Abstract
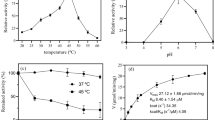
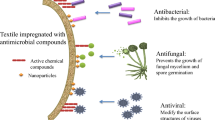
Despite advances achieved in the health field over the last decade, infections caused by resistant bacterial strains are an increasingly important societal issue that needs to be addressed. New approaches have already been developed to overcome this problem. Photodynamic antimicrobial chemotherapy (PACT) could provide a promising alternative method to eradicate microbes. This approach has already inspired the development of innovative surfaces. Interesting results were achieved against Gram-positive bacteria, but it also appeared that Gram-negative strains, especially Pseudomonas aeruginosa, were less sensitive to PACT. However, materials coated with cationic porphyrins have already proven their wide-spectrum activity, but these materials were not suitable for industrial-scale production. The main aim of this work was the design of a large-scale evolutionary material based on PACT and antibiotic prophylaxis. Transparent regenerated cellulose has been simply impregnated with a usual cationic porphyrin (N-methylpyridyl) and an antimicrobial peptide (polymyxin B). In addition to its photophysical properties, this film exhibited a wide-spectrum bactericidal activity over 4 days despite daily application of fresh bacterial inoculums. The efficiency of PACT and polymyxin B combination could help to reduce the emergence of bacterial multi-resistant strains and we believe that this kind of material would provide an excellent opportunity to prevent bacterial contamination of bandages or packaging.
Graphical abstract








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bolognia, J. L., Jorizzo, J. L., & Schaffer, J. V. (2012). Dermatology: 2-Volume set (3rd ed.). Elsevier.
Carrascosa, C., Raheem, D., Ramos, F., Saraiva, A., & Raposo, A. (2021). Microbial biofilms in the food industry—A comprehensive review. International Journal of Environmental Research and Public Health, 18(4), 2014. https://doi.org/10.3390/ijerph18042014
Balikci, E., Yilmaz, B., Tahmasebifar, A., Baran, E. T., & Kara, E. (2021). Surface modification strategies for hemodialysis catheters to prevent catheter-related infections: A review. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 109(3), 314–327. https://doi.org/10.1002/jbm.b.34701
Chouirfa, H., Bouloussa, H., Migonney, V., & Falentin-Daudré, C. (2019). Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomaterialia, 83, 37–54. https://doi.org/10.1016/j.actbio.2018.10.036
Campoccia, D., Montanaro, L., & Arciola, C. R. (2013). A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials, 34(34), 8533–8554. https://doi.org/10.1016/j.biomaterials.2013.07.089
Kim, B. H., Seo, H. S., Jung, S. C., Ohk, S. H., Kim, K. H., Cho, D. L., & Ko, Y. M. (2011). Study in bactericidal properties of chlorhexidine grafting on the modified titanium. Journal of Nanoscience and Nanotechnology, 11(2), 1530–1533. https://doi.org/10.1166/jnn.2011.3314
Wang, S., Yang, Y., Li, W., Wu, Z., Li, J., Xu, K., Zhang, W., Zheng, X., & Chen, J. (2019). Study of the relationship between chlorhexidine-grafted amount and biological performances of micro/nanoporous titanium surfaces. ACS Omega, 4(19), 18370–18380. https://doi.org/10.1021/acsomega.9b02614
Mei, L., Ren, Y., Loontjens, T. J. A., van der Mei, H. C., & Busscher, H. J. (2012). Contact-killing of adhering streptococci by a quaternary ammonium compound incorporated in an acrylic resin. The International Journal of Artificial Organs, 35(10), 854–863. https://doi.org/10.5301/ijao.5000149
Makvandi, P., Jamaledin, R., Jabbari, M., Nikfarjam, N., & Borzacchiello, A. (2018). Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dental Materials, 34(6), 851–867. https://doi.org/10.1016/j.dental.2018.03.014
Zubris, D. L., Minbiole, K. P. C., & Wuest, W. M. (2017). Polymeric quaternary ammonium compounds: Versatile antimicrobial materials. Current Topics in Medicinal Chemistry, 17(3), 305–318.
