Abstract
BRI1-ASSOCIATED KINASE 1 (BAK1/SERK3) and its closest homolog BAK1-LIKE 1 (BKK1/SERK4) are leucine-rich repeat receptor kinases (LRR-RKs) belonging to the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family. They act as co-receptors of various other LRR-RKs and participate in multiple signaling events by complexing and transphosphorylating ligand-binding receptors. Initially identified as the brassinosteroid receptor BRASSINOSTEROID INSENSITIVE 1 (BRI1) co-receptor, BAK1 also functions in plant immunity by interacting with pattern recognition receptors. Mutations in BAK1 and BKK1 cause severely stunted growth and cell death, characterized as autoimmune cell death. Several factors play a role in this type of cell death, including RKs and components of effector-triggered immunity (ETI) signaling pathways, glycosylation factors, ER quality control components, nuclear trafficking components, ion channels, and Nod-like receptors (NLRs). The Shan lab has recently discovered a novel RK BAK-TO-LIFE 2 (BTL2) that interacts with BAK1 and triggers cell death in the absence of BAK1 and BKK1. This RK compensates for the loss of BAK1-mediated pattern-triggered immunity (PTI) by activating phytocytokine-mediated immune and cell death responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Main text
Plant pattern recognition receptors (PRRs) are cell surface localized and get stimulated by microbe-associated molecular patterns (MAMPs). This leads to the activation of various defense mechanisms, including the production of reactive oxygen species (ROS), activation of MAP kinases, and enhanced expression of pathogenesis-related proteins. This ultimately results in pattern-triggered immunity (PTI) (Bender and Zipfel 2023). Additionally, intracellular NOD-like immune receptors (NLR) can detect microbial effector proteins or their actions and trigger strong defense responses, also including ROS burst, defense gene activation, and a hypersensitive cell death reaction (HR) referred to as effector-triggered immunity (ETI) (Ngou et al. 2022). Both immune systems are interconnected and potentiate each other resulting in robust immunity (Ngou et al. 2021; Pruitt et al. 2021; Tian et al. 2021; Yuan et al. 2021) (Fig. 1).
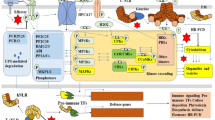
Model of BTL2 function in autoimmune signaling in the absence of BAK1/BKK1 PRR co-receptors. In the absence of BAK1/BKK1, severe autoimmune cell death is activated. The receptor kinase BAK-TO-LIFE 2 (BTL2) can interact with BAK1 and is required for autoimmune cell death in the absence of BAK1 and BKK1. When MAMPs are detected, BAK1/BKK1 dissociate from BIR proteins and are recruited to pattern recognition receptors, activating PTI responses. BTL2 does not contribute to this early process. In the presence of BAK1, BTL2 can be transphosphorylated on residue S676, and BTL2 cell death-inducing capacities are suppressed, suggesting that BAK1/BKK1 negatively regulates autoimmune cell death by suppressing activation of BTL2 by transphosphorylation. Upon perturbation of BAK1/BKK1, either by pathogen effectors like HopB1 or genetic inactivation of these co-receptors, PTI is impaired, but this also results in the activation of BTL2. Autophosphorylation at T669 is required for cell death activation and phosphorylation of CNGC20. Activated BTL2 triggers the activation of CNGC20/CNGC19 calcium channels, leading to Ca2+ influx and subsequent autoimmune cell death. The autoimmune cell death is mediated by ETI components such as NLRs (CSA1, ADR1s, and potentially others), SA, and the lipase-like proteins EDS1 and PAD4. PTI signaling induces phytocytokines recognized by cell surface receptors, amplifying PTI responses and establishing robust plant immunity. These receptors also rely on BAK1 as a co-receptor. The massive production of phytocytokines in the absence of BAK1/BKK1 can initiate a feedback loop, exacerbating the cell death reaction. BTL2 is required for these secondary effects and contributes to SCOOP and PEP-triggered responses, including the novel SCOOPL1 peptide.
