Abstract
Neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and multiple sclerosis affect millions of people around the world. In addition to age, which is a key factor contributing to the development of all neurodegenerative diseases, genetic and environmental components are also important risk factors. Current methods of treating neurodegenerative diseases are mostly symptomatic and do not eliminate the cause of the disease. Many studies focus on searching for natural substances with neuroprotective properties that could be used as an adjuvant therapy in the inhibition of the neurodegeneration process. These compounds include flavonoids, such as luteolin, showing significant anti-inflammatory, antioxidant, and neuroprotective activity. Increasing evidence suggests that luteolin may confer protection against neurodegeneration. In this review, we summarize the scientific reports from preclinical in vitro and in vivo studies regarding the beneficial effects of luteolin in neurodegenerative diseases. Luteolin was studied most extensively in various models of Alzheimer’s disease but there are also several reports showing its neuroprotective effects in models of Parkinson’s disease. Though very limited, studies on possible protective effects of luteolin against Huntington’s disease and multiple sclerosis are also discussed here. Overall, although preclinical studies show the potential benefits of luteolin in neurodegenerative disorders, clinical evidence on its therapeutic efficacy is still deficient.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases are a heterogeneous group of chronic, usually age-related disorders that are characterized by the progressive loss of neurons, deformation of the neuron structure, or loss of neuron function [1]. They remain a major focus of scientific and clinical interest due to their increasing medical and social importance. Majority of the neurodegenerative diseases are characterized by the aggregation of intracellular proteins or their extracellular depositions (plaques) such as α-synuclein in Parkinson’s disease (PD), amyloid beta (Aβ)/tau aggregates in Alzheimer’s disease (AD), superoxide dismutase 1 (SOD 1) in amyotrophic lateral sclerosis (ALS), or huntingtin protein with tandem glutamine repeats in Huntington’s disease (HD) [2]. However, the etiology and exact pathogenesis of these devastating diseases is largely unknown.
The cause of most neurodegenerative diseases is considered as a combination of genetic and environmental factors. Both early and late-onset AD are dominated by genetic mutations on chromosome 1, 14, 21, and chromosome 19q13, respectively [3]. Similarly in PD, autosomal dominant genetic mutations such as leucine-rich repeat kinase 2 (LRRK2) and autosomal recessive mutations in the parkin genes (PRKN and PINK1) are common [4, 5]. Mutations at the HD gene include multiple repeats of the CAG repeat sequence [6]. Environmental factors associated with neurodegeneration are diet, lifestyle, and chemical exposures such as metals mercury, cadmium, inorganic arsenic, and pesticides (e.g., deltamethrin, paraquat, dieldrin and rotenone) [3, 7], whereas environmental factors that have been shown to reduce the incidence of neurodegeneration are nicotine, caffeine, statins, post-menopausal hormonal treatment and non-steroidal anti-inflammatory drugs such as ibuprofen [3, 8].
Although neurodegeneration is tightly bound with the concept of protein aggregation, it is not the sole contributor. Oxidative stress, neuroinflammation, programmed cell death, and abnormalities in the ubiquitin-proteasomal and autophagosomal/lysosomal systems are some key factors that can lead to neuronal cell death [9]. Briefly, oxidative stress is described as a condition where the natural antioxidant machinery in the body cannot overcome the increasing generation of reactive oxygen species (ROS) such as hydroxyl, alkoxyl, peroxyl, and hydrogen peroxide anions. The generation of ROS can be the result of various events such as free radical generation in the substantia nigra, abnormal metabolism of dopamine, abnormalities in the respiratory chain of the mitochondria, and increased production of Fe2+ as a result of Fenton’s reaction. Additionally, nitrosative stress can take place due to the generation of peroxynitrite from radical nitric oxide (NO) in the absence of antioxidant systems. The low availability of antioxidant enzymes (low SOD, glutathione peroxidase, and catalase) can make a cell vulnerable to oxidative stress [10, 11]. Neuroinflammation is a natural defense mechanism against pathogens; however prolonged neuroinflammation can be detrimental as it leads to neuronal death. Microglia and astrocytes are the two main types of cells associated with neuroinflammation. They produce pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, -16, and chemokines, including the C-C motif chemokine ligand 2 (CCL2) and IL-18 to initiate neuroinflammation. The triggers that lead to the loss of neuroprotective functions and gain of neurotoxic functions are not exactly known but may depend on a variety of factors such as the stage and severity of neurodegeneration [12]. Programmed cell death is another area that is closely monitored with respect to neurodegeneration. A defect in the mechanism that otherwise only eliminates damaged or old populations of cells, may start a cascade of events that leads to the death of healthy neuronal cell populations. A group of cell stressors leading to axonal degeneration such as the c-Jun N-terminal kinase (JNK) pathway is a key event [13, 14]. Finally, it has been shown that the activity of the ubiquitin-proteasome system and the autophagy-lysosomal pathway declines, two of the major catabolic pathways of eukaryotic cells, progressively declines with aging. As a consequence, cells become overloaded with deleterious proteins contributing to the development of age-related diseases such as AD or PD [15, 16].
Thus, neurodegenerative diseases are multifactorial conditions involving various pathological mechanisms, and accordingly, they require complex therapeutic approaches. Most of the currently available drugs work symptomatically, without affecting the underlying cause of the disease [1]. Moreover, long-term use of these medications frequently results in significant adverse effects and a decrease in therapeutic efficacy. The development of new treatment strategies for most progressive neurodegenerative diseases is however challenging due to their above-mentioned multi-factorial pathogenesis and diverse disease course [17]. Plants have been valuable source of medicines for centuries and bioactive phytochemicals continue to represent a very attractive research field [18].
Luteolin (3′,4′,5,7-tetrahydroxyflavone; C15H10O6) belongs to a family of naturally occurring secondary metabolites called flavonoids characterized by a diphenylpropane structure (C6-C3-C6) [19], more specifically to the sub-group of flavonoids categorized as flavones. Structurally, it is a 15 C flavone with 2 benzene rings and one oxygen-containing ring arranged in the typical flavonoid structure mentioned above. As a tetrahydroxyflavone, luteolin has two hydroxyl groups on each of the two benzene rings [20, 21]. It was first isolated from a flowering plant called, Reseda odorata L. as a traditional medicinal potion in parts of Asia [22]. It is commonly found in vegetables from the Apiaceae family such as carrots (37.5 mg/kg dry weight), dried parsley (19.75 mg/100 g), and other vegetables including broccoli (74.5 mg/kg), green chilly (33.0 mg/kg), spinach (1.11 mg/100 g), and cabbage (0.4–0.6 mg/100 g). Herbs from the family of Lamiaceae, e.g., thyme (51 mg/100 g), fresh peppermint (11.33 mg/100 g), and others such as Perilla frutescem can be great sources of luteolin as well [21, 23]. Moreover, chrysanthemum flowers are consumed in the form of herbal teas and processed foods in East Asia as a source of antioxidants. The source of this antioxidant activity stems from the presence of luteolin in the flowers (1 mg of luteolin/100 mg of flowers) [24].
Luteolin occurs in the form of either aglycone or glucosides but glycosylated forms are more common. Its bioavailability was reported to be between 4.1% and 26% [20]. In the intestines, luteolin aglycone is absorbed into enterocytes by diffusion as it is lipophilic, whereas luteolin glycosides are converted first into luteolin aglycones. In the enterocytes and/or hepatocytes, luteolin is extensively metabolized into luteolin glucuronides and/or luteolin sulfates [20, 25]. Luteolin-3′-O-β-d-glucuronide and luteolin-3′-O-sulfate were identified as the most abundant metabolites in rat and human plasma, respectively [26]. After oral intake in rats, luteolin and its metabolites have been mostly distributed in the gastrointestinal tract, liver, kidneys, and lungs and biliary excretion was the main elimination pathway of the conjugated luteolin [25]. It has been demonstrated that luteolin given intraperitoneally (ip) to mice can readily cross the blood-brain barrier (BBB) and enter the brain [27]. To increase the bioavailability of luteolin, several delivery methods have been developed; the most thoroughly studied include lipid carriers like liposomes and nanoformulations [21].
Numerous studies show that luteolin possesses various health-beneficial properties including anti-apoptotic, anti-oxidant, anti-inflammatory, and neuroprotective effects. Consequently, it has gained considerable interest as a potential therapeutic agent in many disorders such as cancer and central nervous system (CNS) disorders including neurodegenerative diseases, traumatic brain injury, stroke epilepsy, schizophrenia, autism, and depression (Fig. 1) [11, 20, 21, 28,29,30,31,32]. In this review, we aimed to summarize the current knowledge on the potential beneficial effects of luteolin in four neurodegenerative diseases. We review and discuss data from both in vitro and in vivo preclinical experiments highlighting the potential mechanisms by which luteolin may confer neuroprotection in the aforementioned diseases.
Mechanisms of protective action of luteolin in neurodegenerative diseases
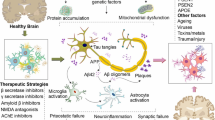
Luteolin can exhibit beneficial effects in neurodegenerative disorders through various mechanisms including suppression of inflammatory processes, oxidative stress, and apoptosis. Moreover, some evidence shows that luteolin may reduce formation of Aβ plaques and enhance neuronal growth [33].