Huang, Z., Nazifi, S., Cheng, K., Karim, A., & Ghasemi, H. (2021). Scalable inter-diffused zwitterionic polyurethanes for durable antibacterial coatings. Chemical Engineering Journal, 422, 130085. https://doi.org/10.1016/j.cej.2021.130085
Li, D., Wei, Q., Wu, C., Zhang, X., Xue, Q., Zheng, T., & Cao, M. (2020). Superhydrophilicity and strong salt-affinity: Zwitterionic polymer grafted surfaces with significant potentials particularly in biological systems. Advances in Colloid and Interface Science, 278, 102141. https://doi.org/10.1016/j.cis.2020.102141
Stillger, L., & Müller, D. (2022). Peptide-coating combating antimicrobial contaminations: A review of covalent immobilization strategies for industrial applications. Journal of Materials Science, 57(24), 10863–10885. https://doi.org/10.1007/s10853-022-07266-w
Nicolas, M., Beito, B., Oliveira, M., Tudela Martins, M., Gallas, B., Salmain, M., Boujday, S., & Humblot, V. (2022). Strategies for antimicrobial peptides immobilization on surfaces to prevent biofilm growth on biomedical devices. Antibiotics, 11(1), 13. https://doi.org/10.3390/antibiotics11010013
Rizwan, M., Alias, R., Zaidi, U. Z., Mahmoodian, R., & Hamdi, M. (2018). Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: A review. Journal of Biomedical Materials Research. Part A, 106(2), 590–605. https://doi.org/10.1002/jbm.a.36259
Birkett, M., Dover, L., Cherian Lukose, C., Wasy Zia, A., Tambuwala, M. M., & Serrano-Aroca, Á. (2022). Recent advances in metal-based antimicrobial coatings for high-touch surfaces. International Journal of Molecular Sciences, 23(3), 1162. https://doi.org/10.3390/ijms23031162
Zainul Armir, N. A., Zulkifli, A., Gunaseelan, S., Palanivelu, S. D., Salleh, K. M., Che Othman, M. H., & Zakaria, S. (2021). Regenerated cellulose products for agricultural and their potential: A review. Polymers, 13(20), 3586. https://doi.org/10.3390/polym13203586
Tu, H., Li, X., Liu, Y., Luo, L., Duan, B., & Zhang, R. (2022). Recent progress in regenerated cellulose-based fibers from alkali/urea system via spinning process. Carbohydrate Polymers, 296, 119942. https://doi.org/10.1016/j.carbpol.2022.119942
Trache, D., Tarchoun, A. F., Derradji, M., Hamidon, T. S., Masruchin, N., Brosse, N., & Hussin, M. H. (2020). Nanocellulose: From fundamentals to advanced applications. Frontiers in Chemistry. https://doi.org/10.3389/fchem.2020.00392
Borbély, É. L. (2008). The new generation of regenerated cellulose. Acta Polytechnica Hungarica, 5(3), 11–18.
