Abstract
Nickel (Ni), a component of urease, is a micronutrient essential for plant growth and development, but excess Ni is toxic to plants. Tomato (Solanum lycopersicum L.) is one of the important vegetables worldwide. Excessive use of fertilizers and pesticides led to Ni contamination in agricultural soils, thus reducing yield and quality of tomatoes. However, the molecular regulatory mechanisms of Ni toxicity responses in tomato plants have largely not been elucidated. Here, we investigated the molecular mechanisms underlying the Ni toxicity response in tomato plants by physio-biochemical, transcriptomic and molecular regulatory network analyses. Ni toxicity repressed photosynthesis, induced the formation of brush-like lateral roots and interfered with micronutrient accumulation in tomato seedlings. Ni toxicity also induced reactive oxygen species accumulation and oxidative stress responses in plants. Furthermore, Ni toxicity reduced the phytohormone concentrations, including auxin, cytokinin and gibberellic acid, thereby retarding plant growth. Transcriptome analysis revealed that Ni toxicity altered the expression of genes involved in carbon/nitrogen metabolism pathways. Taken together, these results provide a theoretical basis for identifying key genes that could reduce excess Ni accumulation in tomato plants and are helpful for ensuring food safety and sustainable agricultural development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel (Ni) is an essential trace element in plants; however, excess Ni is toxic to plant growth and development (Ghasemi et al. 2009). Ni toxicity inhibits plant growth by affecting photosynthesis, root elongation and nutrient uptake, thereby reducing crop yields (Hassan et al. 2019). Ni is one of the 23 metallic pollutants that make up 3% of the total composition of the earth (Duda-Chodak and Blaszczyk 2008). In recent years, with the acceleration of urbanization and industrialization, Ni toxicity has become a worldwide problem that threatens sustainable agricultural development (Yusuf et al. 2011; Pan et al. 2018). Ni deposition in agricultural soils occurs mainly through natural (wind and sand, volcanic eruptions, etc.) and anthropogenic activities (composting, increased use of greenhouses, low recycling of mulch, etc.) (Ameen et al. 2019; Xu et al. 2022). In China, a national survey showed that 4.8% of farmland soil was contaminated with Ni, making it the second most important soil pollutant (Zhao et al. 2015). As a mobile element, Ni can migrate from soil to edible parts of crops. Bioaccumulation of Ni in edible parts of crops increases food chain contamination (Cempel and Nikel 2006). Excessive exposure to Ni can lead to diseases such as cancer, and fibrosis of the lungs, posing a serious threat to human health (Genchi et al. 2020). Therefore, reducing the uptake of Ni by plants in Ni-enriched soils and increasing the tolerance of plants to Ni toxicity are highly important for ensuring ecosystem health and sustainable agricultural development.
The plant root system absorbs Ni in ionic form from the soil by active and passive migration (Ameen et al. 2019). Ni uptake is an active process via ZRT/IRT-like (ZIP) transporters and Natural resistance-associated macrophage proteins (NRAMPs) with low specificity in plants (Mizuno et al. 2005). In addition, members of the Mg2+-transporting MRS/MGT family in Arabidopsis exhibit Ni2+ uptake activity (Li et al. 2001). Ni translocation and accumulation are facilitated by binding to intracellular metal chelators, such as nicotinamide (NA), histidine (His) and organic acids (citric acid and malate). The maize yellow stripe-like (YSL) transporter ZmYS1 mediates long-distance transport of the Ni(II)-NA complex in plants (Schaaf et al. 2004). After Ni enters plants, it is usually stored in epidermal cells and vesicles rather than within the cell wall (Ahmad and Ashraf 2011). Arabidopsis IRON REGULATED 2 (IREG2), which is localized in the vacuolar membrane, is a core gene involved in the Ni toxicity response and in the transport of Ni into root vacuoles. Overexpression of IREG2 results in increased Ni tolerance and increased Ni accumulation in roots (Schaaf et al. 2006).
Phytohormones play crucial roles in coordinating stress and growth to survive heavy metal toxicity (Saini et al. 2021; Cha et al. 2022; Bhat et al. 2023). The exogenous auxin indole-3-acetic acid (IAA) alleviates Ni toxicity, and overexpression of the auxin biosynthesis-related gene YUC6 improves Ni toxicity tolerance by enhancing peroxidase (PRX) activity and reducing reactive oxygen species (ROS) accumulation through thiol-reductase (TR) activity in YUC6 in Arabidopsis (Cha et al. 2022). Gibberellic acid (GA) promotes Ni sequestration in vesicles and transport by upregulating the expression of GmPC1 in soybean plants (Bhat et al. 2023); moreover, GA also upregulates the expression of catalase (CAT), iron superoxide dismutase (Fe-SOD), ascorbate peroxidase (APX) and glutathione 1 (GSH1), thus alleviating excess Ni-induced oxidative damage in soybean plants and ultimately improving yield (Bhat et al. 2023). Exogenous abscisic acid (ABA) can effectively reduce root Ni absorption and alleviate Ni-induced oxidative damage through the nitric oxide (NO) and hydrogen peroxide (H2O2) signaling pathways (Parwez et al. 2023). Inhibition of ethylene production improves Ni toxicity tolerance by reducing ROS overaccumulation (Khan and Khan 2014). JA (jasmonic acid) and SA (salicylic acid) both improve Ni toxicity tolerance by increasing the content of osmoregulatory substances and antioxidant enzyme activities (Wang et al. 2009; Sirhindi et al. 2016).
Tomato (Solanum lycopersicum L.) is one of the popular vegetables worldwide (Vats et al. 2022). Excessive use of fertilizers and pesticides, sewage irrigation and manure has resulted in Ni contamination in agricultural soils (Hassan et al. 2019; Roccotiello et al. 2022). However, the mechanisms underlying the Ni toxicity response in tomato plants have not been fully elucidated. In this study, we investigated the molecular regulatory network of tomato plants in response to Ni stress. Our results provide a theoretical basis for identifying key genes and signaling pathways that could reduce excess Ni accumulation in tomato plants and are helpful for ensuring food safety and sustainable agricultural development. These results obtained in this study provide a theoretical basis for an in-depth investigation of the adaptive mechanisms of tomatoes in response to Ni toxicity.
Results
Physiological effects of Ni toxicity on tomato seedling growth
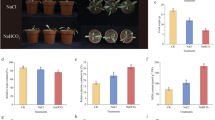
Ni toxicity markedly inhibited plant growth (Fig. 1). The tomato plants exhibited severe dwarfing under Ni toxicity (Fig. 1A and B). Compared with those of the control, Ni toxicity reduced the fresh weight (FW), dry weight (DW) and the water contents of the leaves and roots of tomato plants (Fig. 1C-H). Ni toxicity also inhibits stem growth (Fig. 1I). Ni toxicity inhibited primary root (PR) growth but induced lateral root (LR) formation (Fig. 1J-M). Notably, Ni toxicity induced the formation of brush-like LRs in the region originally occupied by the mature zone of the root tips, especially under 30 μM Ni toxicity (Fig. 1K), suggesting that excess Ni leads to the premature differentiation of the root apical meristem in tomato plants. In addition, Ni toxicity also markedly inhibited leaf growth (Fig. 1N and O).