Neuroinflammation is one of the major hallmarks of neurodegeneration; therefore therapeutics with anti-inflammatory effects are beneficial in the treatment of these diseases. Scientists have studied the structure of luteolin to find evidence for its anti-inflammatory activities [34]. As a flavonoid, luteolin’s strength lies in the number of hydroxyl groups attached to the A and B rings. To be more precise, in the 5′ and 7′ positions of the A ring and 3′ and 4′ of the B ring. Many studies have been conducted to investigate the anti-inflammatory effects of luteolin [35,36,37,38,39,40]. Luteolin mediates anti-inflammatory responses by suppressing microglia and astrocytes and their downstream targets such as pattern recognition receptors; toll-like receptors 2 and 4 (TLR2 and TLR4) [41]. Luteolin has exerted its anti-inflammatory effect by inhibiting pro-inflammatory mediators such as cyclooxygenase-2 (COX-2), nitric oxide (NO), TNF-α, IL-β, IL-6, IL-8, IL-31, and IL-33 in several in vitro models of AD [42], PD [43, 44], MS [45], and in vivo models of AD [27, 46]. The inflammatory response produced by our immune systems is a well-coordinated mechanism including several pathways. Among them, the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinase/activator protein-1 (MAPK/AP-1), and the Janus kinase signal transducer and activator of transcription (JAK-STAT) pathways have been identified as the targets of luteolin [40, 46,47,48,49,50,51,52]. The inhibition of the NF-кB pathway leads to the inhibition of a downstream target– β-site amyloid precursor protein cleaving enzyme (BACE1), which is a key mediator in forming Aβ fibrils in AD pathology [48, 53].
Luteolin exerts an anti-oxidant activity mainly by reducing ROS levels and increasing SOD activity in in vitro models of AD [54,55,56]. Luteolin can also increase the expression of antioxidant enzymes such as heme oxygenase-1 (HO-1) via the nuclear factor erythroid 2–related factor 2/ antioxidant responsive element (Nrf-2/ARE) complex activation, as it was observed in in vitro models of PD [44, 57] and H2O2-induced toxicity in in vitro models [58,59,60].
The anti-apoptotic effects of luteolin can be mediated via several mechanisms such as reducing the levels of caspase-3 and − 9 and improving the B-cell lymphoma protein 2/Bcl-2-associated X protein (Bcl-2/Bax) ratio, as it was reported in in vitro models of AD [61] and PD [62], as well as in vivo models of AD [63, 64] and PD [65, 66]. Among other flavones, luteolin exhibits the strongest cytoprotective activity by reducing the level of caspase-3 and PARP-1 activation and enhancing the unfolded protein response (UPR) pathway, leading to an increase in endoplasmic reticulum (ER) chaperone GRP78 and a decrease in the expression of UPR-targeted pro-apoptotic genes via the MAPK pathway. Additionally, a significant reduction in the p53 transcription factors such as p21, PUMA, and GADD45α were observed [62, 67, 68].
Luteolin also exerts neuroprotective effects by preventing Aβ-induced cell death via an anti-oxidant mechanism and upregulation of ER/ERK/MAPK signaling pathway in in vitro models of AD [55, 61, 69, 70]. There is also evidence that suggests that luteolin can directly influence the formation of Aβ plaques by selectively inhibiting the activity of N-acetyl-α-galactosaminyltransferase (ppGalNAc-T) isoforms [71] and in in vivo models of AD by selectively binding to Aβ fibrils, inactivating the glycogen synthase kinase-3 alpha (GSK-3α) isoform, suppressing Aβ and promoting tau disaggregation [27, 48, 63, 72, 73].
Additionally, luteolin has also a positive effect on neuronal growth and maintenance. Lin et al. [74] found that luteolin dose-dependently promoted the growth and differentiation of neurons both in terms of number and length and the up-regulation of growth-associated protein-43(GAP-43 expression) via the ERK-dependent pathway in PC12 cells. In a similar study, luteolin promoted phosphorylation and activation of cAMP response element-binding protein (CREB) leading to the increased miR-132 expression, and eventually neurite outgrowth in PC12 cells via MAPK/ERK- and cAMP-dependent protein kinase A (PKA) pathways [75].
More details on the possible mechanisms underlying the protective effects of luteolin in various in vitro and in vivo models of neurodegenerative diseases are provided in the next sections and summarized in Figs. 2 and 3.
Schematic overview of the possible mechanisms underlying the neuroprotective effects of luteolin
Abbreviations: BBB: blood-brain barrier; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma protein 2; cAMP: cyclic adenosine monophosphate; ER: endoplasmic reticulum; ERK: extracellular signal-regulated kinase; ERK-dependent CREB: extracellular signal-regulated kinase-dependent cAMP response element-binding protein; FITC; fluorescein isothiocyanate; IRF-1: interferon regulatory factor 1; MAPK: mitogen-activated protein kinase; NaF: sodium fluoride; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PKA; protein kinase A; ROS: reactive oxygen species; SOD: superoxide dismutase; STAT: signal transducer and activator of transcription; TEER: transepithelial electrical resistance
A summary of the effect of luteolin on the prevention of apoptosis in the 6-OHDA-indcued model of PD in vitro
Abbreviations: ATF4: activating transcription factor 4; BIM: Bcl-2-like protein 11; CHOP: C/EBP homologous protein; ER: endoplasmic reticulum; GRP78: glucose-regulated protein 78; HO-1: heme oxygenase-1; p53: tumor protein p53; TRB3: tribbles homolog 3
Preclinical studies on luteolin in neurodegenerative diseases
Luteolin and Alzheimer’s disease
AD is the most common type of neurodegenerative disease and is responsible for the majority of dementia cases (60–80%) [1, 76]. The disease is progressive and it is marked by a gradual decline in memory, along with cognitive and executive dysfunction [76]. The pathology identified is Aβ peptide’s accumulation in the medial temporal lobe and neocortical structures of the brain resulting in neuritic plaques and neurofibrillary tangles [77]. It is estimated that more than 47 million individuals suffer from dementia globally, and the figure is expected to increase to 150 million by 2050 [78, 79]. AD can be classified into two types based on the age of onset. The first type is early-onset AD (EOAD), which is a rare form of the disease that accounts for around 1–6% of cases. EOAD typically affects people between the age group of 30–65 and is usually familial [77]. EOAD mutations consist of three primary autosomal dominant mutations; found on 3 different loci, the amyloid precursor protein (APP) gene on chromosome 21 which encodes for the amyloid precursor protein from which Aβ is derived, PSEN1 on chromosome 14 and PSEN2 gene on chromosome 1, the latter two genes encode the presenilin 1 and 2 subunits of the γ-secretase enzyme which catalyzes the cleavage of APP [80]. The second type is late-onset AD (LOAD), which is more common and typically affects people above the age of 65 [77]. The causes of LOAD are still unknown, but several factors have been shown to modify the risk of developing LOAD, such as environmental, lifestyle, ethnic, socioeconomic, and genetic factors. Both EOAD and LOAD can occur in families with a positive history of AD [79].
The two primary pathological features of end-stage AD are Aβ plaques and neurofibrillary tangles (NFTs). Amyloid plaques consist of aggregated misfolded Aβ protein that assembles from monomers into oligomers and fibrillar species which accumulate in the extracellular space [81]. In contrast, NFTs are made up of hyperphosphorylated tau protein and are formed within neuronal cell bodies and axons which can directly spread to other parts of the brain. These pathological changes begin to occur many years before onset of cognitive impairment and lead to significant neuronal loss in the hippocampal area and neocortex. NFTs are directly linked to progressive neuronal death, whereas Aβ species’ presence and accumulation are associated with other critical aspects of AD pathology as well, for example, soluble Aβ oligomers can cause mitochondrial and ER dysfunction, resulting in the overproduction of ROS, oxidative stress, and neuronal damage. Aβ oligomers and fibrils can also hinder functional synaptic connections and activity, which is believed to be a significant factor in cognitive decline, especially in the prodromal and early stages of AD [79]. The prevailing hypothesis is that the accumulation of Aβ peptide triggers a cascade of events that leads to tau hyperphosphorylation, tangle formation, neuronal dysfunction, and finally symptom development [82]. The amyloid cascade hypothesis which is based on the molecular defects observed in autosomal-dominant EOAD has been severely criticized due to the unsolved paradox: ‘Why do Aβ aggregate into fibrils?’, but it is clear that the Aβ sequence, Aβ concentration, and conditions that destabilize Aβ are important factors in aggregation [83, 84].
As discussed before, AD is a multifactorial condition and therefore numerous factors (both hereditary and lifestyle) apart from Aβ accumulation itself can contribute to or pose a risk to the development of this neurodegenerative disease [85, 86]. As the more common form is LOAD (99%), most research goes into combating the non-hereditary causes of AD. Oxidative stress, glucose metabolism, lipid metabolism, and inflammation are the main non-hereditary factors leading to the development of AD. Among all the mentioned, inflammation seems to have a heavy implication on the pathogenesis and progression of AD [86] and luteolin with its potent anti-inflammatory properties may produce beneficial effects in AD.
Prominent inflammation in AD has been observed through increased levels of proinflammatory cytokines, specifically TNF-α and IL-6, in both serum and brain tissue of AD patients compared to controls. Several studies have demonstrated the presence of immune cells and related proteins near Aβ plaques [87]. Latest clinical studies have reported higher expression of markers of astrogliosis and microgliosis, as well as of both peripheral and central neuroinflammation, in young AD cases as compared with old ones, indicating that all these phenomena are prominent at the earliest AD stage, and can even decrease with aging. These astonishing results have prompted scientists to rethink and approach different therapeutic targets [88].