Vatansever, F., de Melo, W. C. M. A., Avci, P., Vecchio, D., Sadasivam, M., Gupta, A., Chandran, R., Karimi, M., Parizotto, N. A., Yin, R., Tegos, G. P., & Hamblin, M. R. (2013). Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiology Reviews, 37(6), 955–989. https://doi.org/10.1111/1574-6976.12026
Wang, K.-K., Song, S., Jung, S.-J., Hwang, J.-W., Kim, M.-G., Kim, J.-H., Sung, J., Lee, J.-K., & Kim, Y.-R. (2020). Lifetime and diffusion distance of singlet oxygen in air under everyday atmospheric conditions. Physical Chemistry Chemical Physics: PCCP, 22(38), 21664–21671. https://doi.org/10.1039/D0CP00739K
Fu, X., Fang, Y., & Yao, M. (2013). Antimicrobial photodynamic therapy for methicillin-resistant staphylococcus aureus infection. BioMed Research International, 2013, 1–9. https://doi.org/10.1155/2013/159157
Alves, E., Costa, L., Carvalho, C. M., Tomé, J. P., Faustino, M. A., Neves, M. G., Tomé, A. C., Cavaleiro, J. A., Cunha, Â., & Almeida, A. (2009). Charge effect on the photoinactivation of gram-negative and gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiology, 9, 70. https://doi.org/10.1186/1471-2180-9-70
Le Guern, F., Sol, V., Ouk, C., Arnoux, P., Frochot, C., & Ouk, T.-S. (2017). Enhanced photobactericidal and targeting properties of a cationic porphyrin following the attachment of polymyxin B. Bioconjugate Chemistry, 28(9), 2493–2506. https://doi.org/10.1021/acs.bioconjchem.7b00516
Dosselli, R., Tampieri, C., Ruiz-González, R., De Munari, S., Ragàs, X., Sánchez-García, D., Agut, M., Nonell, S., Reddi, E., & Gobbo, M. (2013). Synthesis, characterization, and photoinduced antibacterial activity of porphyrin-type photosensitizers conjugated to the antimicrobial peptide apidaecin 1b. Journal of Medicinal Chemistry, 56(3), 1052–1063. https://doi.org/10.1021/jm301509n
Gourlot, C., Gosset, A., Glattard, E., Aisenbrey, C., Rangasamy, S., Rabineau, M., Ouk, T.-S., Sol, V., Lavalle, P., Gourlaouen, C., Ventura, B., Bechinger, B., & Heitz, V. (2022). Antibacterial photodynamic therapy in the near-infrared region with a targeting antimicrobial peptide connected to a π-extended porphyrin. ACS Infectious Diseases, 8(8), 1509–1520. https://doi.org/10.1021/acsinfecdis.2c00131
Bellin, J. S., Lutwick, L., & Jonas, B. (1969). Effects of photodynamic action on E. coli. Archives of Biochemistry and Biophysics, 132(1), 157–164. https://doi.org/10.1016/0003-9861(69)90348-8
Krouit, M., Granet, R., Branland, P., Verneuil, B., & Krausz, P. (2006). New photoantimicrobial films composed of porphyrinated lipophilic cellulose esters. Bioorganic & Medicinal Chemistry Letters, 16(6), 1651–1655. https://doi.org/10.1016/j.bmcl.2005.12.008
Krouit, M., Granet, R., & Krausz, P. (2008). Photobactericidal plastic films based on cellulose esterified by chloroacetate and a cationic porphyrin. Bioorganic & Medicinal Chemistry, 16(23), 10091–10097. https://doi.org/10.1016/j.bmc.2008.10.010
Ringot, C., Saad, N., Granet, R., Bressollier, P., Sol, V., & Krausz, P. (2010). Meso-functionalized aminoporphyrins as efficient agents for photo -antibacterial surfaces. Journal of Porphyrins and Phthalocyanines, 14, 926–931. https://doi.org/10.1142/S1088424610002719
Feese, E., Sadeghifar, H., Gracz, H. S., Argyropoulos, D. S., & Ghiladi, R. A. (2011). Photobactericidal porphyrin-cellulose nanocrystals: Synthesis, characterization, and antimicrobial properties. Biomacromolecules, 12(10), 3528–3539.
Chen, W., Chen, J., Li, L., Wang, X., Wei, Q., Ghiladi, R. A., & Wang, Q. (2019). Wool/acrylic blended fabrics as next-generation photodynamic antimicrobial materials. ACS Applied Materials & Interfaces, 11, 29557–29568.
Jiang, C., Dejarnette, S., Chen, W., Scholle, F., Wang, Q., & Ghiladi, R. A. (2023). Color-variable dual-dyed photodynamic antimicrobial polyethylene terephthalate (PET)/cotton blended fabrics. Photochemical & Photobiological Sciences, 22, 1573–1590. https://doi.org/10.1007/s43630-023-00398-1
Grammatikova, N. A., George, L., Ahmed, Z., Candeias, N. R., Durandin, N. A., & Efimov, A. (2019). Zinc phthalocyanine activated by conventional indoor light makes a highly efficient antimicrobial material from regular cellulose. Journal of Materials Chemistry B, 7, 4379–4384. https://doi.org/10.1039/c9tb01095e
Carpenter, B. L., Scholle, F., Sadeghifar, H., Francis, A. J., Boltersdorf, J., Weare, W. W., Argyropoulos, D. S., Maggard, P. A., & Ghiladi, R. A. (2015). Synthesis, characterization, and antimicrobial efficacy of photomicrobicidal cellulose paper. Biomacromolecules, 16, 2482–2492.