Nickel toxicity inhibited tomato seedling growth. Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland solution supplemented with or without 30 μM Ni or 50 μM Ni for 5 days. A-I, Representative images showing the plant phenotype (bar = 5 cm) (A), plant height (B), root fresh weight (FW) (C), root dry weight (DW) (D), leaf FW (E), leaf DW (F), root water content (G), leaf water content (H) and stem diameter (I) were measured. J-O, Representative images showing the root phenotype (bar = 5 cm) (J), the outgrowth of lateral roots (LRs) at the root tips (bar = 1 mm) (K), the primary root (PR) length (L) and the average LR number (M). N and O, Representative images showing the leaf phenotype (bar = 1 cm) (N), and the leaf area was measured (O). The values are given as the means ± SDs (n = 3, 6 seedlings/treatment), (* P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ANOVA)
Ni toxicity results in leaf chlorosis in tomato seedlings. Compared with those in the control, the chlorophyll contents in the 30 and 50 μM Ni treatment groups were reduced by 14.3% and 18.4%, respectively (Fig. 2A). Subsequently, we examined chlorophyll fluorescence in tomato leaves (Fig. 2B-G). Under 30 and 50 μM Ni treatments, qN increased by 15.9% and 15.0%, respectively (Fig. 2C); Y(NPQ) increased by 22.1% and 25.3%, respectively (Fig. 2D); Fv/Fm decreased by 0.8% and 2.3%, respectively (Fig. 2E); and Y(II) decreased by 5.0% and 9.2%, respectively (Fig. 2F). Furthermore, qP decreased by 3.1% under 50 μM Ni toxicity (Fig. 2G).
Effects of nickel toxicity on photosynthesis in tomato seedlings. Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland solution supplemented with or without 30 μM Ni or 50 μM Ni for 5 days. A, SPAD values. B-G, Representative images showing the chlorophyll fluorescence parameters (B) and the quantification of qN (C), Y (NPQ) (D), Fv/Fm (E), Y(II) (F) and qP (G). The values are given as the means ± SDs (n = 3, 6 seedlings/treatment). One-way analysis of variance (* P < 0.05, ANOVA)
We then examined the trace element contents in the tomato plants. After 30 μM Ni treatment, the Ni content in the leaves and roots increased by 23.5-fold and 41.2-fold, respectively, compared to that in the control (Fig. 3A and B). Under Ni stress, the Fe content decreased by 30.4% and 20.5% in the leaves and roots, respectively (Fig. 3C and D); the copper (Cu) content decreased by 34.8% in the leaves but increased by 1.07-fold in the roots (Fig. 3E and F); the manganese (Mn) content decreased by 4.2% and 55.5%, respectively, in the leaves and roots (Fig. 3G and H); and the zinc (Zn) content decreased by 19.5% in the leaves but increased by 11.5% in the roots (Fig. 3I and J).
Nickel toxicity affects micronutrient element contents in tomato seedlings. A-J, Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland solution with or without 30 μM Ni for 5 d, the content of Ni in leaves (A) and roots (B), Fe in leaves (C) and roots (D), Cu in leaves (E) and roots (F), Mn in leaves (G) and roots (H), and Zn in leaves (I) and roots (J) were determined. The values are given as the means ± SDs (n = 3, 6 seedlings/treatment), (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ANOVA)
Transcriptome analysis
A transcriptome analysis was performed to detect the differentially expressed genes (DEGs) in the roots of tomato plants after 0, 4, 6, 12, or 24 h of 50 μM Ni treatment (Supplementary Fig. 1; Supplementary Table 1). A total of 94.94 Gb of clean data was obtained, and the percentage of Q30 bases in each sample was not less than 93.42%. Hierarchical clustering (Supplementary Fig. 1A) and intragroup correlation analysis were performed using Pearson’s correlation coefficient (Supplementary Fig. 1B), which revealed clear differences among the five treatment groups and good similarity among the three biological replicates in each group. A total of 2,713 DEGs were identified in Ni-4 h/control (1,775 upregulated and 938 downregulated genes), 1,804 DEGs were identified in Ni-6 h/control (1,248 upregulated genes, 556 downregulated genes), 1,690 DEGs were identified in Ni-12 h/control (767 upregulated, 923 downregulated genes), and 1,561 DEGs were identified in Ni-1d/control (800 upregulated, 761 downregulated genes) (false discovery rate (FDR) < 0.01 and log2 FC > 1 or < -1) (Supplementary Fig. 1C). We randomly selected seven genes to verify the accuracy of the transcriptome data, and the RT‒qPCR results showed good consistency between the RT‒qPCR and transcriptome data, indicating that the transcriptome data were reliable (Supplementary Fig. 1D and E).
We then performed weighted gene coexpression network analysis (WGCNA). A power value of 25 was selected as the optimal soft threshold in the network topology (Supplementary Fig. 2). A total of eight modules were generated from the WGCNA, and four significant modules were screened by correlation analysis between the modules and samples (correlation coefficient > 0.9, P < 0.05) (Supplementary Fig. 3A-E). Subsequently, a network diagram of the gene ontology (GO) enrichment analysis was constructed based on these significant modules. The four major regions were divided according to different biological functions in these modules, including metabolism, signaling pathways, response to the stimulus and transport (Supplementary Fig. 4). DEGs in the green module, which were involved mainly in “transport” and “response to stimulus”, were generally upregulated after Ni treatment; DEGs in the blue module, which were involved mainly in “hormone signaling”, “oxidative stress”, “metal ion transport”, “channel activity” and “nitrogen compounds”, were generally downregulated after Ni treatment; and DEGs in the brown module, which were involved mainly in “amino acid metabolism”, “signaling pathways” and “hormone signaling”, were continuously upregulated after Ni treatment, reaching a peak at 4 h and then downregulated. No significant GO enrichment category was obtained from the pink module (Supplementary Figs. 3 and 4).
Ni toxicity alters the expression of genes involved in metal ion accumulation in roots
The above results showed that Ni toxicity affected the accumulation of trace elements (Fig. 3). GO enrichment analysis also revealed that Ni toxicity modulated metal ion transport (Supplementary Fig. 4). We thus investigated the genes involved in metal ion accumulation, and 25 DEGs were identified in the tomato roots (Supplementary Fig. 5; Supplementary Table 2). bHLH100 is an iron (Fe) deficiency-responsive transcription factor that upregulates the expression of genes related to Fe uptake and accumulation, such as iron-regulated transporter 1 (IRT1) and FRO2, in plants (Hirayama et al. 2018; Wang et al. 2020). Ni toxicity downregulated bHLH100-like (bHLH100L) expression (Supplementary Fig. 5). Moreover, the expression of FRO1 and FRO2 was also significantly downregulated after Ni treatment (Supplementary Fig. 5). Vacuolar iron transfer proteins (VITs) are responsible for Fe storage in vacuoles (Cao 2019). NRAMPs modulate the uptake and compartmentalization of divalent ions such as Fe2+, Mn2+, Cu2+, Zn2+, Cd2+ and Ni2+ in plants (Cun et al. 2014). Metal tolerance proteins (MTPs) mediate ionic homeostasis by regulating the uptake of Zn2+, Fe2+, Co2+, Ni2+, Cd2+ and Mn2+ in plants (Socha and Guerinot 2014). Similarly, the expression of NRAMP1, five VIT and three MTP genes was significantly downregulated after Ni treatment (Supplementary Fig. 5). bZIP23 is a Zn sensor that modulates Zn uptake in cells by inducing the expression of ZIPs (Lilay et al. 2019). Ni toxicity upregulated bZIP23 expression (Supplementary Fig. 5). Furthermore, the expression of the three ZIP genes was also significantly upregulated after Ni treatment (Supplementary Fig. 5). IREG3 is involved in Fe export to mitochondria (Kim et al. 2021). The metal-nicotianamine transporter YSLs play a role in the long-distance transport of metal ions (Curie et al. 2009). The copper transporters (CTRs) are involved in Cu ion uptake (Vatansever et al. 2017). The expression of the IREG3, YSL2 and two CTRs was also significantly upregulated after Ni treatment (Supplementary Fig. 5).