Possible neuroprotective effects of luteolin have been studied in various preclinical models of AD. Firstly, a few in vitro studies investigated the effect of luteolin on the Aβ-induced toxicity. For example, luteolin was found to inhibit Aβ25–35-induced cell death and oxidative stress in rat cerebral microvascular endothelial cells and [89] and mouse cortical cells [69]. The protective effects of luteolin against Aβ25−35-induced toxicity have also been thoroughly investigated and confirmed by Wang et al. [61], who demonstrated that luteolin inhibited Aβ25−35-induced apoptosis of rat pheochromocytoma (PC12) cells by significantly downregulating the expression of Bax and caspase-3 and upregulating the expression of Bcl-2. Additionally, luteolin significantly upregulated the expression of estrogen receptor beta (Erβ) and phosphorylated extracellular signal-regulated protein kinases 1 and 2 (p-ERK1/2). These results indicate that luteolin activated the ER/ERK/MAPK signaling pathway to protect PC12 cells against Aβ25−35-induced cell apoptosis by selectively acting on Erβ [61].
Several investigations also tested the effect of luteolin on cell lines transfected with the ‘Swedish mutation of amyloid precursor protein’ (APPsw). Rezai-Zadeh et al. [73] carried out such an investigation to test the effect of luteolin on Aβ peptide generation in human ‘Swedish’ mutant APP transgene-bearing neuron-like cells (SweAPP) and Tg2576 mouse-derived neuronal cells. The results showed a strong inhibition of both Aβ peptides by luteolin, which was associated with a downregulation of γ-secretase cleavage activity. To establish the mechanism of action, the proteins involved with γ-secretase were studied and the obtained results showed that luteolin increased the level of serine 21 phosphorylated GSK-3α isoform as well as increased presenilin-1 carboxyl-terminal fragment phosphorylation in a time-dependent manner. These two events consequently disrupt the enzyme-substrate association with APP resulting in an anti-amyloidogenic effect. The experiment with the Tg2576 mouse model further confirmed the anti-amyloidogenic effect of luteolin (reduction of both soluble Aβ1–40, 42 isoforms) [73].
Luteolin also exhibited anti-oxidant, anti-apoptotic, and anti-amyloidogenic activities in the copper-induced neurotoxicity model in the human neuroblastoma SH-SY5Y cells carrying the APPsw [56]. In the study by Dragicevic et al. [54], the effects of 25 natural compounds (including luteolin) on amyloid-induced mitochondrial dysfunction in murine neuroblastoma N2a cells expressing the Swedish mutation were evaluated. Three separate assays were performed to determine overall mitochondrial function: ROS production, mitochondrial membrane potential (MMP), and ATP levels were assessed. Luteolin was on top of the list as it almost completely restored the mitochondrial function in the N2a-APPsw neuroblastoma cells (ROS production was decreased by 40%, MMP levels were restored close to control N2a levels (202%), and ATP levels were improved by 444%).
An interesting study was performed by Zhang et al. [42], who investigated the protective effects of luteolin on the BBB using human brain microvascular endothelial cells and human astrocytes under fibrillary Aβ1–40 (fAβ1–40)-damaged conditions. Luteolin improved BBB functioning and reduced the fAβ1−40-induced release of cytokines and inflammatory mediators. The anti-inflammatory activity was mediated through the p38 MAPK/NF-κB pathway [42].
It is also worth to mention about beneficial effect of luteolin on the inhibition of zinc-induced tau phosphorylation in human neuroblastoma SH-SY5Y cells. The mechanism of action was associated with the recovery of total phosphatase activity [70].
Furthermore, it has been shown that O-GalNAc glycosylation (initiated by the members of ppGalNAc-T family of APP is associated with the production of Aβ in AD [90]. Liu et al. [71] studied the effect of luteolin on the inhibition of ppGalNAc-T in several cell lines was studied. Luteolin showed a concentration-dependent inhibitory effect on the GalNAz signal in O-GalNAc glycosylation deficient (CHO-IdID) cells, indicating a potent inhibitory effect on O-GalNAc glycosylation. It also showcased specific inhibitory effects against O-GalNAc in HEK 293T and Jurkat cells. The evaluation of the inhibitory effects of luteolin on APP protein showed reduced ratio of the high-molecular-weight band of APP (APP-H; O-GalNAc-glycosylated APP) with a 75 and 80% reduction in HEK 293T cells and A549 cells, respectively. The mechanism of action was coupled with the inhibition of specific ppGalNAc-T isoforms in cells. Luteolin also inhibited Aβ production in Swedish-mutation APP stable cell line (HEK 293TAPP Swe cell). Finally, possible correlation between the inhibition of ppGalNAc-T and Aβ production by luteolin was investigated. In CHO-IdID cells, luteolin was able to reduce the level of restoration of Aβ, Aβ40, and Aβ42 by approximately 30% when treated with Gal and GalNAc, confirming the hypothesis about the correlation between the inhibition of ppGalNAc-T and Aβ production [71].
Not only in vitro but also in vivo studies provide evidence that luteolin may produce beneficial effects in AD. Animal studies showed that luteolin may improve cognitive decline which is a key symptom of AD. For instance, prolonged treatment with luteolin was shown to attenuate the cognitive impairment induced by chronic cerebral hypoperfusion (CCH) in rats [48, 91, 92]. Xu et al. [92] found that CCH caused dramatic inhibition of LTP formation in the hippocampus and luteolin was able to prevent the LTP impairment. It not only facilitated the phosphorylation of CREB in the hippocampus of naïve rats but also rescued the CCH-induced impairment of CREB activation. In the study by Fu et al. [48], attenuated cognitive dysfunction in CCH rats treated with luteolin was accompanied by reduced oxidative stress and neuroinflammation. Luteolin also down-regulated the expression of NF-κB and BACE1, as well as diminished the deposition of Aβ in the cortex and hippocampus. In addition, He and Chen [91] showed that luteolin ameliorated cognitive impairment in CCH rats through the modulation of the PI3K/Akt pathway.
The protective effect of luteolin against cognitive dysfunction was also reported in the streptozotocin- [93] and Aβ25–35-induced models of AD [94, 95]. In the Aβ25–35-induced model of AD in mice, luteolin attenuated cognitive impairment by, at least in part, modulating the microvascular function. Luteolin increased regional cerebral blood flow values, alleviated the leakage of the lumen of vessels, and protected the integrity of BBB [95]. Luteolin could also ameliorate cognitive deficits by regulating the cholinergic system activity, inhibiting oxidative stress [94, 95], and increasing the level of brain-derived neurotrophic factor (BDNF) and tyrosine kinase receptor (TrkB) expression in the cerebral cortex [95].
Furthermore, several reports showed neuroprotective effects of luteolin in genetic models of AD. Sawmiller et al. [27] studied the effects of luteolin on AD-like pathologies induced by traumatic brain injury in Tg2576 mice overexpressing the human APP gene carrying the Swedish mutation. Within 3 days, the untreated injured mice had significant increases in Aβ deposition, phosphorylated tau accumulation, GSK-3 activation, as well as elevated TNF-α and IL-1β levels, which is consistent with AD pathology. The luteolin-treated mice exhibited none of the AD-associated pathologies or elevations in the inflammatory cytokines [27]. The Tg2576 mouse model of AD was also used by Rezai-Zadeh et al. [73] who found that luteolin reduced GSK-3 activation and cerebral Aβ levels. In contrast to the study by Sawmiller et al. [27], luteolin supplementation for 29 weeks failed to improve cognitive impairment in APP23 mice that also overexpress human APP with the Swedish mutation. It did, however, alleviate the depression-like behavior and inhibited microglial activation by regulating the ER stress [96].
The neuroprotective effects of luteolin were also studied in a triple-transgenic mouse model of AD (3 × Tg-AD). This model is different from the previous ones as it exhibits both Aβ and tau pathology, as well as synaptic dysfunction [97]. In the study by Kou et al. [41], luteolin treatment dose-dependently improved spatial learning and alleviated memory deficits in 3 × Tg-AD mice. Luteolin also reduced histopathological changes and the number of dense Aβ plaques, inhibited over-activation of astrocytes, decreased expression of ER stress-related proteins, and reduced neuroinflammation. Specifically, it restored the increased levels of inflammatory markers and reduced the expression of p-NF-κB and phospho-p38 in the brain of AD mice. Likewise, He et al. [64] showed that luteolin supplementation significantly ameliorated memory and cognitive deficits in 3 × Tg-AD mice. This effect was accompanied by reduced generation and accumulation of Aβ, inhibited neuronal apoptosis, decreased oxidative stress, and attenuated mitochondrial dysfunction via peroxisome proliferator-activated receptor gamma (PPARγ) activation.
A similar positive effect of luteolin was observed in the transgenic Drosophila model of AD where the transgenic flies fed on different concentrations of luteolin showed a dose-dependent delay in the loss of climbing ability, increased lifespan, reduced oxidative stress, acetylcholinesterase activity and Aβ42 peptides accumulation [63].
Palmitoylethanolamide (PEA) is an endogenous molecule that acts as an anti-inflammatory mediator on several cells such as astroglia, microglia, oligodendroglia, and mast cells to maintain cellular homeostasis in the CNS and peripheral nervous system. Facchinetti et al. [88] conducted a study to demonstrate the effects of ultra-micronized PEA combined with luteolin (co-ultra PEALut; 10:1 by mass) in the Aβ1–42-induced model of AD in rats. The study was able to showcase the neuroprotective mechanisms of co-ultra PEALut by observing a decrease in GFAP levels (a biomarker of astrocytes) and a reduced increase in CD11b gene expression (a marker for activated microglial cells) in the hippocampus of the Aβ1–42-inoculated rats. Moreover, co-ultra PEALut prevented the Aβ1–42-induced increase in mRNA expression of inflammatory biomarkers as well as Aβ1–42-induced reduction of BDNF and GDNF mRNA levels [88].