Dong, J., Ghiladi, R. A., Wang, Q., Cai, Y., & Wei, Q. (2018). Protoporphyrin-IX conjugated cellulose nanofibers that exhibit. High antibacterial photodynamic inactivation efficacy. Nanotechnology, 29, e265601.
Bonnett, R., & Galia, A. (1994). Photobactericidal films based on regenerated cellulose. Biotechnology and Biotechnological Equipment, 8(1), 68–74. https://doi.org/10.1080/13102818.1994.10818756
Yin, R., & Hamblin, M. (2015). Antimicrobial photosensitizers: Drug discovery under the spotlight. Current Medicinal Chemistry, 22(18), 2159–2185. https://doi.org/10.2174/0929867322666150319120134
Zavascki, A. P., Goldani, L. Z., Li, J., & Nation, R. L. (2007). Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. Journal of Antimicrobial Chemotherapy, 60(6), 1206–1215. https://doi.org/10.1093/jac/dkm357
Velkov, T., Thompson, P. E., Nation, R. L., & Li, J. (2010). Structure−activity relationships of polymyxin antibiotics. Journal of Medicinal Chemistry, 53(5), 1898–1916. https://doi.org/10.1021/jm900999h
Mohorčič, M., Jerman, I., Zorko, M., Butinar, L., Orel, B., Jerala, R., & Friedrich, J. (2010). Surface with antimicrobial activity obtained through silane coating with covalently bound polymyxin B. Journal of Materials Science. Materials in Medicine, 21(10), 2775–2782. https://doi.org/10.1007/s10856-010-4136-z
Nitzan, Y., Gutterman, M., Malik, Z., & Ehrenberg, B. (1992). Inactivations of gram-negative bacteria by photosensitized porphyrins. Photochemistry and Photobiology, 55(1), 89–96.
Le Guern, F., Ouk, T.-S., Grenier, K., Joly, N., Lequart, V., & Sol, V. (2017). Enhancement of photobactericidal activity of chlorin-e6-cellulose nanocrystals by covalent attachment of polymyxin B. J Mater Chem B, 5(33), 6953–6962. https://doi.org/10.1039/C7TB01274H
Schweizer, H. P. (2003). Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genetics and Molecular Research, 2(1), 48–62.
Pamp, S. J., Gjermansen, M., Johansen, H. K., & Tolker-Nielsen, T. (2008). Tolerance to the antimicrobial peptide colistin in pseudomonas aeruginosa biofilms is linked to metabolically active cells, and Depends on the Pmr and mexAB-oprM Genes. Molecular Microbiology, 68(1), 223–240. https://doi.org/10.1111/j.1365-2958.2008.06152.x
Padilla, E., Llobet, E., Doménech-Sánchez, A., Martínez-Martínez, L., Bengoechea, J. A., & Albertí, S. (2010). Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrobial Agents and Chemotherapy, 54(1), 177–183. https://doi.org/10.1128/AAC.00715-09
Stallivieri, A., Le Guern, F., Vanderesse, R., Meledje, E., Jori, G., Frochot, C., & Acherar, S. (2015). Synthesis and photophysical properties of the photoactivatable cationic porphyrin 5-(4-N-dodecylpyridyl)-10,15,20-tri(4-N-methylpyridyl)-21H,23H-porphyrin tetraiodide for anti-malaria PDT. Photochemical & Photobiological Sciences, 14(7), 1290–1295. https://doi.org/10.1039/C5PP00139K
Gouterman, M., Wagnière, G. H., & Snyder, L. C. (1963). Spectra of porphyrins. Journal of Molecular Spectroscopy, 11(1), 108–127. https://doi.org/10.1016/0022-2852(63)90011-0
Juhász, M. L., Levin, M. K., & Marmur, E. S. (2017). A review of available laser and intense light source home devices: A dermatologist’s perspective. Journal of Cosmetic Dermatology, 16(4), 438–443. https://doi.org/10.1111/jocd.12371
O’Neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations. May 2016.