Ni toxicity affects phytohormone levels and the expression of genes involved in phytohormone signaling pathways
GO enrichment analysis revealed that several pathways involved in the response to phytohormones were enriched in the roots of the Ni-treated tomato plants (Supplementary Fig. 4). Therefore, we investigated DEGs associated with phytohormone biosynthesis (Fig. 4; Supplementary Table 3). Ni toxicity downregulated the expression of 9-cis-epoxycarotenoid dioxygenase (NCED), a key ABA biosynthesis gene, whereas it upregulated the expression of phenylalanine ammonia-lyase (PAL), a SA biosynthesis-related gene (Fig. 4). ACC synthase (ACS) and ACC oxidase (ACO) are the key genes involved in the biosynthesis of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) and ethylene in plants (Houben and Van de Poel 2019). The expression of two ACS genes and three ACO genes was upregulated, whereas the other three ACO genes were downregulated (Fig. 4). In the brassinolide (BR) biosynthesis pathway, the expression of one sterol-C24-methyltransferase 1 (SMT1) gene and one cytochrome P450 CYP92A6 gene was upregulated (Fig. 4). In the GA biosynthesis pathway, the expression of ent-kaurenoic acid monooxygenase (KAO) was downregulated, whereas two gibberellin 2 beta-dioxygenase (GA2ox) genes involved in the deactivation of bioactive GAs were upregulated (Fig. 4). Tryptophan aminotransferase (TAA) and aldehyde dehydrogenase (NAD +) (ALDH) are the key genes involved in IAA biosynthesis. The expression of one TAA gene was downregulated, whereas one ALDH gene was upregulated (Fig. 4). In the cytokinin biosynthesis pathway, the expression of two adenylate dimethylallyl transferase (IPT) genes involved in cytokinin biosynthesis and one cytokinin dehydrogenase (CKX) gene involved in irreversible degradation of cytokinin was downregulated, while the expression of one cis-zeatin O-glucosyl transferase (CISZOG) and two glucosyltransferase 73C (UGT73C) involved in inactivation of cytokinin was upregulated (Fig. 4). In the JA biosynthesis pathway, the expression of one OPC-8:0 CoA ligase 1 (OPCL1), one secretory phospholipase A2 (PLA2G) and two lipoxygenase 2S (LOX2S) was upregulated, whereas one hydroperoxide dehydratase (AOS) and one 12-oxophytodienoic acid reductase (OPR) gene were downregulated by Ni toxicity (Fig. 4).
We subsequently determined the phytohormone contents in the roots of the Ni-treated tomato plants. As shown in Fig. 5A-E, after 12 h of Ni treatment, the levels of IAA, ABA, GA and the two cytokinins trans-zeatin riboside (tZR) and isopentenyl-adenine (iP) decreased by 63.1%, 62.8%, 7.3%, 19.1% and 49%, respectively, while they decreased by 69.0%, 65.0%, 11.0%, 25.2% and 55.6%, respectively, after 24 h of Ni treatment.
Ni toxicity affects phytohormone levels in the roots of tomato seedlings. Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland solution supplemented with or without 50 μM Ni for 12 h or 1 d, after which the contents of IAA (A), ABA (B), tZR (C), iP (D) and GA (E) were determined. The values are given as the means ± SDs (n = 3, 6 seedlings/treatment), (* P < 0.05, ANOVA)
Subsequently, we investigated the expression of DEGs involved in phytohormone signaling pathways (Fig. 6; Supplementary Table 4). In the auxin pathway, the expression of two GH3 genes, one auxin influx carrier AUXIN 1 (AUX1), two PIN-FORMED (PIN) auxin exporters PIN4 and PIN9, one AUX/IAA and four SAUR genes was downregulated, whereas two PIN genes (PIN5 and PIN10), one AUX/IAA gene and five SAUR genes was upregulated. Ni toxicity inhibits the cytokinin, GA, JA and SA signaling pathways in the roots of Ni-treated tomatoes. In the cytokinin pathway, the expression of two A-ARR genes, one B-type response regulator (B-ARR) and one cytokinin response 1 (CRE1) was downregulated. In the GA pathway, the expression of one GA receptor gene, gibberellin insensitive dwarf 1 (GID1), and one GA-responsive gene, phytochrome-interacting factor (PIF), was downregulated. In the JA pathway, the expression of two jasmonate resistant 1 (JAR1) genes involved in the biosynthesis of JA-Ile, and one jasmonate ZIM-domain (JAZ) gene, which is the repressor of JA signaling, was downregulated, whereas the other two JAZ genes were upregulated. In the SA pathway, the expression of three TGA and two NPR1 was downregulated. In addition, Ni toxicity affects the signaling pathways of ABA, ethylene and BR. In the ABA pathway, the expression of one ABRE-binding factor (ABF), one protein phosphatase 2C (PP2C) and two pyrabactin resistance/PYR-like (PYR/PYC) was upregulated, whereas the expression of two other PYR/PYC genes and one core component of ABA signaling, the sucrose nonfermenting-1-related protein kinase 2 (SnRK2) gene, was downregulated. In the ethylene pathway, the expression of one positive regulator of one EIN2, two ETR genes and one EBF1 gene was downregulated, whereas the other one ethylene receptor ethylene response (ETR), one negative regulator of ethylene signaling constitutive triple response 1 (CTR1) and two positive regulators of ethylene signaling EIN3 genes was upregulated. In the BR pathway, the expression of two downstream BR-responsive genes, cyclin D3 (CYCD3) and xyloglucan:xyloglucosyl transferase (TCH4), was downregulated and upregulated, respectively (Fig. 6).
Ni toxicity induces oxidative stress response
GO enrichment analysis revealed that Ni toxicity induced oxidative stress responses in tomato roots (Supplementary Fig. 4). Therefore, we investigated the DEGs associated with the antioxidant system (Fig. 7A; Supplementary Table 5). Ni toxicity altered the expression patterns of the peroxidase (POD) and catalase (CAT) genes (Fig. 7A). The expression of the CAT3 gene was upregulated after 12 h of Ni treatment. Ni toxicity also markedly induces the expression of lignin-forming anionic POD genes and cationic POD genes, thereby regulating cell wall metabolism and root system growth in response to excess Ni (Tamás et al. 2007). Furthermore, after excess Ni treatment, 50% of the POD genes were upregulated, whereas 47% of the POD genes were downregulated in the tomato roots (Fig. 7A).