The combination of luteolin with another compound– l-theanine (an amino acid found in tea) also improved AD-like symptoms in the Aβ25–35-treated rats. Luteolin in combination with l-theanine significantly improved memory function by potentiating the insulin signaling in the hippocampus and by reducing inflammation. Luteolin potentiated insulin signaling mainly via the pAkt/pGSK/pTau pathway, whereas l-theanine primarily reduced TNF-α [98].
Various formulations and drug delivery systems have been introduced to overcome limitations associated with poor solubility in water, low oral bioavailability, and extensive first-pass metabolism of luteolin. For example, luteolin-loaded bile-salt-based nano-vesicles (bilosomes) and luteolin-loaded chitosan decorated nanoparticles delivered intranasally have been developed as a strategy to enhance luteolin solubility and BBB permeability. Both luteolin bilosomes [99] and luteolin chitosomes [72] were found to ameliorate cognitive impairment in the streptozotocin-induced model of AD in mice. They also reduced oxidative stress and neuroinflammation, decreased Aβ aggregation and hyperphosphorylated-tau levels in the hippocampus, and increased neuronal survival [72, 99].
In summary, AD is the most common neurodegenerative disease in the world and its pathogenesis is tightly coupled with neuroinflammation and is estimated to rise in future shortly. This puts a big weight on the shoulders of scientists in the field of neurodegeneration to look for potential candidates to combat AD. As one of the key outcomes of luteolin is anti-inflammation, it consequently makes luteolin a worthy candidate. Upon in vitro and in vivo experimentation (summarized in Tables 1 and 2, respectively), it is evident that luteolin was able to inhibit microglial-associated inflammation pathways such as NF-κB, MAPK/AP-1 which produce pro-inflammatory cytokines which are associated with activating β-secretase and γ-secretase leading to stimulating Aβ formation. It was also observed that luteolin can restore cognitive impairment. Unfortunately, although preclinical data show clear-cut neuroprotective effects of luteolin in AD models, there are no human intervention studies or clinical trials investigating its potential beneficial effects in AD patients. Thus, clinical trials investigating its possible beneficial effects in AD patients are highly warranted.
Luteolin and Parkinson’s disease
PD is the second most common neurodegenerative disease. It is a complex, progressive disorder dominated by movement disabilities such as tremors and bradykinesia but also includes cognitive abnormalities such as PD dementia, depression, and other non-motor symptoms related to the autonomic nervous system. Statistically, in Europe, prevalence and incidence rates for PD are estimated at approximately 108–257/100 000 and 11–19/100 000 per year, respectively [5, 100]. The etiology of PD is complex as there is both the aspect of genetic and environmental factors that have been shown to contribute to the disease [101]. The most frequently occurring autosomal dominant gene mutation is the G2019S missense mutation in LRRK2. Autosomal recessive mutations associated with PD include mutations in the parkin genes such as PRKN and PINK1. Missense mutations in these genes lead to mitophagy. There are also examples of sporadic PD such as microtubule associated protein Tau (MAPT mutations) and glucocerebrosidase loss of function mutations [4, 5]. Pesticides such as rotenone and paraquat and other environmental toxins have been linked with causing PD by inducing oxidative stress.
α-Synuclein is a key protein involved in PD pathology. It is a 140 amino acid-long monomer found in the cytosol. In the presence of lipid droplets or lipid bilayers, it undergoes a conformational change by binding to the lipid components to give a folded α-helical secondary structure that is capable of forming dimers and oligomers. Post-translational modifications such as phosphorylation, ubiquitination and nitration have been associated with α-synuclein aggregation. Together with the lipid association and post-translational modifications, α-synuclein aggregates to form the Lewy bodies [102].
Luteolin has been shown to counteract key aspects of PD pathogenesis including apoptosis and neuronal death, oxidative stress, mitochondrial dysfunction [11, 103], and neuroinflammation that is directly linked to the non-motor symptoms of PD. The anti-inflammatory properties of luteolin have been thoroughly studied in in vitro and in vivo models, as reviewed elsewhere [50, 52]. Here, we focus on experimental data from in vitro and in vivo PD models.
Firstly, the protective effects of luteolin were studied in in vitro 1-methyl-4-phenylpyridinium (MPP+)-induced model of PD. Luteolin attenuated the MPP+-induced apoptosis in PC12 cells [57], rat glioblastoma cells [57], and human SH-SY5Y cells [104]. The protection against MPP⁺-induced neurotoxicity was associated with activation of Nrf2 pathway (a crucial defense mechanism against oxidative stress), via the ERK1/2 signaling pathway [57], inhibition of the mitochondrial ROS-dependent oxidative stress, and enhancement of the Erk1/2/Drp1 and Fak/Akt/GSK3β signaling pathways [104]. In addition, Qin et al. [65] conducted a study on the effect of luteolin-7-O-glucoside (LUT-7G) on the MPP+-treated SH-SY5Y cells. According to the obtained data, LUT-7G protected SH-SY5Y cells against MPP+-induced apoptosis by regulating apoptosis-related protein expression. Specifically, LUT-7G treatment decreased nuclear condensation, elevated the MPP+-induced decline of Bcl-2/Bax ratio, and suppressed the MPP+-induced increase of cleaved caspases 3, which are both markers of apoptosis.
Several in vitro studies also investigated the neuroprotective effects of luteolin in the 6-hydroxydopamine (6-OHDA) model of PD (Fig. 3). Guo et al. [67] demonstrated the impact of luteolin on the reduction of nuclear condensation and fragmentation decreasing PC12 cell apoptosis induced by 6-OHDA. In the case of neuronal cell apoptosis, luteolin significantly reduced the expression of the pro-apoptotic (Bax) gene, while increasing the anti-apoptotic (Bcl) gene. It also significantly suppressed the enhanced Bax/Bcl-2 ratio and down-regulated the enhanced mRNA expression of p53 induced by 6-OHDA. Hu et al. [62] conducted a similar investigation on the effect of luteolin on a 6-OHDA model of PD in PC12 cells, in which luteolin reduced the negative effects of 6-OHDA exposure, including ROS overproduction, cytotoxicity, and caspase-3 activation leading to apoptosis. Additionally, luteolin reduced cell cycle arrest and transcription of p53 target genes, as well as downregulated the unfolded protein response. A recent study revealed that luteolin significantly reduced the 6-OHDA-induced cell death, apoptosis, and ER stress in SH-SY5Y cells by targeting ER stress responses [105].
While numerous in vitro experiments demonstrated that luteolin may be effective against PD, to date only two studies have been conducted to evaluate the beneficial effects of luteolin in animal models of PD. Siddique et al. [66] investigated the effect of luteolin on different aspects associated with PD in the transgenic Drosophila melanogaster expressing human α-synuclein. They found that a 24-day treatment with luteolin dose-dependently delayed the loss of climbing ability and improved the activity pattern in transgenic fly. The behavioral effects were accompanied by reduced oxidative stress markers and caspase-3 and 9 activities in the brains of PD flies. Importantly, luteolin was also able to increase the dopamine level by increasing the activity of tyrosine hydroxylase (TH), which is a key enzyme responsible for generating dopamine [66]. In another study, a protective effect of LUT-7G in the 1-methyl-4-phenyl-1, 2, 3,6-tetrahydropyridine (MPTP)-induced model of PD in mice was studied. A 15-day treatment with LUT-7G attenuated the MPTP-evoked bradykinesia, and improved muscle strength, and balancing capacity. LUT-7G also protected dopaminergic neurons from MPTP-induced toxicity. It increased the number of TH-positive cells in the substantia nigra and the density of TH positive neurofibers in the striatum. In addition, a significant suppression of gliosis in substantia nigra was observed, which suggests the anti-neuroinflammatory effects of LUT-7G in the MPTP-induced model of PD [65].
To sum up, PD is another good example of a disease that falls into the category of ‘cannot be cured’. This problem stems primarily due to the lack of sufficient knowledge of the pathogenesis of the disease. However, as we keep discovering the molecular mechanisms that underlie PD, we keep looking for effective treatment with a lack of adverse effects (a key issue with most available drug-based treatment). Luteolin has a big advantage over synthetic drugs as it is a naturally occurring flavonoid found in commonly consumed fruits and vegetables and has shown very few to no side effects. The range of neuroprotective roles luteolin plays is quite evident in in vitro models (Table 1). However, a lack of sufficient data still exists concerning the in vivo preclinical (Table 2) and clinical studies; therefore, the question of using luteolin as a drug against PD is still under experimentation.
Luteolin and Huntington’s disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease manifesting by uncontrolled body movements and cognitive impairment. It is an inherited disorder that causes gradual breakdown of neurons in parts of the brain and may ultimately result in death within 15 to 20 years after diagnosis. The pathophysiology of HD is based on CAG triplet repeats in the huntingtin gene (HTT), leading to an expanded polyglutamine stretch in the huntingtin (Htt) protein [106]. Although there has been great progress in HD pathogenesis, currently available neuroprotective treatment strategies are still insufficient.