The Arabidopsis proteins BRI1-ASSOCIATED RECEPTOR KINASE 1 (BAK1) and BAK1-LIKE 1 (BKK1) are both receptor kinases (RKs) localized on plant plasma membranes. They have crucial roles in plant immunity, developmental processes, and autoimmune responses (Ma et al. 2016). Both proteins act as co-receptors interacting with various leucine-rich repeat receptor kinases (LRR-RKs) such as the brassinosteroid receptor BRASSINOSTEROID INSENSITIVE 1 (BRI1) (Li et al. 2002; Nam and Li 2002), the flagellin receptor FLAGELLIN-SENSING 2 (FLS2)(Chinchilla et al. 2007) and the receptor-like protein-interacting RK SUPPRESSOR OF BIR1 (SOBIR1) (Liebrand et al. 2013; Albert et al. 2015). These interactions are crucial for activating downstream signaling pathways involved in plant immunity and development.
RKs can also perceive endogenous plant peptides that function as danger signals, such as SERINE-RICH ENDOGENOUS PEPTIDE (SCOOP) peptides by MALE DISCOVERER 1-INTERACTING RECEPTOR-LIKE KINASE 2 (MIK2) (Coleman et al. 2021; Hou et al. 2021; Rhodes et al. 2021) and PLANT ELICITOR PEPTIDE (PEP) by the PEP RECEPTORS 1 and 2 (PEPR1 and PEPR2) (Yamaguchi et al. 2006, 2010; Krol et al. 2010). These peptides function as phytocytokines that amplify defense responses in a non-cell-autonomous manner. Upon activation, they also recruit BAK1 as a co-receptor and positive regulator of phytocytokine responses. Therefore, BAK1 is involved in primary and secondary events under natural infection conditions.
BAK1 and BKK1 negatively regulate autoimmune cell death. The proper balance of BAK1 and BKK1 and their associated partners is crucial in regulating cell death (Belkhadir et al. 2012; Halter et al. 2014; Dominguez-Ferreras et al. 2015; Imkampe et al. 2017). Any alterations in the amount of BAK1 or its partners can impact the activation of cell death, indicating that this is a quantitatively controlled process.
This type of cell death involves key components of ETI, including ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1), PHYTOALEXIN DEFICIENT 4 (PAD4), and the ACTIVATED DISEASE RESISTANCE 1 (ADR1) helper NLR family (de Oliveira et al. 2016; Du et al. 2016; Yu et al. 2019a; Wu et al. 2020). These components indicate that an ETI-type surveillance system is monitoring BAK1 and BKK1. CONSTITUTIVE SHADE AVOIDANCE 1 (CSA1), a TIR-domain containing NLR (TNL), has recently been found to play a role in cell death in bak1 bkk1 and bak1 bir3 mutants (Schulze et al. 2022; Yang et al. 2022). It interacts directly with BIR3 and indirectly with BAK1 to sense their integrity. When BAK1 is disrupted by genetic measures or following MAMP or effector activation, CSA1 triggers cell death, suggesting that CSA1 guards BAK1 and initiates ETI-type cell death in the absence or imbalance of BAK1 complexes. Additional components are necessary for bak1 bkk1 cell death, such as nuclear transport components, glycosylation factors, and CYCLIC NUCLEOTIDE GATED ION CHANNELs 20 and 19 (CNGC20/19) (de Oliveira et al. 2016; Du et al. 2016; Gao et al. 2017; Yu et al. 2019a; Wu et al. 2020).
Multiple effector proteins, including HopB1, target BAK1 (Gao et al. 2009). During infection, HopB1 cleaves activated BAK1, resulting in reduced PTI-type defense responses (Li et al. 2016). However, overexpression of HopB1 leads to autoimmune cell death, indicating that perturbation of BAK1 during infection can induce ETI-like autoimmune cell death. HopB1-induced autoimmune responses require the NLR CSA1 and the helper NLR ADR1 family proteins (Wu et al. 2020; Schulze et al. 2022).