Nickel toxicity induced oxidative damage and affected antioxidant enzyme activities in tomato seedlings. A, Heatmaps showing the expression patterns of antioxidative enzyme-encoding genes according to the log2-fold change, and the asterisks in the heatmaps represent the DEGs. B-G, Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland solution supplemented with or without 30 μM Ni or 50 μM Ni for 12 h, 1 d, 3 d, or 5 d, after which the leaf superoxide dismutase (SOD) activity (B), root SOD activity (C), leaf catalase (CAT) activity (D), root CAT activity (E), leaf peroxidase (POD) activity (F) and root POD activity (G) were determined. The values are given as the means ± SDs. Different letters indicate significant differences (P < 0.05). H-N, Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland nutrient solution supplemented with or without 30 μM Ni or 50 μM Ni for 5 days. H, DAB staining. I, NBT staining. Bar = 1 cm. J, DCFH-DA fluorescence staining showing ROS levels in the root tips (bar = 500 μm). K-N, Twenty-five-day-old tomato seedlings were transferred to fresh 1/4 Hoagland nutrient solution supplemented with or without 30 μM Ni or 50 μM Ni for 12 h, 1 d, 3 d, or 5 d, after which the H2O2 (K, L) and MDA (M, N) contents in the leaves (K, M) and roots (L, N) were determined. The values are given as the means ± SDs (n = 3, 6 seedlings/treatment). Different letters indicate significant differences (P < 0.05, ANOVA)
We then examined antioxidative enzyme activities in tomato plants. Ni stress increased the activities of superoxide dismutase (SOD) and peroxidase (POD) both in leaves and roots; however, it did not significantly affect catalase (CAT) activity (Fig. 7B-G). Next, we detected ROS levels in tomato seedlings under Ni toxicity. 3,3'-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining further revealed that Ni toxicity induced H2O2 and O2.− accumulation, respectively, in the leaves (Fig. 7H and I). Visualization of ROS levels by a 2,7-dichlorofluorescein diacetate (DCFH-DA) fluorescence probe showed that Ni toxicity induced ROS accumulation in roots (Fig. 7J). The quantitative detection of H2O2 content also confirmed these results (Fig. 7K and L). The malondialdehyde (MDA) content reflects the degree of oxidative damage in plants (Hodges et al. 1999). Ni toxicity did not significantly induce MDA accumulation in the leaves (Fig. 7M). However, the root MDA content increased by 51.1% and 70% after 5 days of 30 and 50 μM Ni treatment, respectively (Fig. 7N).
Ni toxicity alters the expression of genes associated with the primary metabolism
GO enrichment analysis revealed that Ni toxicity altered metabolic processes in tomato roots (Supplementary Fig. 4). Therefore, we analyzed the DEGs involved in carbon and primary nitrogen metabolism (Fig. 8; Supplementary Table 6). In glycolysis pathway, the expression of one hexokinase (HK), one fructose-bisphosphate aldolase (ALDO), one enolase (ENO) and one pyruvate kinase (PK) gene was upregulated under Ni toxicity, but one HK and one 6-phosphofructokinase A (pfkA) gene were downregulated (Fig. 8). In the pentose phosphate pathway, the expression of one G6PD gene and one 6-phosphogluconate dehydrogenase (PGD) gene was downregulated, whereas one ribose 5-phosphate isomerase A (rpiA) gene was upregulated (Fig. 8). In the glyoxylate and C4-dicarboxylic acid cycle pathway, the expression of one glutamic-oxaloacetic transaminase 2 (GOT2), one malate synthase (MS), one malate dehydrogenase (ME2), one glutamate: glyoxylate aminotransferase (GGAT) and two phosphoenolpyruvate carboxylase (PPC) genes was downregulated, whereas one ME2 gene was upregulated (Fig. 8). In the biosynthetic pathway of cysteine, the expression of one serine acetyltransferase (cysE) gene was upregulated after Ni treatment, whereas the expression of one cysteine synthase (cysK) gene was upregulated after 4 and 6 h of Ni treatment but downregulated after 1 d of Ni treatment (Fig. 8). In the primary nitrogen metabolism pathway, the expression of two ferredoxin-nitrite reductases (nirA), two glutamine synthetases (glnA) and one carbonic anhydrase (CA) gene was downregulated, whereas one CA gene was upregulated, and one high-affinity nitrate transporter (NRT) gene was downregulated after 6 and 12 h of Ni treatment and subsequently upregulated after 1 d of Ni treatment (Fig. 8).
Discussion
Excess Ni inhibits growth and development and reduces yield and quality in crops (Ameen et al. 2019). Our study also indicated that excess Ni inhibits plant growth by decreasing the water content, chlorophyll level and PSII activity (Figs. 1 and 2). Ni toxicity inhibits root system growth and reduces the number of LRs in rice (Seregin et al. 2003; Rizwan et al. 2022), whereas it increases the density of LRs in Arabidopsis (Leškovï et al. 2020). We found that although excess Ni (30 or 50 μM) inhibited PR growth, it markedly induced LR formation and the formation of brush-like LRs at the root tips in tomato plants (Fig. 1J-M). This difference suggested that there were different response mechanisms involved in modulating root system growth in response to Ni toxicity between dicotyledons and monocotyledons. Increased LRs improve the absorption of water and nutrients, which is beneficial for enhancing plant tolerance to heavy metal toxicity. Therefore, this may be an adaptative mechanism for Arabidopsis and tomato plants in response to Ni toxicity. However, the underlying molecular mechanisms still need further clarification. Heavy metal toxicity interferes with the carbon and nitrogen supplies in plants (Ghori et al. 2019). A previous study demonstrated that Ni toxicity affects carbon and nitrogen metabolism in wheat (Gajewska and Skłodowska 2009; Gajewska et al. 2013). In support of these results, our results indicated that Ni toxicity altered the expression of genes involved in primary carbon and nitrogen metabolism (Fig. 8), ultimately modulating the adaptation of tomato plants to Ni toxicity.
Ni toxicity induced the expression of divalent metal cation long-distance transporter YSL2, metal transmembrane transporter NRAMP3-Like and divalent ion transporters ZIPs in roots (Mizuno et al. 2005; Oomen et al. 2009; Chu 2010) (Supplementary Fig. 5), thereby maintaining the uptake and accumulation of metal micronutrients in plants (Fig. 3A and B). Ni toxicity also upregulates the expression of two Cu transporter CTRs in roots. Consistent with these results, the Cu concentration in the roots was elevated in the Ni-treated tomato plants (Fig. 3F). FRO is responsible for Fe reduction from Fe3+ to Fe2+ (Bernal et al. 2012); subsequently, IRT1 transports Fe2+ into roots (Brumbarova et al. 2015). The Fe deficiency-responsive bHLH100-like gene encodes a transcription factor that directly upregulates the expression of IRT1 and FRO2, thereby positively regulating Fe uptake. Ni toxicity downregulated the expression of bHLH100-like, FRO1/2 and IRT1 in roots (Supplementary Fig. 5); consistent with these results, the Fe levels in the leaves and roots of the Ni-treated plants were reduced (Fig. 3C and D). VITs are excess Fe-responsive genes that are involved in Fe compartmentalization in vacuoles (Kim et al. 2006; Peng and Gong 2014); moreover, Ni toxicity decreases Fe accumulation; therefore, VIT expression was downregulated in Ni-treated tomato roots (Fig. 3C and D; Supplementary Fig. 5). Ni toxicity induced the Zn sensor bZIP23 expression, and three ZIP genes was also induced in the roots (Supplementary Fig. 5). Consistent with this result, Ni toxicity increased Zn accumulation in roots (Fig. 3J). Fe, Cu and Mn are essential components for maintaining chlorophyll structure and activity (Ahmad and Ashraf 2011). Ni toxicity reduces the levels of Fe, Cu and Mn in leaves (Fig. 3C, E and G), thereby repressing photosynthetic efficiency in tomato plants (Fig. 2).