Neuroinflammation plays a key role in the development and progression of HD, and the disease may begin before significant neuronal loss occurs during the disease. It is closely linked with a wide range of biological impairments such as oxidative stress, which may induce mitochondrial dysfunction, and mitochondrial damages and lead to gliosis, with loss of astrocytes and oligodendrocytes, and in neuronal death and atrophy of brain tissues [107].
The type of HD treatment depends primarily on the patient’s clinical symptoms: (1) a progressive motor disorder; (2) progressive cognitive disorder culminating in dementia; and (3) psychiatric disorders including depression, anxiety, apathy, obsessive-compulsive behaviors, outbursts, addictions, and occasionally psychosis. Some of these symptoms can be treated non-pharmacologically, whereas the others require the use of medications [106] as well as add-on therapy with natural substances, e.g., flavonoids [108].
Only a few studies (Table 1) investigated the potential beneficial effects of luteolin in HD. The protective effect of luteolin and four luteolin derivatives was indicated by Oliveira et al. [109] in striatal cells derived from HD knock-in mice expressing mutant Htt versus wild-type striatal cells. Results they obtained showed a significant decrease of caspase-3-like activity and intracellular ROS and increased nuclear levels of phospho(Ser40)- Nrf2 and Nrf2/ARE transcription after treatment with 2 out 4 luteolin derivatives. Additionally, it was confirmed that one of luteolin derivatives enhanced SOD1 mRNA and SOD activity and glutamate-cysteine ligase catalytic subunit (GCLc) mRNA and protein levels, whereas another one induced mRNA levels of GCLc only in mutant striatal cells. The obtained results suggest that also luteolin derivatives may be important in the search for new antioxidant strategies in HD.
Molecular docking studies by Hasan Siddique et al. [110] have shown the effectiveness of luteolin as an anti-inflammatory agent. The results obtained for free energy of binding along with the non-covalent interactions proved a good interaction between luteolin and the mutant huntingtin protein (mHTT). Additionally, using a transgenic Drosophila model of HD they observed a dose-dependent delay in the loss of climbing ability and a decrease in the oxidative stress compared to the control flies after 33 days of luteolin supplementation [110] (Table 2).
Luteolin as an anti-oxidant plays a key role in the reduction and elimination of oxidative stress which is one of the major factors affecting the CNS (increased cell death, mitochondrial dysfunction, reduced neuronal plasticity and neurogenesis, increased autoimmune responses) in neurodegenerative diseases including HD [103, 111]. Taking into consideration the antioxidant properties of luteolin (protection from oxidative damage by scavenging free radicals and increasing the activity of antioxidant enzymes) it can be concluded that the use of this natural supplement in the treatment of HD may slow down or even stop the progression of the disease. Nevertheless, there are not enough scientific reports so far regarding the relationship between luteolin and mHTT and further advanced studies are needed to develop a new potent drug candidate able to reduce the aggregation of this pathogenic protein in the body.
Luteolin and multiple sclerosis
Confirmed antioxidant, anti-inflammatory, and neuroprotective activities of luteolin certainly enabled research to assess the effectiveness of this substance in other neurodegenerative disorders multiple sclerosis (MS). MS is a chronic demyelinating disease of the CNS, in which progressive neuroinflammation induces lesions throughout the white and gray matter of the brain and spinal cord. It affects approximately 2.5 million people worldwide [112, 113]. The most important symptoms of MS are visual disorders (double vision, vestibular symptoms, dysphagia, dysarthria), and motor, cognitive, and mental disorders (depression, anxiety) [113].
First, in vitro investigations provided by Sternberg et al. [45] on peripheral blood mononuclear cells (PBMCs) isolated from MS patients showed that luteolin dose-dependently reduced PBMCs proliferation as well as production of IL-1β, TNF-α, and matrix metalloproteinase-9 (MMP-9), the inflammatory factors that are crucial in MS. Additionally, luteolin in combination with interferon-beta therapy, had and additive impact on cell proliferation, IL-1β, TNF-α, MMP-9 and tissue inhibitor of metalloproteinase-1.
Considering the problem of the remyelination of the myelin-producing oligodendrocytes in MS disease, Barbierato et al. [114] examined the ability of co-ultra PEALut to promote progression of oligodendrocyte progenitor cells (OPCs) into a more differentiated phenotype. The results they obtained showed that co-ultra PEALut promoted the morphological development of OPCs without affecting proliferation, which suggests that co-ultra PEALut may be a potential new drug candidate in the treatment of inflammatory demyelinating disorders.
Further, in vitro research on the OPC culture derived from rat pup cortex by Facci et al. [115] indicated an association between co-ultra PEALut-induced OPC maturation and tyrosine-protein kinase (Tyro3) receptor upregulation, which may be of great significance for the remyelination process.
While the above-mentioned findings (summarized in Table 1) are very promising, it is important to note that studies on the potential neuroprotective effects of luteolin in MS are at a very preliminary stage as yet.
Human studies on luteolin in CNS disorders
Despite promising findings from preclinical in vitro and in vivo studies, the potential of luteolin in the treatment and/or prevention of neurodegenerative diseases has not been widely studied in clinical settings thus far.
Camptocormia, also known as the bent spine syndrome, when the trunk of a patient bends forward when walking or standing, is a common axial symptom of PD [116]. Camptocormia mainly arises in the later stages of PD. Its response to levodopa is very poor and there are no other effective treatment options [117]. Interestingly, a formulation which combined luteolin with PEA was found to significantly improve dyskinesia and reduce camptocormia in a 68-year PD patient. It was concluded that luteolin in this combination increases the stability of PEA and enhance the neuroprotective effects. Thus, co-ultra PEALut may be a promising adjuvant therapy for patients with PD to treat both dyskinesia and camptocormia but this needs confirmation in large-scale studies [116].
Frontotemporal dementia is a devastating neurodegenerative disorder manifested by progressive impairment in behavior, executive function, and language [118]. Recently, a randomized controlled trial on co-ultra PEALut in frontotemporal dementia has been completed. This study aimed to evaluate a global disease severity and various executive functions including cognition changes (ClinicalTrials.gov Identifier: NCT04489017). Unfortunately, the outcomes are not available as yet (as of May 2024).
It is noteworthy that there is also some initial clinical evidence on the beneficial effects of luteolin in other CNS conditions. Theoharides et al. [119] reported an uncontrolled open case series of 37 children aged 4–14 years (29 boys and 8 girls) suffering from autism spectrum disorder (ASD) who received a mixture of luteolin (100 mg) with the related flavonoids quercetin (70 mg) and rutin (30 mg). After 4 months of treatment, a significant improvement in various areas was reported. Specifically, 50% of the children had increased eye contact and attention to directions, 30–50% of children showed improvement in retaining learned tasks, 10% of the children started speaking words and even sentences, and lower incidence of hyperactivity and aggression. The same luteolin-containing dietary formulation was tested in a group of 50 children (4–10 years old; 42 boys and 8 girls) with ASD in a prospective open-label clinical trial. Positive changes in adaptive functioning and in the overall behavior were reported after a 26-week treatment [120]. Children that improved the most after dietary supplementation with this luteolin-containing formulation showed reduced IL-6 and TNF serum levels [121], which suggest that the beneficial effects were associated with anti-inflammatory action.
Brain fog is a clinical symptom where an individual has difficulty with memory, concentration, decision-making, and subjective cognitive impairment or behavioral changes. It was discovered as a persistent symptom by almost a third of COVID-19 patients. In a clinical study where De Luca et al. [122] investigated the effect of co-ultra PEALut on ‘brain fog’ in COVID-19 patients, some light was shed on the potential of luteolin on cognitive impairment (mainly memory loss) in humans. The study group was patients with a confirmed history of COVID-19 in the age group 40–50 years (43 women and 26 men). Three months after treatment with co-ultra PEALut a statistical reduction in brain fog was reported. The mechanism of action was deduced to be interconnected to neuroinflammation [122].
Co-ultra PEALut was also reported to improve the neurological status of human stroke patients. In an observational study, a cohort of 250 stroke patients (118 women and 132 men) undergoing neurorehabilitation were treated with co-ultra PEALut. After 30 days of supplementation, a significant improvement in neurological status, cognitive abilities, the degree of spasticity, independence in daily living, and pain was reported [123].
Although the above-mentioned data should be interpreted with caution due to their observational nature, they do show that luteolin-containing formulations are well-tolerated [119, 120, 123] and have the potential to attenuate cognitive deficits. This encourages further clinical studies, also in patients suffering from neurodegenerative disorders.
In January 2022, a double-blind, placebo-controlled, randomized clinical trial of the efficacy of luteolin (300 mg daily for 12 weeks) in patients with schizophrenia has been registered at clinicaltrials.gov (NCT05204407). The study will measure the effect of luteolin treatment on global psychopathology, cognitive impairments; antioxidant stress, levels of inflammatory markers, as well as positive and negative symptoms of schizophrenia. It is worth noticing here that luteolin has not been extensively studied in schizophrenia. Molecular docking and functional assays showed that luteolin has a potent antagonist effect on dopamine D4 receptors– the prime target for antipsychotic drugs [124]. In in vitro model of maternal immune activation (MIA), luteolin reduced astrogliosis and produced neuroprotective effects suggesting that it may attenuate the MIA-induced neurodevelopmental abnormalities occurring in ASD or schizophrenia [125]. Thus, the anti-inflammatory and neuroprotective action of luteolin may bring positive effects in schizophrenia that shares some features of neurodegenerative diseases [126].
