treated in vitro shoots at 8 and 16 d (Fig. 4f).
Endogenous hormone contents of in vitro shoots cultured on medium containing nitrate and ammonium during adventitious root (AR) formation (9.4 mM), (a)–(f). (a) Indole‐3‐acetic acid (IAA), (b) zeatin riboside (ZR), (c) abscisic acid (ABA), (d) jasmonic acid (JA), (e) gibberellic acid 3 (GA3), and (f) brassinosteroids (BRs). The x-axis indicates the different time points of AR formation (0, 3, 8, and 16 d), and the y-axis shows the endogenous hormone concentration. Error bars refer to the average value ± SD from three biological replicates. Different asterisks indicate significant differences at *P<0.05, **P<0.01, and ****P<0.0001
Expression analysis of ammonium and nitrate transporter genes
The expression level of Malus domestica ammonium transporter (MdAMT1.2) was elevated in ammonium-treated shoots only at 3 d, and no difference was observed at other time points. At 3 and 8 d, MdAMT2.1 expression was elevated in nitrate-treated shoots; however, there was no significant difference at 3 d in either in vitro shoot (Fig. 5a, b). The Malus domestica nitrate transporter (MdNRT1.1) was highly expressed at 3 and 8 d in ammonium-treated shoots, but the expression was sharply induced in nitrate-treated shoots at 16 d. Compared to nitrate-treated shoots, MdNRT2.1 was expressed at all time points except at 16 d in ammonium-treated shoots (Fig. 5c, d).
Effect of nitrate and ammonium (9.4 mM) treatments on the relative expressions of ammonium transporter genes: (a) Malus domestica ammonium transporter (MdAMT1.2) and (b) MdAMT2.1 and nitrate transporter genes: (c) Malus domestica nitrate transporter (MdNRT1.1) and (d) MdNRT2.1 during adventitious root (AR) formation in GL-3 apple in vitro shoots. Error bars refer to the average value ± SD from three biological replicates. Different asterisks indicate significant differences at *P< 0.05, **P< 0.01, ***P<0.001, and ****P<0.0001
Expression analysis of auxin signal transduction-related genes
MdPIN1 expression peaked at 3 d in ammonium-treated shoots and was induced at day 16 in nitrate-treated shoots (Fig. 6a). MdAUX1 and MdIAA14 showed consistently higher expression in nitrate-treated shoots, except at 16 d (Fig. 6b, c). Interestingly, MdIAA23 exhibited elevated expression at all time points in nitrate-treated shoots compared to ammonium-treated shoots (Fig. 6d). Furthermore, MdARF7 and MdARF19 were more expressed in nitrate-treated shoots at all time points, except for MdARF7 at 16 d (Fig. 6e, f).
Effect of nitrate and ammonium (9.4 mM) treatments on the relative expressions of auxin signal transduction-related genes: (a) Malus domestica pin-formed1 (MdPIN1) (b) Malus domestica auxin resistance1 (MdAUX1), (c) Malus domestica indole-3-acetic acid 14 (MdIAA14), (d) MdIAA23, (e) Malus domestica auxin response factor 7 (MdARF7), and (f) MdARF19 at different time points during adventitious root (AR) formation in GL-3 apple in vitro shoots. Error bars refer to the average value ± SD from three biological replicates. Different asterisks indicate significant differences at *P<0.05, **P<0.01, ***P<0.001, and ****P< 0.0001
Expression analysis of AR development- and cell cycle-related genes
MdWOX5 and Malus domestica short root (MdSHR) expression levels were elevated at all time points in response to ammonium treatment, with no significant difference at 16 d (Fig. 7a, e). MdWOX11 expression was significantly higher at all time points in response to nitrate treatment (Fig. 7b). Furthermore, MdLBD16 expression was induced in nitrate-treated shoots at 3 and 8 d but was significantly reduced at 16 d compared to ammonium-treated shoots (Fig. 7c). MdLBD29 levels were higher at 3 and 16 d (Fig. 7d), whereas Malus domestica adventitious rooting related oxygenase (MdARRO1) expression was only higher at 16 d in nitrate-treated shoots (Fig. 7f). The expression of Malus domestica cell cycle-related gene (MdCYCD1;1) was elevated at 8 d but increased sharply at 16 d in nitrate-treated shoots (Fig. 7g). Both MdCYCD3;1 and MdCYCD4;1 showed similar expression patterns and were elevated at 3 and 8 d in response to nitrate treatment (Fig. 7h, i).