Ni toxicity induces ROS overproduction in tomato plants (Fig. 7H-L). This result is consistent with previous reports that Ni toxicity induces oxidative damage in plants (Gajewska et al. 2006; Gajewska and Skłodowska 2007). ROS accumulation in roots promotes LR formation (Orman-Ligeza et al. 2016). Indeed, we observed brush-like LRs in the root tips of the Ni-treated tomato plants (Fig. 1J-M). Antioxidative enzymes play an important role in maintaining ROS homeostasis in vivo and preventing oxidative damage in plants under abiotic stresses (Dubey and Pandey 2011). Ni toxicity upregulated the expression of several POD genes and increased POD activity in tomato plants (Fig. 7A, F and G). Moreover, Ni toxicity also increased SOD activity in tomato plants (Fig. 7B and C) but did not affect the expression of SOD genes in roots. In addition, Ni toxicity upregulated CAT3 expression in roots (Fig. 7A) but did not alter CAT activity (Fig. 7D and E). These results collectively indicated that Ni toxicity modulates antioxidative enzyme activities at both the transcriptional and posttranscriptional levels.
In this study, we found that excess Ni decreased the concentrations of auxin, cytokinin and GA in tomato roots (Fig. 5A-E), thereby slowing plant growth. Leškovï et al. (2020) reported that a high concentration of Ni (100 and 150 μM) inhibits PR growth by repressing PIN2 abundance in root tips. We found that Ni toxicity (50 μM) upregulated the expression of PIN5 and PIN10 but downregulated the expression of PIN4, PIN9 and AUX1 in roots (Fig. 6), suggesting that excess Ni interferes with root auxin transport and distribution, thus modulating root system architecture. However, the detailed molecular mechanisms need to be further elucidated. Excess Ni downregulated IPT gene expression (Fig. 4). In support of these results, the contents of tZR and iP decreased in the roots of the Ni-treated plants (Fig. 5C-D). In addition, Ni toxicity also upregulated the expression of the GA-inactivating enzyme GA2ox; consistent with these results, the GA content decreased in the roots (Figs. 4 and 5E). ABA plays important roles in modulating abiotic stress tolerance in plants. However, we found that Ni toxicity reduces ABA levels in roots (Fig. 5). Moreover, Ni toxicity upregulated the expression of PP2C genes, which are negative regulators of ABA signaling, while it downregulated the expression of ABA biosynthesis-related NCED genes, as well as the SnRK2 gene, the core component of ABA signaling, thereby potentially inhibiting the ABA signaling pathway (Figs. 4 and 6). However, the detailed molecular mechanism by which excess Ni represses ABA levels and the ABA signaling pathway in tomato roots still need to be further elucidated.
Conclusion
In summary, this study investigated the physiological and molecular mechanisms underlying excess Ni-mediated growth in tomato plants via physio-biochemical and transcriptomic analyses. The results indicated that (i) excess Ni reprogrammed root system architecture by inducing the formation of brush-like LRs in tomato plants; (ii) excess Ni interfered with micronutrient accumulation and photosynthesis efficiency; (iii) excess Ni altered the expression of genes involved in primary metabolic processes; (iv) excess Ni reduced the levels of IAA, cytokinin and GA, ultimately maintaining tomato plant survival under Ni toxicity (Fig. 9). This work provides a basis for future in-depth studies of the molecular mechanisms involved in the Ni toxicity response in tomato plants.
Materials and methods
Plant materials and growth conditions
Tomato (Solanum lycopersicum L.) cv. micro-Tom seeds were soaked in sterile water for 30 min, surface sterilized with 75% (v/v) alcohol for 40 s, washed with 50% (v/v) bleach for 6 min, and then rinsed 5 times with sterile water. The sterilized seeds were placed on sprouting trays for germination. Twelve-day-old tomato plants were subsequently transferred to 1/4 Hoagland nutrient solution for 10 days. Twenty-five-day-old tomato plants were subsequently transferred to fresh 1/4 Hoagland solution supplemented with or without 30 or 50 μM NiCl2 for 5 days.
Phenotypic analysis
The plant stem thickness was measured with a Vernier caliper. The root and leaf images were obtained by scanning with an Epson Perfection V500 Photo scanner (Epson, Japan), and ImageJ (version 1, 44) software was subsequently used to measure PR length, plant height and leaf area. The FW and DW were measured, and the plant water content was calculated using the following formula: plant water content (%) = (FW-DW)/DW.
The roots were immersed in FAA fixative (5 ml of 38% formaldehyde, 5 ml of glacial acetic acid, 90 ml of 50% alcohol, and 5 ml of glycerol) and fixed for more than 24 h. The fixed roots were first rehydrated by a gradient of 50% ethanol and 30% ethanol for 5 min, soaked in distilled water for 10 min, and then transferred to 0.01% methylene blue staining solution for 7–10 min. The roots were washed three times with distilled water, and the number of LRs was observed and counted using an optical microscope (Leica, Germany).
Determination of chlorophyll contents and photosynthetic indices
The chlorophyll contents were determined using a SPAD-502 chlorophyll meter (Minolta Camera Co., Ltd., Japan). Fluorescence detection was performed using a MAXI imaging PAM instrument (Heinz Walz GmbH, 91,090 Effeltrich, Germany). The seedlings were pretreated in the dark for 30 min and subsequently placed in the instrument to determine the chlorophyll fluorescence parameters. The fluorescence parameters were calculated as follows: PS II maximum activity parameter Fv/Fm = (Fm-Fo)/Fm; nonphotochemical quenching coefficient Y(NPQ) = 1-Y(II)-1/[NPQ + 1 + qL-(Fm/F0-1); nonphotochemical quenching parameter qN = (Fm-Fm′)/(Fm-F0); PS(II) effective electron yield Y(II) = (Fm'—F)/Fm'; and photochemical bursting coefficient qP = (Fm'—F)/(Fm'—Fo').