Future perspectives and conclusions
As reviewed above, accumulating evidence shows that luteolin may confer neuroprotection against various neurodegenerative disorders, especially in Alzheimer’s disease. Although the results of the current preclinical studies are very promising, much effort is needed to fully evaluate the therapeutic potential of luteolin. Such studies should include not only the assessment of the neuroprotective effects of luteolin across validated animal models of neurodegenerative disease but also the evaluation of its safety, tolerability, long-term adverse effects, pharmacokinetic profile, and interaction with CNS drugs. Moreover, the potential bidirectional interference of luteolin with the gut microbiota should be characterized since intestinal microbiomes can influence the bioavailability of orally taken polyphenols. Luteolin may also alter the gut microbiota composition, which consequently can affect its bioavailability and efficacy. In further perspective, well-designed clinical studies are warranted to verify the possible benefits of luteolin in neurodegenerative diseases.
Given the large increase in the aging population in recent decades, there is no doubt that the growing number of patients suffering from neurodegenerative diseases has become a major health problem. Apart from aging, there are many other causes of irreversible neuronal damage and death such as genetic mutations, pathologic proteins, and environmental factors [127]. From molecular point of view, the process of neurodegeneration is strongly related to neuroinflammation and oxidative stress [128].
Finding effective therapies that can delay the onset or progression of neurodegenerative diseases, especially AD and PD, is a primary goal and challenge for neuroscience research. Certainly, searching for and testing natural substances with neuroprotective, antioxidant, and anti-inflammatory properties may be an extremely important stage in the development of potential add-on therapies supporting the treatment of a given neurodegenerative disease. Flavones, including luteolin have shown therapeutic potential and can contribute to the treatment of neurodegenerative diseases. Current studies involving luteolin as a therapeutic agent are promising and strongly encourage further research on flavonoids showing neuroprotective activity.
Data availability
Data sharing does not apply to this article as no new data were generated or analyzed in this study.
Abbreviations
- 6-OHDA:
-
6-hydroxydopamine
- Aβ:
-
Amyloid beta
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- AP-1:
-
Activator protein-1
- APP:
-
Amyloid precursor protein
- APPsw:
-
Swedish mutation of amyloid precursor protein
- ARE:
-
Antioxidant responsive element
- ASD:
-
Autism spectrum disorder
- BACE-1:
-
Beta site amyloid precursor protein cleaving enzyme
- Bax:
-
Bcl-2-associated X protein
- BBB:
-
Blood-brain barrier
- Bcl-2:
-
B-cell lymphoma protein 2
- BDNF:
-
Brain-derived neurotrophic factor
- CCH:
-
Chronic cerebral hypoperfusion
- CNS:
-
Central nervous system
- co-ultra PEALut:
-
Ultra-micronized PEA combined with luteolin
- COX-2:
-
Cyclooxygenase-2
- CREB:
-
Camp response element-binding protein
- EOAD:
-
Early-onset Alzheimer’s disease
- Erβ:
-
Estrogen receptor beta
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated protein kinase
- fAβ1–40 :
-
Fibrillary amyloid beta (1–40)
- GCLc:
-
Glutamate-cysteine ligase catalytic subunit
- GSK-3α:
-
Glycogen synthase kinase-3 alpha
- HD:
-
Huntington’s disease
- HO-1:
-
Heme oxygenase-1
- HTT:
-
Huntingtin
- IL-1β:
-
Interleukin-1 beta
- IL-16:
-
Interleukin-16
- JAK-STAT:
-
Janus kinase signal transducer and activator of transcription
- LOAD:
-
Late-onset Alzheimer’s disease
- LRRK2:
-
Leucine-rich repeat kinase 2
- LUT-7G:
-
Luteolin-7-O-glucoside
- MAPK:
-
Mitogen-activated protein kinase
- mHTT:
-
Mutant huntingtin protein
- MIA:
-
Maternal immune activation
- MMP-9:
-
Matrix metalloproteinase-9
- MMP:
-
Mitochondrial membrane potential
- MPTP:
-
1-methyl-4-phenyl-1, 2, 3,6-tetrahydropyridine
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NFTs:
-
Neurofibrillary tangles
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- OPCs:
-
Oligodendrocyte progenitor cells
- p-ERK1/2:
-
Phosphorylated extracellular signal-regulated protein kinases 1 and 2
- PBMCs:
-
Peripheral blood mononuclear cells
- PC12:
-
Pheochromocytoma cell line 12
- PD:
-
Parkinson’s disease
- PEA:
-
Palmitoylethanolamide
- ppGalNAc-T:
-
Polypeptide N-acetyl-α-galactosaminyltransferase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TH:
-
Tyrosine hydroxylase
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- TrkB:
-
Tyrosine kinase receptor
References
Lamptey RNL, Chaulagain B, Trivedi R, Gothwal A, Layek B, Singh J. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of nanotherapeutics. Int J Mol Sci. 2022;23(3):1851.
Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, Kopito R, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener. 2017;6:6.
Elbaz A, Dufouil C, Alpérovitch A. Interaction between genes and environment in neurodegenerative diseases. C R Biol. 2007;330:318–28.
Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS. Emerging therapies in Parkinson disease– repurposed drugs and new approaches. Nat Rev Neurol. 2019;15:204–23.
Kouli A, Torsney KM, Kuan WL. Parkinson’s disease: etiology, neuropathology, and pathogenesis. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and clinical aspects. Codon Publications, Brisbane (AU); 2018.
Myers RH. Huntington’s disease genetics. NeuroRx. 2004;1:255–62.
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124.
Oertel W, Schulz JB. Current and experimental treatments of Parkinson disease: a guide for neuroscientists. J Neurochem. 2016;139(Suppl 1):325–37.
Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9.
Knight JA. Reactive oxygen species and the neurodegenerative disorders. Ann Clin Lab Sci. 1997;27:11–25.
Siddique YH. Role of luteolin in overcoming Parkinson’s disease. BioFactors. 2021;47:198–206.
Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42.
Andreone BJ, Larhammar M, Lewcock JW. Cell death and neurodegeneration. Cold Spring Harb Perspect Biol. 2020;12.
Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28:2029–44.
Behl T, Kumar S, Althafar ZM, Sehgal A, Singh S, Sharma N, et al. Exploring the role of ubiquitin-proteasome system in Parkinson’s disease. Mol Neurobiol. 2022;59:4257–73.
Bustamante HA, González AE, Cerda-Troncoso C, Shaughnessy R, Otth C, Soza A, et al. Interplay between the autophagy-lysosomal pathway and the ubiquitin-proteasome system: a target for therapeutic development in Alzheimer’s disease. Front Cell Neurosci. 2018;12:126.
Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. 2022;17:23.
Srivastava P, Yadav RS. Efficacy of natural compounds in neurodegenerative disorders. Adv Neurobiol. 2016;12:107–23.
Gendrisch F, Esser PR, Schempp CM, Wölfle U. Luteolin as a modulator of skin aging and inflammation. BioFactors. 2021;47:170–80.
Daily JW, Kang S, Park S. Protection against Alzheimer’s disease by luteolin: role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors. 2021;47:218–31.
Taheri Y, Sharifi-Rad J, Antika G, Yılmaz YB, Tumer TB, Abuhamdah S, et al. Paving luteolin therapeutic potentialities and agro-food-pharma applications: emphasis on in vivo pharmacological effects and bioavailability traits. Oxid Med Cell Longev. 2021;2021:1987588.
Chagas M, Behrens MD, Moragas-Tellis CJ, Penedo GXM, Silva AR, Gonçalves-de-Albuquerque CF. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid Med Cell Longev. 2022;2022:9966750.
Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–12.
Takara T, Yamamoto K, Suzuki N, Yamashita SI, Lio SI, Kakinuma T, et al. Effects of luteolin-rich chrysanthemum flower extract on purine base absorption and blood uric acid in Japanese subjects. Funct Foods Health Dis. 2022;12:12.
Deng C, Gao C, Tian X, Chao B, Wang F, Zhang Y, et al. Pharmacokinetics, tissue distribution and excretion of luteolin and its major metabolites in rats: metabolites predominate in blood, tissues and are mainly excreted via bile. J Funct Foods. 2017;35:332–40.
Hayasaka N, Shimizu N, Komoda T, Mohri S, Tsushida T, Eitsuka T, et al. Absorption and metabolism of luteolin in rats and humans in relation to in vitro anti-inflammatory effects. J Agric Food Chem. 2018;66:11320–9.
Sawmiller D, Li S, Shahaduzzaman M, Smith AJ, Obregon D, Giunta B, et al. Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int J Mol Sci. 2014;15:895–904.
Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K, et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Res Bull. 2015;119:1–11.
Goyal A, Solanki K, Verma A, Luteolin. Nature’s promising warrior against Alzheimer’s and Parkinson’s disease. J Biochem Mol Toxicol. 2024;38:e23619.
Savino R, Medoro A, Ali S, Scapagnini G, Maes M, Davinelli S. The emerging role of flavonoids in autism spectrum disorder: a systematic review. J Clin Med. 2023;12.
Sur B, Lee B. Luteolin reduces fear, anxiety, and depression in rats with post-traumatic stress disorder. Anim Cells Syst (Seoul). 2022;26:174–82.
Wu X, Xu H, Zeng N, Li H, Yao G, Liu K, et al. Luteolin alleviates depression-like behavior by modulating glycerophospholipid metabolism in the hippocampus and prefrontal cortex of LOD rats. CNS Neurosci Ther. 2024;30:e14455.