Effect of nitrate and ammonium (9.4 mM) treatments on the relative expressions of root development-related genes: (a) Malus domestica WUSCHEL-related Homeobox gene 5 (MdWOX5), (b) MdWOX11, (c) Malus domestica lateral organ boundaries domain gene 16 (MdLBD16), (d) MdLBD29, (e) Malus domestica short root (MdSHR), and (f) Malus domestica adventitious rooting related oxygenase (MdARRO1) and cell cycle-related genes: (g) MdCYCD1;1, (h) MdCYCD3;1, and (i) MdCYCD4;1 at different time points during adventitious root (AR) formation in GL-3 apple in vitro shoots. Error bars refer to the average value ± SD from three biological replicates. Different asterisks indicate significant differences at *P< 0.05, **P<0.01, ***P<0.001, and ****P<0.0001
Analysis of cis-acting elements of key gene promoters
To investigate the cis-acting elements of several important genes' promoter and their potential functions, we analyzed the 2000 bp upstream sequence of the start codon of the genes (Fig. 8). The type, number, and distribution of cis-acting elements in these promoters were diverse, indicating their different potential functions. We selected some elements associated with stress and plant growth and development for further analysis (Fig. 8a, b). Almost all the genes contained many TATA-boxes and CAAT-boxes, essential for transcription initiation. The other common elements were the ABRE elements (involved in abscisic acid responses) and the G-box elements (involved in light responsiveness). These results suggest that these genes are involved in regulating various signaling pathways.
Molecular docking simulations
We utilized PyRx to set up the molecular docking simulations. PyRx provides an intuitive graphical user interface that simplifies the process of defining the search space within the protein binding site and selecting docking algorithms and scoring functions. We carefully defined the binding site based on known binding interactions and structural information. In a molecular docking simulation using PyRx, the ligand IAA displayed a substantial binding affinity to the protein KNO3 with a binding energy of –7.4 kcal/mol, indicating a strong interaction. The docking conformation had a root mean square deviation (RMSD) of 0.97, suggesting a relatively good fit with the native structure, although lower RMSD values are preferable for greater accuracy. Interactions were observed with specific residues, including K424, V420, H159, L160, Q161, and L423, indicating potential binding sites within the protein (Fig. 9).
Molecular docking simulation analysis. The outcomes of molecular docking simulations conducted using PyRx showcase the predicted binding poses and interactions between a small-molecule ligand and a target protein. The color-coded representations highlight key binding interactions and provide insights into the binding affinity and orientation of the ligand within the protein’s active site
Discussion
Nitrate promotes better AR formation relative to ammonium
Adventitious rooting is extremely important in the vegetative propagation of herbaceous and woody horticultural plants. The AR formation process is a complicated biological pathway encompassing several stages: induction (0–3 d), initiation (3–8 d), and emergence (8–16 d) (Tahir et al. 2022c). Nitrate and ammonium are two inorganic sources that significantly influence these stages by regulating the formation and expansion of new AR founder cells. Cell formation aids rooting by providing cells as a raw material resource for growth. In this study, nitrate treatment increased cell division and differentiation at the initiation stage, promoting AR formation, and high rates of AR primordia formation were also observed. While both groups developed AR at day 16, the number and length of AR in nitrate-treated shoots were higher than those in ammonium-treated shoots. The effect of ammonium on cell formation is primarily evident in shorter root meristem lengths and reduced number of dividing cells. Kinematic analysis reveals that ammonium limits the elongation zone and the maximum rate of elemental expansion (Liu et al. 2013). Ammonium progressively reduced the number of dividing cells, and this response became more intense over time. After 5 d of ammonium treatment, the rate of cell formation was dramatically reduced, thereby restricting the number of cells available in the elongation zone. Interpreting the restriction of cells entering the elongation zone as solely restricting the number of elongating cells oversimplifies the impact on the length of the elongation zone and root rate growth intensity (Beemster and Baskin 1998). Although the length of the elongation zone can be regulated by variables other than the rate of cell formation, the flux of cells into the elongation zone plays an important part in controlling root growth (Baskin 2013). These results suggest that AR formation arrest in response to ammonium treatment is a primary factor contributing to the decline in cell formation.