Carpenter, B. L., Feese, E., Sadeghifar, H., Argyropoulos, D. S., & Ghiladi, R. A. (2012). Porphyrin-cellulose nanocrystals: A photobactericidal material that exhibits broad spectrum antimicrobial activity. Photochemistry and Photobiology, 88(3), 527–536.
Anaya-Plaza, E., van de Winckel, E., Mikkilä, J., Malho, J.-M., Ikkala, O., Gulías, O., Bresolí-Obach, R., Agut, M., Nonell, S., Torres, T., Kostiainen, M. A., & de la Escosura, A. (2017). Photoantimicrobial biohybrids by supramolecular immobilization of cationic phthalocyanines onto cellulose nanocrystals. Chemistry: A European Journal. https://doi.org/10.1002/chem.201605285
Perni, S., Piccirillo, C., Kafizas, A., Uppal, M., Pratten, J., Wilson, M., & Parkin, I. P. (2010). Antibacterial activity of light-activated silicone containing methylene blue and gold nanoparticles of different sizes. Journal of Cluster Science, 21(3), 427–438. https://doi.org/10.1007/s10876-010-0319-5
Le Guern, F., Ouk, T.-S., Yerzhan, I., Nurlykyz, Y., Arnoux, P., Frochot, C., Leroy-Lhez, S., & Sol, V. (2021). Photophysical and bactericidal properties of pyridinium and imidazolium porphyrins for photodynamic antimicrobial chemotherapy. Molecules, 26(4), 1122. https://doi.org/10.3390/molecules26041122
Vergeldt, F. J., Koehorst, R. B., van Hoek, A., & Schaafsma, T. J. (1995). Intramolecular interactions in the ground and excited states of tetrakis (N-methylpyridyl) porphyrins. Journal of Physical Chemistry, 99(13), 4397–4405.
Seliger, H. H. (1964). Chemiluminescence of H2O2–NaOCl solutions. The Journal of Chemical Physics, 40(10), 3133–3134. https://doi.org/10.1063/1.1724975
Taylor, R. B., Richards, R. M. E., Low, A. S., & Hardie, L. (1994). Chemical stability of polymyxin b in aqueous solution. International Journal of Pharmaceutics, 102, 201–206.
Reddi, E., Ceccon, M., Valduga, G., Jori, G., Bommer, J. C., Elisei, F., Latterini, L., & Mazzucato, U. (2002). Photophysical properties and antibacterial activity of meso-substituted cationic porphyrins. Photochemistry and Photobiology, 75(5), 462–470. https://doi.org/10.1562/0031-8655(2002)075%3c0462:PPAAAO%3e2.0.CO;2
Ringot, C., Saad, N., Brégier, F., Bressollier, P., Poli, E., Chaleix, V., Ouk, T.-S., & Sol, V. (2018). Antibacterial activity of a photosensitive hybrid cellulose fabrics. Photochemical & Photobiological Sciences, 17(11), 1780–1786. https://doi.org/10.1039/c8pp00212f
Kumar, A., & Ting, Y. P. (2015). Presence of pseudomonas aeruginosa influences biofilm formation and surface protein expression of Staphylococcus aureus: Bacterial biofilm formation in co-culture. Environmental Microbiology, 17(11), 4459–4468. https://doi.org/10.1111/1462-2920.12890
Acknowledgements
The authors thank the “Conseil Regional du Limousin” for financial support (FLG) and are indebted to Dr. Michel Guilloton for manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Guern, F., Ouk, TS., Arnoux, P. et al. Easy and versatile cellulosic support inhibiting broad spectrum strains: synergy between photodynamic antimicrobial therapy and polymyxin B. Photochem Photobiol Sci 23, 395–407 (2024). https://doi.org/10.1007/s43630-023-00526-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00526-x