Kempuraj D, Thangavel R, Kempuraj DD, Ahmed ME, Selvakumar GP, Raikwar SP, et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors. 2021;47:190–7.
Odontuya G, Hoult JR, Houghton PJ. Structure-activity relationship for antiinflammatory effect of luteolin and its derived glycosides. Phytother Res. 2005;19:782–6.
Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA. 2008;105:7534–9.
Kang OH, Choi JG, Lee JH, Kwon DY. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules. 2010;15:385–98.
Kao TK, Ou YC, Lin SY, Pan HC, Song PJ, Raung SL, et al. Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J Nutr Biochem. 2011;22:612–24.
Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology. 2005;115:375–87.
Lv L, Lv L, Zhang Y, Kong Q. Luteolin prevents LPS-induced TNF-α expression in cardiac myocytes through inhibiting NF-κB signaling pathway. Inflammation. 2011;34:620–9.
Park CM, Song YS. Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice. Nutr Res Pract. 2019;13:473–9.
Kou JJ, Shi JZ, He YY, Hao JJ, Zhang HY, Luo DM, et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol Sin. 2022;43:840–9.
Zhang JX, Xing JG, Wang LL, Jiang HL, Guo SL, Liu R. Luteolin inhibits fibrillary β-Amyloid(1–40)-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules. 2017;22(3):334.
Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett. 2008;448:175–9.
Elmazoglu Z, Yar Saglam AS, Sonmez C, Karasu C. Luteolin protects microglia against rotenone-induced toxicity in a hormetic manner through targeting oxidative stress response, genes associated with Parkinson’s disease and inflammatory pathways. Drug Chem Toxicol. 2020;43:96–103.
Sternberg Z, Chadha K, Lieberman A, Drake A, Hojnacki D, Weinstock-Guttman B, et al. Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: additive effects of IFN-beta. J Neuroinflammation. 2009;6:28.
Ahmad S, Jo MH, Ikram M, Khan A, Kim MO. Deciphering the potential neuroprotective effects of luteolin against Aβ(1)-(42)-induced Alzheimer’s disease. Int J Mol Sci. 2021;22(17):9583.
Che DN, Cho BO, Kim JS, Shin JY, Kang HJ, Jang SI. Luteolin and apigenin attenuate LPS-induced astrocyte activation and cytokine production by targeting MAPK, STAT3, and NF-κB signaling pathways. Inflammation. 2020;43:1716–28.
Fu X, Zhang J, Guo L, Xu Y, Sun L, Wang S, et al. Protective role of luteolin against cognitive dysfunction induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2014;126:122–30.
Zhu L, Bi W, Lu D, Zhang C, Shu X, Lu D. Luteolin inhibits SH-SY5Y cell apoptosis through suppression of the nuclear transcription factor-κB, mitogen-activated protein kinase and protein kinase B pathways in lipopolysaccharide-stimulated cocultured BV2 cells. Exp Ther Med. 2014;7:1065–70.
Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–58.
Caporali S, De Stefano A, Calabrese C, Giovannelli A, Pieri M, Savini I, et al. Anti-inflammatory and active biological properties of the plant-derived bioactive compounds luteolin and luteolin 7-glucoside. Nutrients. 2022;14(6):1155.
Hussain MS, Gupta G, Goyal A, Thapa R, Almalki WH, Kazmi I et al. From nature to therapy: luteolin’s potential as an immune system modulator in inflammatory disorders. J Biochem Mol Toxicol. 2023:e23482.
Zheng N, Yuan P, Li C, Wu J, Huang J. Luteolin reduces BACE1 expression through NF-κB and through estrogen receptor mediated pathways in HEK293 and SH-SY5Y cells. J Alzheimers Dis. 2015;45:659–71.
Dragicevic N, Smith A, Lin X, Yuan F, Copes N, Delic V, et al. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J Alzheimers Dis. 2011;26:507–21.
Li J-R, Sun M-J, Ping Q-N, Chen X-J, Qi J-P, Han D-E, Metabolism. Excretion and bioavailability of hydroxysafflor yellow A after oral administration of its lipid-based formulation and aqueous solution in rats. Chin J Nat Med. 2010;8:0233–40.
Liu R, Meng F, Zhang L, Liu A, Qin H, Lan X, et al. Luteolin isolated from the medicinal plant Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Molecules. 2011;16:2084–96.
Wruck CJ, Claussen M, Fuhrmann G, Römer L, Schulz A, Pufe T et al. Luteolin protects rat PC12 and C6 cells against MPP + induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J Neural Transm Suppl. 2007:57–67.
Kim S, Chin YW, Cho J. Protection of cultured cortical neurons by luteolin against oxidative damage through inhibition of apoptosis and induction of heme oxygenase-1. Biol Pharm Bull. 2017;40:256–65.
Lin P, Tian XH, Yi YS, Jiang WS, Zhou YJ, Cheng WJ. Luteolin-induced protection of H2O2-induced apoptosis in PC12 cells and the associated pathway. Mol Med Rep. 2015;12:7699–704.
Zhao G, Yao-Yue C, Qin GW, Guo LH. Luteolin from purple perilla mitigates ROS insult particularly in primary neurons. Neurobiol Aging. 2012;33:176–86.
Wang HR, Pei SY, Fan DX, Liu YH, Pan XF, Song FX, et al. Luteolin protects pheochromocytoma (PC-12) cells against Aβ (25–35)-induced cell apoptosis through the ER/ERK/MAPK signalling pathway. Evid Based Complement Alternat Med. 2020;2020:2861978.
Hu LW, Yen JH, Shen YT, Wu KY, Wu MJ. Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS ONE. 2014;9:e97880.
Ali F, Rahul, Jyoti S, Naz F, Ashafaq M, Shahid M, et al. Therapeutic potential of luteolin in transgenic drosophila model of Alzheimer’s disease. Neurosci Lett. 2019;692:90–9.
He Z, Li X, Wang Z, Cao Y, Han S, Li N, et al. Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer’s disease. Redox Biol. 2023;66:102848.
Qin L, Chen Z, Yang L, Shi H, Wu H, Zhang B, et al. Luteolin-7-O-glucoside protects dopaminergic neurons by activating estrogen-receptor-mediated signaling pathway in MPTP-induced mice. Toxicology. 2019;426:152256.
Siddique YH, Jyoti S, Naz F. Protective effect of luteolin on the transgenic drosophila model of Parkinson’s disease. Braz J Pharm Sci. 2018;54.
Guo DJ, Li F, Yu PH, Chan SW. Neuroprotective effects of luteolin against apoptosis induced by 6-hydroxydopamine on rat pheochromocytoma PC12 cells. Pharm Biol. 2013;51:190–6.
Wu PS, Yen JH, Kou MC, Wu MJ. Luteolin and apigenin attenuate 4-hydroxy-2-nonenal-mediated cell death through modulation of UPR, Nrf2-ARE and MAPK pathways in PC12 cells. PLoS ONE. 2015;10:e0130599.
Choi SM, Kim BC, Cho YH, Choi KH, Chang J, Park MS, et al. Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med J. 2014;50:45–51.
Zhou F, Chen S, Xiong J, Li Y, Qu L. Luteolin reduces zinc-induced tau phosphorylation at Ser262/356 in an ROS-dependent manner in SH-SY5Y cells. Biol Trace Elem Res. 2012;149:273–9.
Liu F, Xu K, Xu Z, de Las Rivas M, Wang C, Li X, et al. The small molecule luteolin inhibits N-acetyl-α-galactosaminyltransferases and reduces mucin-type O-glycosylation of amyloid precursor protein. J Biol Chem. 2017;292:21304–19.
Abbas H, Sayed NSE, Youssef N, Mousa PMEG, Fayez MR. Novel luteolin-loaded chitosan decorated nanoparticles for brain-targeting delivery in a sporadic Alzheimer’s disease mouse model: focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceutics. 2022;14(5):1003.
Rezai-Zadeh K, Douglas Shytle R, Bai Y, Tian J, Hou H, Mori T, et al. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer’s disease beta-amyloid production. J Cell Mol Med. 2009;13:574–88.
Lin CW, Wu MJ, Liu IY, Su JD, Yen JH. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J Agric Food Chem. 2010;58:4477–86.
Lin LF, Chiu SP, Wu MJ, Chen PY, Yen JH. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS ONE. 2012;7:e43304.
DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14:32.
Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020;25(24):5789.
Santiago JA, Potashkin JA. The impact of disease comorbidities in Alzheimer’s disease. Front Aging Neurosci. 2021;13:631770.
Yanakiev M, Soper O, Berg DA, Kang E. Modelling Alzheimer’s disease using human brain organoids: current progress and challenges. Expert Rev Mol Med. 2022;25:e3.
Shepherd C, McCann H, Halliday GM. Variations in the neuropathology of familial Alzheimer’s disease. Acta Neuropathol. 2009;118:37–52.
Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017;38:1205–35.
Gulisano W, Maugeri D, Baltrons MA, Fà M, Amato A, Palmeri A, et al. Role of amyloid-β and tau proteins in Alzheimer’s disease: confuting the amyloid cascade. J Alzheimers Dis. 2018;64:S611–31.
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–31.
Small SA, Duff K. Linking abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–42.
Iqbal K, Grundke-Iqbal I. Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 2010;6:420–4.
Kwon Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp Gerontol. 2017;95:39–43.
Ahmad MH, Fatima M, Mondal AC. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: rational insights for the therapeutic approaches. J Clin Neurosci. 2019;59:6–11.