Endogenous hormones contribute to AR formation
Phytohormones are important endogenous activators of AR formation. Several hormones control the complex adventitious rooting process, with auxin being the key controller and bridge in the hormonal crosstalk. The auxin signaling pathway, encompassing polar auxin transport and auxin signal transduction systems, participates in all phases of plant embryonic and postembryonic root development, from hypophysis to meristem initiation, emergence, and elongation (Lavenus et al. 2013; Bellini et al. 2014). Auxin is critical for adventitious rooting; hence, a high level correlates to a higher rooting rate, and CK appears to play a beneficial role in cell division, a negative role in root founder cell differentiation, and a supportive role in AR differentiation with auxin (Bellini et al. 2014; Druege et al. 2019). Thus, higher IAA levels at 3 and 8 d (induction and initiation stages) and lower ZR levels at all time points contributed to the greater rooting in nitrate-treated shoots than in ammonium-treated shoots. Understanding such interactions can highlight the potential physiological effects or agricultural implications of this binding, which may have practical applications in crop growth regulation or other areas. ABA helps plants adapt to various stresses; under drought stress, it accumulates and limits cell cycle progression. Furthermore, ABA inhibits AR formation (Tahir et al. 2022a; Zhang et al. 2021). At the induction and initiation stages, ammonium-treated shoots had higher levels of ABA than in nitrate-treated shoots, which could explain the poor rooting. Because of its higher accumulation at the stem base, JA restricts adventitious rooting in apple stem cuttings (Tahir et al. 2021b, 2022a). Furthermore, it affects AR, which functions downstream of the auxin pathway in Arabidopsis via the GH3 proteins (Gutierrez et al. 2012; Staswick 2009). GH3s catalyze JA conjugation with amino acids, decreasing free IAA content and supporting JA homeostasis (Staswick 2009; Staswick et al. 2005). Furthermore, GH3.11, also known as jasmonate acid resistant 1 (JAR1), suppresses adventitious rooting in Arabidopsis hypocotyls. Consistent with previous findings, higher IAA and lower JA contents may be responsible for increased rooting in response to nitrate.
Nitrate and ammonium transporters contribute to AR formation
Nitrate and ammonium have identical diffusion coefficients in water. However, because of the soil’s complex properties, such as negative ion charge and viscosity, nitrate and ammonium ions behave differently in soil water (Miller and Cramer 2005). The diffusion coefficient of nitrate in certain soil waters is predicted to be 10–100-fold greater than that of ammonium. Rapid mass flow allows nitrate to penetrate the root surface; however, cationic ammonium is quickly absorbed by soil particles (Giehl and von Wirén 2014). Plants alter their root morphology when nitrate or ammonium is added to the soil to maximize nitrogen absorption. Several NRTs and AMTs are expressed in root organs, permitting efficient nitrogen uptake (Tahir et al. 2021c; Huang et al. 2022; Nacry et al. 2013; Kiba and Krapp 2016). Nitrate application stimulates ammonium-related gene expression in Arabidopsis; similarly, MdAMT2.1 expression was induced in this study. At most time points, ammonium application induced MdNRT1.1 and MdNRT2.1 expression. Similarly, in Chinese cabbage, MdNRT1.1 and MdNRT2.1 expression was positively correlated with ammonium and negatively correlated with nitrate. More research is required to determine the roles of these genes in adventitious rooting.