BAK1 and BKK1 are closely monitored by BAK1-INTERACTING RECEPTOR 1 (BIR1), BIR2, and BIR3 to ensure appropriate immune responses and prevent unwanted PTI activation in the absence of microbial patterns (Gao et al. 2009; Halter et al. 2014; Imkampe et al. 2017). Loss of BIR1 results in strong autoimmune cell death and loss of BIR2 and BIR3 in weaker autoimmune responses, suggesting that a control system containing additional RKs is necessary to keep BAK1 and BKK1 under control.
In Arabidopsis, BTL2 has been recently identified in an elegant virus-induced gene silencing-based screen of BAK1- and BKK1-silenced plants as a suppressor of autoimmune cell death in BAK1/BKK1 deficient plants (Yu et al. 2023). BTL2 is a cell surface-localized, active non-RD kinase, and kinase activity is required for its function. It belongs to the RK subfamily LRR XI, has 20 LRRs, and is therefore considered a large, likely ligand-binding RK in contrast to the short, only five LRR-containing, not ligand-binding SERK and BIR family receptor kinases (Xi et al. 2019). Whether it is indeed a ligand-binding receptor and what it perceives is an interesting question for the future. Mutants in BTL2 can partially suppress the growth defects and autoimmune phenotypes caused by BAK1 BKK1 silencing or knockout, but none of the tested immune responses are altered in btl2 single mutants. This shows that BTL2 is not involved in primary plant immunity in the presence of BAK1 and BKK1. In their absence, it is required to induce autoimmune cell death. Overexpression of BTL2 leads to cell death in a significant part of the transformants suggesting a quantitative effect of BTL2 on cell death that BAK1 and BKK1 keep under control in their presence. As shown for BAK1 and BIRs, overexpression of BTL2 leads to enhanced cell death, indicating that imbalances in receptor complexes activate autoimmune cell death (Dominguez-Ferreras et al. 2015; Imkampe et al. 2017; Yu et al. 2023). Other types of autoimmune cell death, such as bir1- and MAPK/ERK kinase kinase 1 mutant- (mekk1) induced phenotypes, are not suppressed in the absence of BTL2, suggesting independent pathways for these also BAK1-related components. Quantitative effects in different alleles of bak1 can cause different outcomes in cell death suppression, this might be the case here as well.
As an autoactive kinase, BTL2 autophosphorylates on residue T669. BTL2T669A mutants cannot restore the growth and autoimmune phenotypes in bak1 bkk1 btl2 triple mutants indicating that autophosphorylation is required for its activity. In addition, BAK1 can phosphorylate BTL2 at residues T657 and S676, and a phosphomimetic mutant S676D cannot induce cell death anymore, suggesting that phosphorylation of this residue leads to a negative regulation of BTL2 supporting the idea that BAK1 (and BKK1) negatively regulate BTL2 in their presence.
BTL2 enhances the cell death-inducing capacity of CNGC20, and BTL2-induced cell death is impaired in cngc20 mutants showing that BTL2 can activate CNGC20 in the absence of BAK1/BKK1 and that this activation is necessary to induce autoimmune cell death. BAK1 co-expression with BTL2 and CNGC20 reduces the channel activity, indicating that BTL2 activates and BAK1 suppresses the channel activity (likely via BTL2 phosphorylation). Loss-of-function cngc20 mutants do not show defects in PTI or ETI though a misregulated gain-of-function allele can affect both (Yu et al. 2019b; Zhao et al. 2021). This supports the model that BAK1 keeps BTL2 and the channel activity under control, and BTL2 activates it in the absence of BAK1/BKK1.
Another previously identified suppressor of bak1 bkk1 cell death, STAUROSPORIN AND TEMPERATURE SENSITIVE 3a (STT3a), necessary for membrane protein glycosylation, is also essential for BTL2 and CNGC20 glycosylation, and BTL2 glycosylation-deficient mutants are not functional. This shows that proper membrane protein glycosylation is critical for the function of BTL2 and CNGC20.