Facchinetti R, Valenza M, Bronzuoli MR, Menegoni G, Ratano P, Steardo L, et al. Looking for a treatment for the early stage of Alzheimer’s disease: preclinical evidence with co-ultramicronized palmitoylethanolamide and luteolin. Int J Mol Sci. 2020;21(11):3802.
Liu R, Lan X, Ying J, Du GH. Protective effects of luteolin against amyloid β25-35-induced toxicity on rat cerebral microvascular endothelial cells. Chin J Nat Med. 2010;8:223–7.
Akasaka-Manya K, Manya H. The role of APP O-glycosylation in Alzheimer’s disease. Biomolecules. 2020;10(11):1569.
He H, Chen X. Luteolin attenuates cognitive dysfunction induced by chronic cerebral hypoperfusion through the modulation of the PI3K/Akt pathway in rats. J Vet Res. 2021;65:341–9.
Xu B, Li XX, He GR, Hu JJ, Mu X, Tian S, et al. Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur J Pharmacol. 2010;627:99–105.
Wang H, Wang H, Cheng H, Che Z. Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Mol Med Rep. 2016;13:4215–20.
Liu R, Gao M, Qiang GF, Zhang TT, Lan X, Ying J, et al. The anti-amnesic effects of luteolin against amyloid beta(25–35) peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience. 2009;162:1232–43.
Yu TX, Zhang P, Guan Y, Wang M, Zhen MQ. Protective effects of luteolin against cognitive impairment induced by infusion of Aβ peptide in rats. Int J Clin Exp Pathol. 2015;8:6740–7.
Tana NT. Luteolin ameliorates depression-like behaviors by suppressing ER stress in a mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2022;588:168–74.
Sterniczuk R, Antle MC, Laferla FM, Dyck RH. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 2. Behavioral and cognitive changes. Brain Res. 2010;1348:149–55.
Park S, Kim DS, Kang S, Kim HJ. The combination of luteolin and l-theanine improved Alzheimer disease-like symptoms by potentiating hippocampal insulin signaling and decreasing neuroinflammation and norepinephrine degradation in amyloid-β-infused rats. Nutr Res. 2018;60:116–31.
Elsheikh MA, El-Feky YA, Al-Sawahli MM, Ali ME, Fayez AM, Abbas H. sA brain-targeted approach to ameliorate memory disorders in a sporadic Alzheimer’s disease mouse model via intranasal luteolin-loaded nanobilosome. Pharmaceutics. 2022;14(3):576.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27:27–42.
Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–90.
Kim WS, Kågedal K, Halliday GM. Alpha-synuclein biology in lewy body diseases. Alzheimers Res Ther. 2014;6:73.
Ashaari Z, Hadjzadeh MA, Hassanzadeh G, Alizamir T, Yousefi B, Keshavarzi Z et al. The flavone luteolin improves central nervous system disorders by different mechanisms: a review. J Mol Neurosci. 65:491–506.
Reudhabibadh R, Binlateh T, Chonpathompikunlert P, Nonpanya N, Prommeenate P, Chanvorachote P, et al. Suppressing Cdk5 activity by luteolin inhibits MPP(+)-induced apoptotic of neuroblastoma through Erk/Drp1 and Fak/Akt/GSK3β pathways. Molecules. 2021;26(5):1307.
Nishiguchi H, Omura T, Sato A, Kitahiro Y, Yamamoto K, Kunimasa J, et al. Luteolin protects against 6-hydoroxydopamine-induced cell death via an upregulation of HRD1 and SEL1L. Neurochem Res. 2023;49:117–28.
Dayalu P, Albin RL. Huntington disease: pathogenesis and treatment. Neurol Clin. 2015;33:101–14.
Ignácio ZM, Quevedo J, Réus GZ. Pathophysiological mechanisms of Huntington’s disease. Pathology. In: Singh S, Joshi N, editors. Prevention and therapeutics of neurodegenerative disease. Singapore: Springer; 2018. pp. 49–60.
Khan H, Ullah H, Tundis R, Belwal T, Devkota H, Daglia M, et al. Dietary flavonoids in the management of Huntington’s Disease: mechanism and clinical perspective. eFood. 2020;1:38–52.
Oliveira AM, Cardoso SM, Ribeiro M, Seixas RS, Silva AM, Rego AC. Protective effects of 3-alkyl luteolin derivatives are mediated by Nrf2 transcriptional activity and decreased oxidative stress in Huntington’s disease mouse striatal cells. Neurochem Int. 2015;91:1–12.
Hasan Siddique Y, Varshney H, Mantasha I, Shahid M. Effect of luteolin on the transgenic Drosophila model of Huntington’s disease. Comput Toxicol. 2021;17:100148.
Chen HI, Hu WS, Hung MY, Ou HC, Huang SH, Hsu PT, et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr Metab Cardiovasc Dis. 2020;30:1032–43.
Bayat P, Farshchi M, Yousefian M, Mahmoudi M, Yazdian-Robati R. Flavonoids, the compounds with anti-inflammatory and immunomodulatory properties, as promising tools in multiple sclerosis (MS) therapy: a systematic review of preclinical evidence. Int Immunopharmacol. 2021;95:107562.
La Rosa G, Lonardo MS, Cacciapuoti N, Muscariello E, Guida B, Faraonio R, et al. Dietary polyphenols, microbiome, and multiple sclerosis: from molecular anti-inflammatory and neuroprotective mechanisms to clinical evidence. Int J Mol Sci. 2023;24(8):7247.
Barbierato M, Facci L, Marinelli C, Zusso M, Argentini C, Skaper SD, et al. Co-ultramicronized palmitoylethanolamide/luteolin promotes the maturation of oligodendrocyte precursor cells. Sci Rep. 2015;5:16676.
Facci L, Barbierato M, Fusco M, Giusti P, Zusso M. Co-ultramicronized palmitoylethanolamide/luteolin-induced oligodendrocyte precursor cell differentiation is associated with Tyro3 receptor upregulation. Front Pharmacol. 2021;12:698133.
Brotini S. Palmitoylethanolamide/luteolin as adjuvant therapy to improve an unusual case of camptocormia in a patient with Parkinson’s disease: a case report. Innov Clin Neurosci. 2021;18:12–4.
Cordaro M, Cuzzocrea S, Crupi R. An update of palmitoylethanolamide and luteolin effects in preclinical and clinical studies of neuroinflammatory events. Antioxid (Basel). 2020;9(3):216.
Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–82.
Theoharides TC, Asadi S, Panagiotidou S. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. Int J Immunopathol Pharmacol. 2012;25:317–23.
Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther. 2013;35:592–602.
Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry. 2015;5:e647.
De Luca P, Camaioni A, Marra P, Salzano G, Carriere G, Ricciardi L, et al. Effect of ultra-micronized palmitoylethanolamide and luteolin on olfaction and memory in patients with long COVID: results of a longitudinal study. Cells. 2022;11(16):2552.
Caltagirone C, Cisari C, Schievano C, Di Paola R, Cordaro M, Bruschetta G, et al. Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: from rodent to man. Transl Stroke Res. 2016;7:54–69.
Park SE, Paudel P, Wagle A, Seong SH, Kim HR, Fauzi FM, et al. Luteolin, a potent human monoamine oxidase-A inhibitor and dopamine D(4) and vasopressin V(1A) receptor antagonist. J Agric Food Chem. 2020;68:10719–29.
Zuiki M, Chiyonobu T, Yoshida M, Maeda H, Yamashita S, Kidowaki S, et al. Luteolin attenuates interleukin-6-mediated astrogliosis in human iPSC-derived neural aggregates: a candidate preventive substance for maternal immune activation-induced abnormalities. Neurosci Lett. 2017;653:296–301.
Rund BR. Is schizophrenia a neurodegenerative disorder? Nord J Psychiatry. 2009;63:196–201.
Armstrong R. What causes neurodegenerative disease? Folia Neuropathol. 2020;58:93–112.
Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, et al. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. 2022;23(11):5938.
Wu Y, Jiang X, Yang K, Xia Y, Cheng S, Tang Q, et al. Inhibition of α-synuclein contributes to the ameliorative effects of dietary flavonoids luteolin on arsenite-induced apoptotic cell death in the dopaminergic PC12 cells. Toxicol Mech Methods. 2017;27:598–608.
Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–8.
Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, et al. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation. 2010;7:3.
Paredes-Gonzalez X, Fuentes F, Jeffery S, Saw CL, Shu L, Su ZY, et al. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm Drug Dispos. 2015;36:440–51.
Parrella E, Porrini V, Iorio R, Benarese M, Lanzillotta A, Mota M, et al. PEA and luteolin synergistically reduce mast cell-mediated toxicity and elicit neuroprotection in cell-based models of brain ischemia. Brain Res. 2016;1648:409–17.
Acknowledgements
Figures were created with BioRender.com.
Funding
This work was not supported by any funding.
Author information
Authors and Affiliations
Contributions
Dunuvilla Kavindi Jayawickreme, conceptualization, writing– original draft, visualization; Cletus Ekwosi, writing– original draft, visualization; Apurva Anand, writing– original draft, visualization; Marta Andres-Mach, writing– original draft, writing– review & editing; Piotr Wlaź, writing– review & editing; Katarzyna Socała, conceptualization, writing– original draft, writing– review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jayawickreme, D.K., Ekwosi, C., Anand, A. et al. Luteolin for neurodegenerative diseases: a review. Pharmacol. Rep (2024). https://doi.org/10.1007/s43440-024-00610-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43440-024-00610-8