Auxin signal transduction pathway genes are involved in AR formation
Several auxin biosynthesis and signaling pathway genes involving transcription factors regulate adventitious rooting in Arabidopsis and other species (Bellini et al. 2014; Lakehal and Bellini 2019). Polar auxin transport (PAT) via auxin influx and efflux carriers is an important step in the AR formation process. The AUX1 mutation reduces IAA deposition in young seedling roots; it acts as an auxin influx carrier and stimulates LR growth by distributing IAA between shoots and roots (Marchant et al. 2002). Endogenous IAA content was also notably elevated at 3 and 8 d (induction and initiation stages). However, these elevated IAA levels were related to increased MdAUX1 and MdIAA23 expression at 3 and 8 d. After AR primordia formation at 8 d (initiation), the establishment and maintenance of auxin peaks in the quiescent center (QC) are crucial for their unrestricted proliferation. High auxin levels promote early AR stages but not emergence (Meng et al. 2019). ARF7 and ARF19 positively regulate adventitious rooting in Arabidopsis hypocotyls by initiating the downstream transcription factors LBD16 and LBD29 (Lee et al. 2015). Moreover, in a previous study, arf7 and arf19 single mutants lowered the number of LRs and ARs, whereas arf7 and arf19 double mutants showed significantly fewer LRs and ARs (Okushima et al. 2007; Wilmoth et al. 2005). High rooting may be associated with nitrate-induced upregulation of these genes.
AR development- and cell cycle-related genes are associated with AR formation
According to a previous study, ARF7 and ARF19 directly activated the expression of LBD16 and LBD29 to induce LR formation (Okushima et al. 2007). Consequently, in this study, we speculate that MdARF7 and MdARF19 may modulate MdLBD16 and MdLBD29 expression, promoting adventitious rooting in nitrate-treated shoots. During AR formation in Arabidopsis, auxin regulates WOX11 at the initiation of cell fate transition, upregulating the transcripts of LBD16 and LBD29 (Liu et al. 2014). We recently discovered that MdWOX11 binds directly to the MdLBD29 promoter and positively regulates its expression in apples (Mao et al. 2023). The combined transcripts of these genes increased the expression of cell cycle-related genes, enhancing adventitious rooting (Tahir et al. 2022b). Therefore, we can speculate that increased IAA accumulation promoted MdWOX11 expression, triggering MdLBD16 and MdLBD29 expression. Furthermore, we hypothesize that the coordinated expression of the mentioned genes enhances AR formation in response to nitrate by upregulating MdCYCD1;1, MdCYCD3;1, and MdCYCD4;1 expression.
Conclusions
This study proposes that nitrate treatment enhances the formation of AR primordia and stimulates adventitious rooting by interacting with IAA and improving MdAUX1 and MdIAA23 expression. MdARF7 and MdARF19 are suggested to regulate MdLBD16 and MdLBD29 transcripts, potentially promoting adventitious rooting. We hypothesize that IAA may activate MdWOX11, influencing the expression of MdLBD16 and MdLBD29. Furthermore, the coordinated activities of these genes may promote adventitious rooting by upregulating the expression of cell cycle-related genes (Fig. 10). While this study highlights the involvement of specific genes and pathways in nitrate-mediated adventitious rooting, functional analyses are crucial to validate the proposed mechanisms.
Availability of data and materials
All data generated or analyzed during this study is included in this published article and its Supplementary information file.
References
Bai T, Dong Z, Zheng X, Song S, Jiao J, Wang M, et al. Auxin and its interaction with ethylene control adventitious root formation and development in apple rootstock. Front Plant Sci. 2020;11:574881. https://doi.org/10.3389/fpls.2020.574881.
Baskin TI. Patterns of root growth acclimation: constant processes, changing boundaries. Wires Dev Biol. 2013;2:65–73. https://doi.org/10.1002/wdev.94.
Beemster GT, Baskin TI. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 1998;116:1515–26. https://doi.org/10.1104/pp.116.4.1515.
Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014;65:639–66. https://doi.org/10.1146/annurev-arplant-050213-035645.