Phytocytokines such as SCOOP or PEP peptides are produced upon PTI activation and amplify immune responses (Yamaguchi et al. 2006, 2010; Krol et al. 2010; Coleman et al. 2021; Hou et al. 2021; Rhodes et al. 2021). A number of these signaling peptides are upregulated in bak1 bkk1 mutants. The RK MIK2 mediates SCOOP signaling, and BTL2-induced cell death is reduced in mik2. SCOOP and PEP-induced defense responses and immune outcome are also impaired in bak1 bkk1 btl2 mutants suggesting a role of BTL2 in the amplification and/or perception of phytocytokines in the absence of BAK1/BKK1. This includes a novel SCOOP-LIKE 1 (SCOOPL1) with SCOOP peptide characteristics.
As BTL2 has no impact on immunity in the presence of BAK1 and BKK1, BTL2 likely functions in the amplification loop mediated by phytocytokines, leading to enhanced cell death in bak1 and bak1 bkk1 mutants. This cell death likely impacts the amplification of primary responses by activating CNGC20. Exploration of natural conditions in which BAK1 and BKK1 are modified or depleted, leading to activation of BTL2 and compensation of impaired PTI by activating ETI-type immune responses, will be a fascinating future question (Yamada et al. 2016; Zhou et al. 2019; Schulze et al. 2022).
Taken together, BTL2 can suppress cell death occurring in the absence of BAK1 and BKK1 and is involved in phytocytokine signaling but not in primary PTI responses in the presence of BAK1 and BKK1. Interesting open questions are i.) which additional components are required; ii.) how are the multiple components participating in autoimmune cell death organized in the plant cells and iii.) how primary and secondary events are sorted and regulated. BTL2 is an essential factor of BAK1 surveillance, and it will be interesting to see iv.) whether it perceives phytocytokines directly, as a co-receptor of multiple DAMP receptors or as a scaffold for receptor complexes. v.) Sorting out primary and secondary effects in natural infections and specificities for PRRs and phytocytokines receptors might solve the jigsaw of BAK1/SERK-related receptor complexes in plant immunity.
Availability of data and materials
Not applicable.
Abbreviations
- ADR1:
-
ACTIVATED DISEASE RESISTANCE 1
- BRI1:
-
BRASSINOSTEROID INSENSITIVE 1
- BAK1:
-
BRI1-ASSOCIATED KINASE 1
- BKK1:
-
BAK1-LIKE 1
- BTL2:
-
BAK-TO-LIFE 2
- CNGC20:
-
CYCLIC NUCLEOTIDE GATED ION CHANNEL 20
- CSA1:
-
CONSTITUTIVE SHADE AVOIDANCE 1
- EDS1:
-
ENHANCED DISEASE SUSCEPTIBILITY 1
- ETI:
-
Effector-triggered immunity
- HR:
-
Hypersensitive response
- LRR-RK:
-
Leucine-rich repeat receptor kinase
- MAMP:
-
Microbe-associated molecular patterns
- MAPKs:
-
MITOGEN-ACTIVATED PROTEIN KINASES
- MEKK1:
-
MAPK/ERK KINASE KINASE 1
- MIK2:
-
MALE DISCOVERER 1-INTERACTING RECEPTOR-LIKE KINASE 2
- NLR:
-
Nucleotide-binding domain leucine-rich repeat-containing receptor
- PAD4:
-
PHYTOALEXIN DEFICIENT 4
- PEP:
-
PLANT ELICITOR PEPTIDE
- PRRs:
-
Pattern-recognition receptors
- PTI:
-
Pattern-triggered immunity
- Pto :
-
Pseudomonas syringae Pv. tomato
- RKs:
-
Receptor kinases
- RLPs:
-
Receptor-like proteins
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SCOOP(L1):
-
SERINE-RICH ENDOGENOUS PEPTIDE (LIKE 1)
- SERK:
-
SOMATIC EMBRYOGENESIS-RELATED KINASE
- STT3a:
-
STAUROSPORIN AND TEMPERATURE SENSITIVE 3a
- SOBIR1:
-