Determination of antioxidant enzyme activities, reactive oxygen species and malondialdehyde contents
The NBT method was used for the determination of SOD activity (Agami and Mohamed 2013). POD activity was determined using guaiacol as a substrate (Chen and Zhang 2016). CAT activity was determined using the hydrogen peroxide method (Du et al. 2017). The H2O2 content was determined using the iodometric method (Wei et al. 2009). In situ H2O2 staining of the roots and leaves was performed using the 3,3'-diaminobenzidine (DAB, 1 mg/mL, pH = 3.8) method as described by Xia et al. (2009). In situ O2.− staining of the roots and leaves was performed using nitroblue tetrazolium (NBT, 0.5 mg/ml in 50 mM PBS, pH = 7.8) as described by Ahammed et al. (2013). The ROS fluorescent probe DCFH-DA (Beyotime, China) was used to detect endogenous ROS levels in the root tips according to the manufacturer's instructions (excitation wavelength of 488 nm and emission wavelength of 530 nm). MDA levels were determined using the thiobarbituric acid (TBA) method (Zeng et al. 2020).
Transcriptome analysis and RT‒qPCR analysis
Transcriptome sequencing libraries were generated using the Hieff NGS Ultima Dual-mode mRNA Library Prep Kit for Illumina (Yeasen Biotechnology (Shanghai) Co., Ltd.). After passing quality control, the libraries were analyzed on the Lumina NovaSeq 6000 platform for PE150 mode sequencing. The raw reads were further processed and analyzed using the bioinformatics platform BMKCloud (www.biocloud.net). The reference genome sequence is Solanum lycopersicum SL4.0_and_ITAG4.0.genome.fa. The raw data were submitted to the National Center for Biotechnology Information (NCBI) Short Read Archive (SRA) under accession number PRJNA952335. A FDR < 0.01 and log2 (fold change) > 1 or < -1 were used as criteria to screen the DEGs. WGCNA was performed according to the methods of Wang et al. (2023). GO network analysis was performed by association analysis between the significant modules and treatment groups using the OmicShare cloud platform (https://www.omicshare.com), and GO network visual presentation was carried out using Cytoscape v3.9.1 (Q value < 0.05).
RNA reverse transcription was performed using the cDNA synthesis kit NovoScript® Plus All-in-one 1st Strand cDNA Synthesis SuperMix (gDNA Purge, Novozymes, China). RT‒qPCR analyses were performed for three biological and technical replicates. The specific primers used are shown in Supplementary Table 7.
Determination of phytohormones
The contents of IAA, GA, and ABA and the cytokinins tZR and iP were determined using high-performance liquid chromatography (HPLC) as described by Gao et al. (2022). Briefly, approximately 1 g of tomato root was thoroughly ground with liquid nitrogen, and the powders were then immersed in 20 ml of 80% methanol (chromatographically pure) for 16 h at 4 ℃. After centrifugation at 1000 rpm for 10 min at 4 °C, the supernatant was collected. The residue was transferred to 20 ml of 80% precooled methanol and centrifuged, after which the supernatants were merged. The supernatant was evaporated at 40 °C to remove the methanol using a rotary evaporator (RE-52AA, Shanghai, China). The supernatant was extracted three times with 10 ml of petroleum ether (chromatographically pure). After adding polyvinylpyrrolidone (PVPP) to the ether phase, the mixture was ultrasonicated for 30 min and then shaken for 30 min. After centrifugation at 13,000 r/min for 10 min, the supernatant was collected. Extraction was carried out three times. Subsequently, the ester phases were evaporated by rotary evaporation at 40 °C, and 1 ml of methanol was added to dissolve the ester phases. After filtering through a 0.45 μM filter membrane, the sample was placed at 4 °C. The separation was performed on a Syncroords C18 250 × 4.6 × 5 μm liquid chromatography column (Thermo Fisher Scientific, China) with 100% methanol as mobile phase A and 0.8% glacial acetic acid as mobile phase B. A total of 10 μl of sample was injected at a flow rate of 1 ml/min, the column temperature was set at 30 ℃, the UV wavelength was 254 nm, and the sample was detected online by a Dionex UltiMate 300 Diode Array Detector (Thermo Fisher Scientific, China).
Trace element determination
The samples were dried in an oven at 65 °C until a constant weight was reached. Subsequently, the samples were ground to powder in a mortar and immersed in nitric acid. The contents of Ni, Fe, Mn, Cu and Zn were determined using ICP‒AES (inductively coupled plasma atomic emission spectroscopy; iCAP6300; Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All the experiments were performed in triplicate, and the data were analyzed and are presented visually using IBM SPSS Statistics 26 and GraphPad Prism 9.5.1. Significant differences were determined by ANOVA and t tests (P < 0.05).
Availability of data and materials
Data and materials will be made available on request.
Abbreviations
- Ni:
-
Nickel
- Fe:
-
Iron
- Mn:
-
Manganese
- Cu:
-
Copper
- Zn:
-
Zinc
- GA:
-
Gibberellic acid
- ABA:
-
Abscisic acid
- IAA:
-
Indole-3-acetic acid
- JA:
-
Jasmonic acid
- SA:
-
Salicylic acid
- tZR:
-
Trans-Zeatin riboside
- ip:
-
Isopentenyl-adenine
- HPLC:
-
High-performance liquid chromatography
- PVPP:
-
Polyvinylpyrrolidone
- NA:
-
Nicotinamide
- His:
-
Histidine
- PRX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- TR:
-
Thiol-reductase
- NO:
-
Nitric oxide
- H2O2 :
-
Hydrogen peroxide
- NBT:
-
Nitroblue tetrazolium
- DAB:
-
3,3'-Diaminobenzidine
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- TCA:
-
Trichloroacetic acid
- DCFH-DA:
-
2,7-Dichlorofluorescein diacetate
- TBA:
-
Thiobarbituric acid
- MDA:
-
Malondialdehyde
- PR:
-
Primary root
- FW:
-
Fresh weight
- DW:
-
Dry weight
- LRs:
-
Lateral roots
- NCBI:
-
National Center for Biotechnology Information
- SRA:
-
Short Read Archive
- FDR:
-
False discovery rate
- DEG:
-
Differentially expressed gene
- WGCNA:
-
Weighted gene coexpression network analysis
- FPKM:
-
Fragments per kilobase million
- GO:
-
Gene ontology
References
Agami RA, Mohamed GF (2013) Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol Environ Saf 94:164–171. https://doi.org/10.1016/j.ecoenv.2013.04.013
Ahammed GJ, Ruan YP, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ (2013) Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 90(11):2645–2653. https://doi.org/10.1016/j.chemosphere.2012.11.041
Ahmad MS, Ashraf M (2011) Essential roles and hazardous effects of nickel in plants. Rev Environ Contam Toxicol 214:125–167. https://doi.org/10.1007/978-1-4614-0668-6_6
Ameen N, Amjad M, Murtaza B, Abbas G, Shahid M, Imran M, Naeem MA, Niazi NK (2019) Biogeochemical behavior of nickel under different abiotic stresses: toxicity and detoxification mechanisms in plants. Environ Sci Pollut Res Int 26(11):10496–10514. https://doi.org/10.1007/s11356-019-04540-4