Bhat K. Nutrient inflows into apple roots. Plant Soil. 1983;71:371–80. https://doi.org/10.1007/BF02182678.
Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, et al. TBtools-II: A"One for All, All for One" bioinformatics platform for biological big-data mining. Mol Plant. 2023;16:1733–42. https://doi.org/10.1016/j.molp.2023.09.010.
Da Costa CT, De Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci. 2013;4:133. https://doi.org/10.3389/fpls.2013.00133.
De Klerk GJ, Van Der Krieken W, De Jong JC. Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev-Plant. 1999;35:189–99. https://doi.org/10.1007/s11627-999-0076-z.
Druege U, Hilo A, Pérez-Pérez JM, Klopotek Y, Acosta M, Shahinnia F, et al. Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Ann Bot. 2019;123:929–49. https://doi.org/10.1093/aob/mcy234.
Fan S, Zhang D, Zhang L, Gao C, Xin M, Tahir MM, et al. Comprehensive analysis of GASA family members in the Malus domestica genome: identification, characterization, and their expressions in response to apple flower induction. BMC Genomics. 2017;18:827. https://doi.org/10.1186/s12864-017-4213-5.
Giehl RF, von Wirén N. Root nutrient foraging. Plant Physiol. 2014;166:509–17. https://doi.org/10.1104/pp.114.245225.
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–6. https://doi.org/10.1038/35104500.
Gutierrez L, Mongelard G, Floková K, Păcurar DI, Novák O, Staswick P, et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell. 2012;24:2515–27. https://doi.org/10.1105/tpc.112.099119.
Hilo A, Shahinnia F, Druege U, Franken P, Melzer M, Rutten T, et al. A specific role of iron in promoting meristematic cell division during adventitious root formation. J Exp Bot. 2017;68:4233–47. https://doi.org/10.1093/jxb/erx248.
Huang L, Li J, Zhang B, Hao Y, Ma F, et al. Genome-wide identification and expression analysis of AMT gene family in apple (Malus domestica Borkh.). Horticulturae. 2022;8:457. https://doi.org/10.3390/horticulturae8050457.
Kiba T, Krapp A. Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol. 2016;57:707–14. https://doi.org/10.1093/pcp/pcw052.
Lakehal A, Bellini C. Control of adventitious root formation: insights into synergistic and antagonistic hormonal interactions. Physiol Plantarum. 2019;165:90–100. https://doi.org/10.1111/ppl.12823.
Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, et al. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450–8. https://doi.org/10.1016/j.tplants.2013.04.006.
Lee HW, Cho C, Kim J. Lateral organ boundaries domain16 and 18 act downstream of the AUXIN1 and LIKE-AUXIN3 auxin influx carriers to control lateral root development in Arabidopsis. Plant Physiol. 2015;168:1792–806. https://doi.org/10.1104/pp.15.00578.
Lei C, Fan S, Li K, Meng Y, Mao J, Han M, et al. iTRAQ-based proteomic analysis reveals potential regulation networks of IBA-induced adventitious root formation in apple. Int J Mol Sci. 2018;19:667. https://doi.org/10.3390/ijms19030667.
Li S, Tahir MM, Wu T, Xie L, Zhang X, Mao J, et al. Transcriptome analysis reveals multiple genes and complex hormonal-mediated interactions with PEG during adventitious root formation in apple. Int J Mol Sci. 2022;23:976. https://doi.org/10.3390/ijms23020976.
Liu Y, Lai N, Gao K, Chen F, Yuan L, Mi G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE. 2013;8:e61031. https://doi.org/10.1371/journal.pone.0061031.
Liu J, Sheng L, Xu Y, Li J, Yang Z, Huang H, et al. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014;26:1081–93. https://doi.org/10.1105/tpc.114.122887.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.
Lund ST, Smith AG, Hackett WP. Cuttings of a tobacco mutant, rae, undergo cell divisions but do not initiate adventitious roots in response to exogenous auxin. Physiol Plantarum. 1996;97:372–80. https://doi.org/10.1034/j.1399-3054.1996.970223.x.