SUPPRESSOR OF BIR 1
- TIR:
-
TOLL/INTERLEUKIN-1 RECEPTOR;
- TNLs:
-
TIR NLRs
References
Albert I, Böhm H, Albert M, Feiler CE, Imkampe J, Wallmeroth N, Brancato C, Raaymakers TM, Oome S, Zhang H, Krol E, Grefen C, Gust AA, Chai J, Hedrich R, van den Ackerveken G, Nürnberger T (2015) An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nat Plants https://doi.org/10.1038/nplants.2015.140
Belkhadir Y, Jaillais Y, Epple P, Balsemao-Pires E, Dangl JL, Chory J (2012) Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci USA 109(1):297–302. https://doi.org/10.1073/pnas.1112840108
Bender KW, Zipfel C (2023) Paradigms of receptor kinase signaling in plants. Biochem J 480(12):835–854. https://doi.org/10.1042/BCJ20220372
Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448(7152):497–500. https://doi.org/10.1038/nature05999
Coleman AD, Maroschek J, Raasch L, Takken FLW, Ranf S, Huckelhoven R (2021) The Arabidopsis leucine-rich repeat receptor-like kinase MIK2 is a crucial component of early immune responses to a fungal-derived elicitor. New Phytol 229(6):3453–3466. https://doi.org/10.1111/nph.17122
de Oliveira MV, Xu G, Li B, de Souza Vespoli L, Meng X, Chen X, Yu X, de Souza SA, Intorne AC, de AMAM, Musinsky AL, Koiwa H, de Souza Filho GA, Shan L, He P (2016) Specific control of Arabidopsis BAK1/SERK4-regulated cell death by protein glycosylation. Nature Plants 215218. https://doi.org/10.1038/nplants.2015.218
Dominguez-Ferreras A, Kiss-Papp M, Jehle AK, Felix G, Chinchilla D (2015) An Overdose of the Arabidopsis Coreceptor BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 or Its Ectodomain Causes Autoimmunity in a SUPPRESSOR OF BIR1–1-Dependent Manner. Plant Physiol 168(3):1106–1121. https://doi.org/10.1104/pp.15.00537
Du J, Gao Y, Zhan Y, Zhang S, Wu Y, Xiao Y, Zou B, He K, Gou X, Li G, Lin H, Li J (2016) Nucleocytoplasmic trafficking is essential for BAK1- and BKK1-mediated cell-death control. Plant J 85(4):520–531. https://doi.org/10.1111/tpj.13125
Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, Zhang Y (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6(1):34–44. https://doi.org/10.1016/j.chom.2009.05.019
Gao Y, Wu Y, Du J, Zhan Y, Sun D, Zhao J, Zhang S, Li J, He K (2017) Both light-induced SA accumulation and ETI mediators contribute to the cell death regulated by BAK1 and BKK1. Front Plant Sci 8622. https://doi.org/10.3389/fpls.2017.00622
Halter T, Imkampe J, Mazzotta S, Wierzba M, Postel S, Bücherl C, Kiefer C, Stahl M, Chinchilla D, Wang X, Nürnberger T, Zipfel C, Clouse S, Borst JW, Boeren S, de Vries SC, Tax F, Kemmerling B (2014) The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr Biol 24(2):134–143. https://doi.org/10.1016/j.cub.2013.11.047
Hou S, Liu D, Huang S, Luo D, Liu Z, Xiang Q, Wang P, Mu R, Han Z, Chen S, Chai J, Shan L, He P (2021) The Arabidopsis MIK2 receptor elicits immunity by sensing a conserved signature from phytocytokines and microbes. Nat Commun 12(1):5494. https://doi.org/10.1038/s41467-021-25580-w
Imkampe J, Halter T, Huang S, Schulze S, Mazzotta S, Schmidt N, Manstretta R, Postel S, Wierzba M, Yang Y, van Dongen W, Stahl M, Zipfel C, Goshe MB, Clouse S, de Vries SC, Tax F, Wang X, Kemmerling B (2017) The arabidopsis leucine-rich repeat receptor kinase BIR3 negatively regulates BAK1 receptor complex formation and stabilizes BAK1. Plant Cell 29(9):2285–2303. https://doi.org/10.1105/tpc.17.00376
Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Postel S, Arents M, Jeworutzki E, Al-Rasheid KA, Becker D, Hedrich R (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 285(18):13471–13479. https://doi.org/10.1074/jbc.M109.097394
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110(2):213–222. https://doi.org/10.1016/s0092-8674(02)00812-7
Li L, Kim P, Yu L, Cai G, Chen S, Alfano JR, Zhou JM (2016) Activation-dependent destruction of a co-receptor by a pseudomonas syringae effector dampens plant immunity. Cell Host Microbe 20(4):504–514. https://doi.org/10.1016/j.chom.2016.09.007
Liebrand TW, van den Berg GC, Zhang Z, Smit P, Cordewener JH, America AH, Sklenar J, Jones AM, Tameling WI, Robatzek S, Thomma BP, Joosten MH (2013) Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc Natl Acad Sci USA 110(24):10010–10015. https://doi.org/10.1073/pnas.1220015110
Ma X, Xu G, He P, Shan L (2016) SERKing Coreceptors for Receptors. Trends Plant Sci 21(12):1017–1033. https://doi.org/10.1016/j.tplants.2016.08.014
Nam KH, Li J (2002) BRI1/BAK1, a Receptor Kinase Pair Mediating Brassinosteroid Signaling. Cell 110(2):203–212. https://doi.org/10.1016/s0092-8674(02)00814-0
Ngou BPM, Ahn HK, Ding P, Jones JDG (2021) Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592(7852):110–115. https://doi.org/10.1038/s41586-021-03315-7
Ngou BPM, Jones JDG, Ding P (2022) Plant immune networks. Trends Plant Sci 27(3):255–273. https://doi.org/10.1016/j.tplants.2021.08.012
Pruitt RN, Locci F, Wanke F, Zhang L, Saile SC, Joe A, Karelina D, Hua C, Frohlich K, Wan WL, Hu M, Rao S, Stolze SC, Harzen A, Gust AA, Harter K, Joosten M, Thomma B, Zhou JM, Dangl JL, Weigel D, Nakagami H, Oecking C, Kasmi FE, Parker JE, Nurnberger T (2021) The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 598(7881):495–499. https://doi.org/10.1038/s41586-021-03829-0
Rhodes J, Yang H, Moussu S, Boutrot F, Santiago J, Zipfel C (2021) Perception of a divergent family of phytocytokines by the Arabidopsis receptor kinase MIK2. Nat Commun 12(1):705. https://doi.org/10.1038/s41467-021-20932-y
Schulze S, Yu L, Hua C, Zhang L, Kolb D, Weber H, Ehinger A, Saile SC, Stahl M, Franz-Wachtel M, Li L, El Kasmi F, Nurnberger T, Cevik V, Kemmerling B (2022) The Arabidopsis TIR-NBS-LRR protein CSA1 guards BAK1-BIR3 homeostasis and mediates convergence of pattern- and effector-induced immune responses. Cell Host Microbe 30(12):1717–1731 e1716. https://doi.org/10.1016/j.chom.2022.11.001
Tian H, Wu Z, Chen S, Ao K, Huang W, Yaghmaiean H, Sun T, Xu F, Zhang Y, Wang S, Li X, Zhang Y (2021) Activation of TIR signalling boosts pattern-triggered immunity. Nature 598(7881):500–503. https://doi.org/10.1038/s41586-021-03987-1
Wu Y, Gao Y, Zhan Y, Kui H, Liu H, Yan L, Kemmerling B, Zhou JM, He K, Li J (2020) Loss of the common immune coreceptor BAK1 leads to NLR-dependent cell death. Proc Natl Acad Sci USA 117(43):27044–27053. https://doi.org/10.1073/pnas.1915339117
Xi L, Wu XN, Gilbert M, Schulze WX (2019) Classification and interactions of LRR receptors and co-receptors within the arabidopsis plasma membrane – An overview. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.00472