Bernal M, Casero D, Singh V, Wilson GT, Grande A, Yang H, Dodani SC, Pellegrini M, Huijser P, Connolly EL, Merchant SS, Krämer U (2012) Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24(2):738–761. https://doi.org/10.1105/tpc.111.090431
Bhat JA, Basit F, Alyemeni MN, Mansoor S, Kaya C, Ahmad P (2023) Gibberellic acid mitigates nickel stress in soybean by cell wall fixation and regulating oxidative stress metabolism and glyoxalase system. Plant Physiol Biochem 198:107678. https://doi.org/10.1016/j.plaphy.2023.107678
Brumbarova T, Bauer P, Ivanov R (2015) Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci 20(2):124–133. https://doi.org/10.1016/j.tplants.2014.11.004
Cao J (2019) Molecular evolution of the Vacuolar Iron Transporter (VIT) family genes in 14 plant species. Genes (Basel) 10(2). https://doi.org/10.3390/genes10020144
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Polish J Environ Stud Sci 15(3):375–382
Cha JY, Jeong SY, Ahn G, Shin GI, Ji MG, Lee SC, Khakurel D, Macoy DM, Lee YB, Kim MG, Lee SY, Yun DJ, Kim WY (2022) The thiol-reductase activity of YUCCA6 enhances nickel heavy metal stress tolerance in Arabidopsis. Front Plant Sci 13:1007542. https://doi.org/10.3389/fpls.2022.1007542
Chen T, Zhang B (2016) Measurements of proline and malondialdehyde content and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-Protoc 6(17):e1913–e1913. https://doi.org/10.21769/bioprotoc.1913
Chu H-H (2010) Analyses of Arabidopsis Yellow Stripe-Like (YSL) family of metal transporters. Dissertation, University of Massachusetts Amherst. https://doi.org/10.7275/1264433
Cun P, Sarrobert C, Richaud P, Chevalier A, Soreau P, Auroy P, Gravot A, Baltz A, Leonhardt N, Vavasseur A (2014) Modulation of Zn/Cd P(1B2)-ATPase activities in Arabidopsis impacts differently on Zn and Cd contents in shoots and seeds. Metallomics 6(11):2109–2116. https://doi.org/10.1039/c4mt00182f
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103(1):1–11. https://doi.org/10.1093/aob/mcn207
Du C, Zhao P, Zhang H, Li N, Zheng L, Wang Y (2017) The Reaumuria trigyna transcription factor RtWRKY1 confers tolerance to salt stress in transgenic Arabidopsis. J Plant Physiol 215:48–58. https://doi.org/10.1016/j.jplph.2017.05.002
Dubey D, Pandey A (2011) Effect of nickel (Ni) on chlorophyll, lipid peroxidation and antioxidant enzymes activities in black gram ( Vigna mungo ) leaves. Int J Sci Nat 2(2):395–401
Duda-Chodak A, Blaszczyk U (2008) The impact of nickel on human health. J Elementol 13(4):685–693
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20(1):27–36. https://doi.org/10.1007/s10534-006-9011-5
Gajewska E, Skłodowska M (2009) Nickel-induced changes in nitrogen metabolism in wheat shoots. J Plant Physiol 166(10):1034–1044. https://doi.org/10.1016/j.jplph.2008.12.004
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plantarum 50:653–659
Gajewska E, Niewiadomska E, Tokarz K, Słaba M, Skłodowska M (2013) Nickel-induced changes in carbon metabolism in wheat shoots. J Plant Physiol 170(4):369–377. https://doi.org/10.1016/j.jplph.2012.10.012
Gao M, Jiang W, Lin Z, Lin Q, Ye Q, Wang W, Xie Q, He X, Luo C, Chen Q (2022) SMRT and illumina RNA-Seq identifies potential candidate genes related to the double flower phenotype and unveils SsAP2 as a key regulator of the double-flower trait in Sagittaria sagittifolia. Int J Mol Sci 23(4). https://doi.org/10.3390/ijms23042240
Genchi G, Carocci A, Lauria G, Sinicropi M S and Catalano A (2020) Nickel: Human health and environmental toxicology. Int J Environ Res Public Health 17(3). https://doi.org/10.3390/ijerph17030679
Ghasemi R, Ghaderian SM, Krämer U (2009) Interference of nickel with copper and iron homeostasis contributes to metal toxicity symptoms in the nickel hyperaccumulator plant Alyssum inflatum. New Phytol 184(3):566–580. https://doi.org/10.1111/j.1469-8137.2009.02993.x
Ghori N-H, Ghori T, Hayat M, Imadi S, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16:1807–1828
Hassan MU, Chattha MU, Khan I, Chattha MB, Aamer M, Nawaz M, Ali A, Khan MAU, Khan TA (2019) Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environ Sci Pollut Res Int 26(13):12673–12688. https://doi.org/10.1007/s11356-019-04892-x
Hirayama T, Lei GJ, Yamaji N, Nakagawa N, Ma JF (2018) The putative peptide gene FEP1 regulates iron deficiency response in Arabidopsis. Plant Cell Physiol 59(9):1739–1752. https://doi.org/10.1093/pcp/pcy145
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Houben M, Van de Poel B (2019) 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): the enzyme that makes the plant hormone ethylene. Front Plant Sci 10:695. https://doi.org/10.3389/fpls.2019.00695
Khan MI, Khan NA (2014) Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 251(5):1007–1019. https://doi.org/10.1007/s00709-014-0610-7
Kim SA, Punshon T, Lanzirotti A, Li L, Alonso JM, Ecker JR, Kaplan J, Guerinot ML (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314(5803):1295–1298. https://doi.org/10.1126/science.1132563
Kim LJ, Tsuyuki KM, Hu F, Park EY, Zhang J, Iraheta JG, Chia JC, Huang R, Tucker AE, Clyne M, Castellano C, Kim A, Chung DD, DaVeiga CT, Parsons EM, Vatamaniuk OK, Jeong J (2021) Ferroportin 3 is a dual-targeted mitochondrial/chloroplast iron exporter necessary for iron homeostasis in Arabidopsis. Plant J 107(1):215–236. https://doi.org/10.1111/tpj.15286
Leškovï A, Zvarï KM, Araya T, Giehl RFH (2020) Nickel toxicity targets cell wall-related processes and PIN2-mediated auxin transport to inhibit root elongation and Gravitropic responses in Arabidopsis. Plant Cell Physiol 61(3):519–535. https://doi.org/10.1093/pcp/pcz217
Li L, Tutone AF, Drummond RS, Gardner RC, Luan S (2001) A novel family of magnesium transport genes in Arabidopsis. Plant Cell 13(12):2761–2775. https://doi.org/10.1105/tpc.010352
Lilay GH, Castro PH, Campilho A, Assunção AGL (2019) The Arabidopsis bZIP19 and bZIP23 activity requires zinc deficiency - insight on regulation from complementation lines. Front Plant Sci 9:1955. https://doi.org/10.3389/fpls.2018.01955
Mizuno T, Usui K, Horie K, Nosaka S, Mizuno N, Obata H (2005) Cloning of three ZIP/Nramp transporter genes from a Ni hyperaccumulator plant Thlaspi japonicum and their Ni2+-transport abilities. Plant Physiol Biochem 43(8):793–801. https://doi.org/10.1016/j.plaphy.2005.07.006
Oomen RJ, Wu J, Lelièvre F, Blanchet S, Richaud P, Barbier-Brygoo H, Aarts MG, Thomine S (2009) Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol 181(3):637–650. https://doi.org/10.1111/j.1469-8137.2008.02694.x
Orman-Ligeza B, Parizot B, de Rycke R, Fernandez A, Himschoot E, Van Breusegem F, Bennett MJ, Périlleux C, Beeckman T, Draye X (2016) RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143(18):3328–3339. https://doi.org/10.1242/dev.136465