Mao J, Zhang D, Meng Y, Li K, Wang H, Han M. Inhibition of adventitious root development in apple rootstocks by cytokinin is based on its suppression of adventitious root primordia formation. Physiol Plantarum. 2019;166:663–76. https://doi.org/10.1111/ppl.12817.
Mao J, Niu C, Li K, Fan L, Liu Z, Li S, et al. Cytokinin-responsive MdTCP17 interacts with MdWOX11 to repress adventitious root primordium formation in apple rootstocks. Plant Cell. 2023;35:1202–21. https://doi.org/10.1093/plcell/koac369.
Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–97. https://doi.org/10.1105/tpc.010354.
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot-London. 2010;105:1141–57. https://doi.org/10.1093/aob/mcq028.
Meng Y, Xing L, Li K, Wei Y, Wang H, Mao J, et al. Genome-wide identification, characterization and expression analysis of novel long non-coding RNAs that mediate IBA-induced adventitious root formation in apple rootstocks. Plant Growth Regul. 2019;87:287–302. https://doi.org/10.1007/s10725-018-0470-9.
Miller AJ, Cramer MD. Root nitrogen acquisition and assimilation. Plant Soil. 2005;274:1–36. https://doi.org/10.1007/s11104-004-0965-1.
Nacry P, Bouguyon E, Gojon A. Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil. 2013;370:1–29. https://doi.org/10.1007/s11104-013-1645-9.
Naija S, Elloumi N, Jbir N, Ammar S, Kevers C. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM 106 cultured in vitro. CR Biol. 2008;331:518–25. https://doi.org/10.1016/j.crvi.2008.04.002.
O’Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, et al. Nitrate transport, sensing, and responses in plants. Mol Plant. 2016;9:837–56. https://doi.org/10.1016/j.molp.2016.05.004.
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–30. https://doi.org/10.1105/tpc.106.047761.
Orman-Ligeza B, Parizot B, Gantet PP, Beeckman T, Bennett MJ, Draye X. Post-embryonic root organogenesis in cereals: branching out from model plants. Trends Plant Sci. 2013;18:459–67. https://doi.org/10.1016/j.tplants.2013.04.010.
Patial S, Chandel J, Sharma N, Verma P. Influence of auxin on rooting in hardwood cuttings of apple (Malus* Domestica borkh.) clonal rootstock’M 116’under mist chamber conditions. Indian J Ecol. 2021;48:429–33.
Sriskandarajah S, Skirvin R, Abu-Qaoud H. The effect of some macronutrients on adventitious root development on scion apple cultivars in vitro. Plant Cell Tiss Orga. 1990;21:185–9. https://doi.org/10.1007/BF00033440.
Sriskandarajah S. Induction of adventitious roots in some scion cultivars of apple (Malus pumila Mill). Sydney, Australia: University of Sydney; 1984.
Staswick P. The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol. 2009;150:1310–21. https://doi.org/10.1104/pp.109.138529.
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, et al. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–27. https://doi.org/10.1105/tpc.104.026690.
Tahir MM, Zhang X, Shah K, Hayat F, Li S, Mao J, et al. Nitrate application affects root morphology by altering hormonal status and gene expression patterns in B9 apple rootstock nursery plants. Fruit Res. 2021;1:1–14. https://doi.org/10.48130/FruRes-2021-0014.
Tahir MM, Chen S, Ma X, Li S, Zhang X, Shao Y, et al. Transcriptome analysis reveals the promotive effect of potassium by hormones and sugar signaling pathways during adventitious roots formation in the apple rootstock. Plant Physiol Bioch. 2021a;165:123–36. https://doi.org/10.1016/j.plaphy.2021.05.015.
Tahir MM, Li S, Mao J, Liu Y, Li K, Zhang X, et al. High nitrate inhibited adventitious roots formation in apple rootstock by altering hormonal contents and miRNAs expression profiles. Scientia Hortic. 2021b;286:110230. https://doi.org/10.1016/j.scienta.2021.110230.