Yamada K, Yamashita-Yamada M, Hirase T, Fujiwara T, Tsuda K, Hiruma K, Saijo Y (2016) Danger peptide receptor signaling in plants ensures basal immunity upon pathogen-induced depletion of BAK1. EMBO J 35(1):46–61. https://doi.org/10.15252/embj.201591807
Yamaguchi Y, Pearce G, Ryan CA (2006) The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA 103(26):10104–10109. https://doi.org/10.1073/pnas.0603729103
Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA (2010) PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22(2):508–522. https://doi.org/10.1105/tpc.109.068874
Yang Y, Kim NH, Cevik V, Jacob P, Wan L, Furzer OJ, Dangl JL (2022) Allelic variation in the Arabidopsis TNL CHS3/CSA1 immune receptor pair reveals two functional cell-death regulatory modes. Cell Host Microbe 30(12):1701–1716 e1705. https://doi.org/10.1016/j.chom.2022.09.013
Yu X, Xu G, Li B, de Souza Vespoli L, Liu H, Moeder W, Chen S, de Oliveira MVV, Ariadina de Souza S, Shao W, Rodrigues B, Ma Y, Chhajed S, Xue S, Berkowitz GA, Yoshioka K, He P, Shan L (2019a) The Receptor Kinases BAK1/SERK4 Regulate Ca(2+) Channel-Mediated Cellular Homeostasis for Cell Death Containment. Curr Biol 29(22):3778–3790 e3778. https://doi.org/10.1016/j.cub.2019.09.018
Yu X, Xu G, Li B, de Souza Vespoli L, Liu H, Moeder W, Chen S, de Oliveira MVV, Ariadina de Souza S, Shao W, Rodrigues B, Ma Y, Chhajed S, Xue S, Berkowitz GA, Yoshioka K, He P, Shan L (2019b) The receptor kinases BAK1/SERK4 regulate Ca(2+) channel-mediated cellular homeostasis for cell death containment. Curr Biol 29(22):3778–3790 e3778. https://doi.org/10.1016/j.cub.2019.09.018
Yu X, Xie Y, Luo D, Liu H, de Oliveira MVV, Qi P, Kim SI, Ortiz-Morea FA, Liu J, Chen Y, Chen S, Rodrigues B, Li B, Xue S, He P, Shan L (2023) A phospho-switch constrains BTL2-mediated phytocytokine signaling in plant immunity. Cell 186(11):2329–2344 e2320. https://doi.org/10.1016/j.cell.2023.04.027
Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou JM, He SY, Xin XF (2021) Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592(7852):105–109. https://doi.org/10.1038/s41586-021-03316-6
Zhao C, Tang Y, Wang J, Zeng Y, Sun H, Zheng Z, Su R, Schneeberger K, Parker JE, Cui H (2021) A mis-regulated cyclic nucleotide-gated channel mediates cytosolic calcium elevation and activates immunity in Arabidopsis. New Phytol 230(3):1078–1094. https://doi.org/10.1111/nph.17218
Zhou J, Wang P, Claus LAN, Savatin DV, Xu G, Wu S, Meng X, Russinova E, He P, Shan L (2019) Proteolytic processing of SERK3/BAK1 regulates plant immunity, development, and cell death. Plant Physiol 180(1):543–558. https://doi.org/10.1104/pp.18.01503
Acknowledgements
The authors apologize for all publications not mentioned in this highlight article.
Funding
This work was supported by the SFB1101 D03 and the TRR356 B02, funded by the German science foundation DFG.
Author information
Authors and Affiliations
Contributions
H.W., V.F.-M., and B.K. conceived and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
H.W., V.F.-M., and B.K. agree for publication.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Dr. Ping He.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fallahzadeh-Mamaghami, V., Weber, H. & Kemmerling, B. BAK-up: the receptor kinase BAK-TO-LIFE 2 enhances immunity when BAK1 is lacking. Stress Biology 3, 42 (2023). https://doi.org/10.1007/s44154-023-00124-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-023-00124-y