Pan L, Wang Y, Ma J, Hu Y, Su B, Fang G, Wang L, Xiang B (2018) A review of heavy metal pollution levels and health risk assessment of urban soils in Chinese cities. Environ Sci Pollut Res Int 25(2):1055–1069. https://doi.org/10.1007/s11356-017-0513-1
Parwez R, Aftab T, Khan MMA, Naeem M (2023) Exogenous abscisic acid fine-tunes heavy metal accumulation and plant’s antioxidant defence mechanism to optimize crop performance and secondary metabolite production in Trigonella foenum-graecum L. under nickel stress. Plant Sci 332:111703. https://doi.org/10.1016/j.plantsci.2023.111703
Peng JS, Gong JM (2014) Vacuolar sequestration capacity and long-distance metal transport in plants. Front Plant Sci 5:19. https://doi.org/10.3389/fpls.2014.00019
Rizwan M, Usman K, Alsafran M, Jabri H A, Samreen T, Saleem M H and Tu S (2022) Nickel toxicity interferes with NO3-/NH4+ uptake and nitrogen metabolic enzyme activity in rice (Oryza sativa L.). Plants (Basel) 11(11). https://doi.org/10.3390/plants11111401
Roccotiello E, Nicosia E, Pierdonà L, Marescotti P, Ciardiello MA, Giangrieco I, Mari A, Zennaro D, Dozza D, Brancucci M, Mariotti M (2022) Tomato (Solanum lycopersicum L.) accumulation and allergenicity in response to nickel stress. Sci Rep 12(1):5432. https://doi.org/10.1038/s41598-022-09107-x
Saini S, Kaur N, Pati PK (2021) Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf 223:112578. https://doi.org/10.1016/j.ecoenv.2021.112578
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279(10):9091–9096. https://doi.org/10.1074/jbc.M311799200
Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, von Wirén N (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem 281(35):25532–25540. https://doi.org/10.1074/jbc.M601062200
Seregin I, Kozhevnikova A, Kazyumina E, Ivanov V (2003) Nickel toxicity and distribution in maize roots. Russ J Plant Physiol 50:711–717. https://doi.org/10.1023/A:1025660712475
Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S (2016) Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci 7:591. https://doi.org/10.3389/fpls.2016.00591
Socha AL, Guerinot ML (2014) Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front Plant Sci 5:106. https://doi.org/10.3389/fpls.2014.00106
Tamás L, Durceková K, Halusková L, Huttová J, Mistrík I, Ollé M (2007) Rhizosphere localized cationic peroxidase from barley roots is strongly activated by cadmium and correlated with root growth inhibition. Chemosphere 66(7):1292–1300. https://doi.org/10.1016/j.chemosphere.2006.07.023
Vatansever R, Ozyigit II, Filiz E (2017) Genome-wide identification and comparative analysis of copper transporter genes in plants. Interdiscip Sci 9(2):278–291. https://doi.org/10.1007/s12539-016-0150-2
Vats S, Bansal R, Rana N, Kumawat S, Bhatt V, Jadhav P, Kale V, Sathe A, Sonah H, Jugdaohsingh R, Sharma TR, Deshmukh R (2022) Unexplored nutritive potential of tomato to combat global malnutrition. Crit Rev Food Sci Nutr 62(4):1003–1034. https://doi.org/10.1080/10408398.2020.1832954
Wang H, Feng T, Peng X, Yan M, Tang X (2009) Up-regulation of chloroplastic antioxidant capacity is involved in alleviation of nickel toxicity of Zea mays L. by exogenous salicylic acid. Ecotoxicol Environ Saf 72(5):1354–1362. https://doi.org/10.1016/j.ecoenv.2009.03.008
Wang M, Gong J, Bhullar NK (2020) Iron deficiency triggered transcriptome changes in bread wheat. Comput Struct Biotechnol J 18:2709–2722. https://doi.org/10.1016/j.csbj.2020.09.009
Wang Y, Xing M, Gao X, Wu M, Liu F, Sun L, Zhang P, Duan M, Fan W, Xu J (2023) Physiological and transcriptomic analyses reveal that phytohormone pathways and glutathione metabolism are involved in the arsenite toxicity response in tomatoes. Sci Total Environ 899:165676. https://doi.org/10.1016/j.scitotenv.2023.165676
Wei K, Jin X, Chen X, Wu F, Zhou W, Qiu B, Qiu L, Wang X, Li C, Zhang G (2009) The effect of H2O2 and abscisic acid (ABA) interaction on beta-amylase activity under osmotic stress during grain development in barley. Plant Physiol Biochem 47(9):778–784. https://doi.org/10.1016/j.plaphy.2009.05.005
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150(2):801–814. https://doi.org/10.1104/pp.109.138230
Xu Z, Shi M, Yu X and Liu M (2022) Heavy metal pollution and health risk assessment of vegetable-soil systems of facilities irrigated with wastewater in Northern China. Int J Environ Res Public Health 19(16). https://doi.org/10.3390/ijerph19169835
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. Bull Environ Contam Toxicol 86(1):1–17. https://doi.org/10.1007/s00128-010-0171-1
Zeng P, Huang F, Guo Z, Xiao X, Peng C (2020) Physiological responses of Morus alba L. in heavy metal(loid)-contaminated soil and its associated improvement of the microbial diversity. Environ Sci Pollut Res Int 27(4):4294–4308. https://doi.org/10.1007/s11356-019-07124-4
Zhao FJ, Ma Y, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49(2):750–759. https://doi.org/10.1021/es5047099
Funding
This research was supported by the China National Natural Sciences Foundation (32070314) to J.X.
Author information
Authors and Affiliations
Contributions
HY: Investigation, Data curation, Formal analysis, Visualization, Writing-original draft, Writing-review & editing; WL: Investigation, Visualization; XL: Investigation; QS: Investigation; JL: Methodology; JX: Writing-review & editing, Supervision, Conceptualization, Methodology, Validation, Supervision, Project administration. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors agree to publish.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Handling Editor: Dr. Huazhong Shi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
Transcriptome analysis. Supplementary Fig. 2. The soft threshold with scale independence (left) and mean connectivity (right). Supplementary Fig. 3. Transcriptome module maps obtained from weighted coexpression network analysis. Supplementary Fig. 4. Integrated network of GO catalogs in the WGCNA modules. Supplementary Fig. 5. Heatmap of DEGs associated with the uptake and accumulation of micronutrients in tomato roots.
Additional file 2: Supplementary Table 1.
DEGs identified in the roots of Ni-treated tomato seedling. Supplementary Table 2. DEGs involved in metal ion transport. Supplementary Table 3. DEGs involved in phytohormone biosynthesis. Supplementary Table 4. DEGs involved in phytohormone signaling pathways. Supplementary Table 5. DEGs involved in antioxidative enzyme biosynthesis. Supplementary Table 6. DEGs involved in carbon/nitrogen metabolism. Supplementary Table 7. List of primers for RT-qPCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, H., Li, W., Liu, X. et al. Physiological and molecular bases of the nickel toxicity responses in tomato. Stress Biology 4, 25 (2024). https://doi.org/10.1007/s44154-024-00162-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-024-00162-0