Tahir MM, Wang H, Ahmad B, Liu Y, Fan S, Li K, et al. Identification and characterization of NRT gene family reveals their critical response to nitrate regulation during adventitious root formation and development in apple rootstock. Sci Hortic. 2021c;275:109642. https://doi.org/10.1016/j.scienta.2020.109642.
Tahir MM, Li S, Liu Z, Fan L, Tang T, Zhang X, et al. Different miRNAs and hormones are involved in PEG-induced inhibition of adventitious root formation in apple. Scientia Hortic. 2022a;303:111206. https://doi.org/10.1016/j.scienta.2022.111206.
Tahir MM, Mao J, Li S, Li K, Liu Y, Shao Y, et al. Insights into factors controlling adventitious root formation in apples. Horticulturae. 2022b;8:276. https://doi.org/10.3390/horticulturae8040276.
Tahir MM, Tong L, Fan L, Liu Z, Li S, Zhang X, et al. Insights into the complicated networks contribute to adventitious rooting in transgenic MdWOX11 apple microshoots under nitrate treatments. Plant Cell Environ. 2022c;45:3134–56. https://doi.org/10.1111/pce.14409.
Tahir MM, Tong L, Xie L, Wu T, Ghani MI, Zhang X, et al. Identification of the HAK gene family reveals their critical response to potassium regulation during adventitious root formation in apple rootstock. Hortic Plant J. 2023;9:45–59. https://doi.org/10.1016/j.hpj.2022.11.001.
Tahir MM, Lu Z, Wang C, Shah K, Li S, Zhang X, et al. Nitrate application induces adventitious root growth by regulating gene expression patterns in apple rootstocks. J Plant Growth Regul. 2021:1–12. https://doi.org/10.1007/s00344-021-10527-8 .
Wang H, Tahir MM, Nawaz MA, Mao J, Li K, Wei Y, et al. Spermidine application affects the adventitious root formation and root morphology of apple rootstock by altering the hormonal profile and regulating the gene expression pattern. Sci Hortic. 2020;266:109310. https://doi.org/10.1016/j.scienta.2020.109310.
Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, et al. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005;43:118–30. https://doi.org/10.1111/j.1365-313X.2005.02432.x.
Zhang X, Tahir MM, Li S, Mao J, Nawaz MA, Liu Y, et al. Transcriptome analysis reveals the inhibitory nature of high nitrate during adventitious roots formation in the apple rootstock. Physiol Plantarum. 2021;173:867–82. https://doi.org/10.1111/ppl.13480.
Acknowledgements
We would like to thank Prof. Zhihong Zhang (Shenyang Agricultural University, Shenyang, Liaoning) for providing tissue-cultured GL‐3 plants. We are also extremely grateful to our teacher, senior experimentalist Liu Hangkong from the National Horticultural Experimental Teaching Demonstration Center, who has kindly provided us with technical support in the course of preparing this paper.
Funding
This work was financially supported by the National Natural Science Foundation of China (32372657), the Xinjiang Production and Construction Corps Science and Technology Plan Project (2023AB077), the Shanxi Apple Industry Science and Technology Project (2020zdzx03-0–04), the Cyrus Tang Foundation and the earmarked fund CARS (CARS-27), and the Research and Development of Virus-Free Seedling Breeding Technology for Apples and Pears (201901020043).
Author information
Authors and Affiliations
Contributions
MMT is the main author. He collected all test data, performed statistical analyses, interpreted the results, and drafted the manuscript. XH, YL, LF, ZA, SL, and SS gave help in the sections of results and discussion of the manuscript. HR and UA mainly helped with the review and editing of the manuscript. DZ and LB helped with the paper’s writing and are the leaders of our team. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tahir, M.M., He, X., Liu, Y. et al. Nitrate stimulates adventitious rooting by increasing auxin content and regulating auxin- and root development-related genes expression in apple. HORTIC. ADV. 1, 18 (2023). https://doi.org/10.1007/s44281-023-00020-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44281-023-00020-5