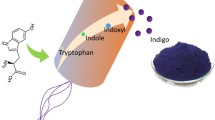
Abstract
Indole is a signalling molecule produced both by bacteria and plants. In this review its signalling role between microbes and in particular in the human gut is discussed. Besides the natural roles, indole also has value for flavour and fragrance applications, for example, in food industry or perfumery. Additionally, indole can be derivatized to several halogenated and oxygenated compounds that can be used as natural colourants or have promising bioactivity with therapeutic potential to treat human diseases. Indole is traditionally obtained from coal tar. Biocatalytic approaches have been developed to convert indole into halogenated and oxygenated derivatives. This review will discuss recent advances in production of indole from glucose or tryptophan by fermentation and the production of derived halogenated and oxygenated derivatives by microbial cell factories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Structure of natural indole
More than 4000 indoles are known to occur in nature (Fig. 1A), and many are biologically active as some compounds exhibit antitumor, antibacterial, antiviral or antifungal activities. The indole ring system is probably the most ubiquitous heterocycle in nature, and it has long inspired organic synthesis chemists [1]. In pharmaceutical chemistry, substituted indoles are considered “privileged structures” as they show high-affinity binding to many receptors. Free indole can give rise to substituted indoles (Fig. 1A). Three examples are given: (a) addition of single groups for functionalization (e.g. 3-bromoindole as synthesised by BrvH, a flavin-dependent halogenase encoded in a metagenome [2]); (b) cascaded enzyme-catalysed and spontaneous reactions, (e.g. hydroxylation by a P450 monooxygenase followed by spontaneous oxidation and dimerization yielding indigo [3]), and (c) complex biosynthetic pathways of plants and fungi (e.g. biosynthesis of benzoxazinoid alkaloids, whereas vinblastine derives from tryptamine [4]).
Indole is important in bacterial signalling
Multiple bacterial species in environmental niches developed quorum sensing (QS) to adapt and survive in natural communities. Many bacteria release diffusible chemical communication signals to sense the local environmental condition and eventually to regulate diverse physiological processes [5]. Indole and its derivatives are among these bacterial signalling molecules, being produced by more than 85 Gram-positive and Gram-negative bacterial species primarily from the phyla Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria [6]. In many cases, however, indoles are involved not just in intra-species signalling, but also in inter-species and even inter-kingdom signalling between bacteria and their eukaryotic hosts [7]. While indole-producing bacteria employ indole for QS, many non-indole-producing bacteria as well as eukaryotes sense and metabolise indole via oxygenases affecting their physiology in different ways [6]. The following section describes the influence of indole in coordinating actions within organisms as well as its ubiquitous role in intercellular communication.
As an extracellular signal molecule in Escherichia coli, indole was observed to activate the transcription of genes gabT and astD involved in the degradation pathway of amino acids to pyruvate or succinate [8], suggesting that indole signalling may play a role in preparing the cells for a nutrient-poor environment when the catabolism of amino acids becomes important for energy production [9]. Aside from being an active signal in metabolic control, indole also participates in cell cycle regulation by delaying cell division in E. coli until plasmid dimers are resolved to monomers [10] contributing to the maintenance of plasmid copies and genetic stability [11]. Bacterial cell division begins with the assembly of a large number of proteins that form a macromolecular complex called divisome [12]. Cells exposed to indole were observed to have FtsZ, filamenting temperature-sensitive mutant Z, fluorescence uniformly distributed throughout the cytoplasm in contrast to cells not exposed to indole which had FtsZ localised at the cell mid-point as the cell cycle progressed [13]. Since formation of the FtsZ ring is a prerequisite for division, prevention of its generation by indole effectively inhibits cell division. Moreover, as early as 1982, it has already been recognised that as little as 0.5 mM indole can uncouple the mitochondrial oxidative phosphorylation, the final biochemical pathway in the production of ATP [14]. Both preventing FtsZ localisation and mitochondrial uncoupling may explain the role of indole in cell cycle regulation.
Several studies also reported that indole modulates bacterial persistence, the resistance and tolerance to antibiotics of some bacteria. Investigations in E. coli show that indole at physiologic concentrations (below 1 mM) induces the transition to a persistent state against lethal concentrations of ofloxacin, ampicillin and kanamycin [15]. By contrast, toxic concentrations of indole, i.e. 1–2 mM where indole behaves as a membrane ionophore, lead to decreased persister frequency with ampicillin, ciprofloxacin [16] and rifampicin [17]. Interestingly, sub-minimal inhibitory concentrations of the aminoglycoside tobramycin elicited increased indole production in the pathogen Vibrio cholerae which causes the deadly disease cholera [18]. This indole secretion induced increased persistence to lethal concentrations of aminoglycoside of V. cholerae through the action of RaiA, increasing the protection of ribosomes during stress, a novel pathway in which indole mediates bacterial persistence. In addition, indole also uses other mechanism to induce persistence namely, by reducing the production of ribosomes and hence, slowing down the metabolism [19]. Notably, another opportunistic pathogen Pseudomonas aeruginosa can be resuscitated with the amino acid l-Pro after inducing the persister phenotype by reducing translation through depleting ATP levels. However, physiological concentrations of indole inhibited cell resuscitation of P. aeruginosa persister cells [20] hinting on the positive role of indole produced by the commensal E. coli in preventing waking of the persister pathogenic P. aeruginosa in the gut [21]. Consequent to the dissimilar observations on the effect of indole in bacterial persistence, more research is needed to demystify the role of indole in pathways leading to persistence of some bacteria.
Studies also pointed to the involvement of indole in biofilm formation. Microbial biofilms are bacterial populations in which cells are embedded in a self-produced matrix of extracellular polymeric substances that are adherent to each other and/or a surface [22]. Due to their resistance to antibiotics, pathogen biofilms pose a major problem for human health [23]. Indole interacts with the transcriptional regulator, LuxR homolog SdiA of E. coli and regulates SdiA-mediated transcription resulting in repressed motility and decreased acid resistance ultimately leading to reduced biofilm formation [24]. The effect of presence of indole in biofilm formation of different microorganisms has also been previously reviewed [6, 25, 26]. As already pointed out in these reviews, there is a lack of consensus on the direction of effect and role of indole in biofilm formation since there are different outcomes for varied experimental parameters like strain, indole concentration and condition like medium and temperature [24, 27,28,29,30,31]. Recently, in a mixed-species co-culture between the non-biofilm forming indole-degrading betaproteobacteria Burkholderia unamae and indole-producing gammaproteobacteria E. coli, indole produced by E. coli facilitated the interspecies biofilm formation [32]. In turn, the B. unamae-derived signalling diketopiperazine cyclo-upregulated indole biosynthesis and enhanced E. coli biofilm formation, an example of a bidirectional, mutually beneficial, cell-to-cell communication involving indole-producing and indole-consuming species [32]. Interspecies QS between the human opportunistic pathogens Acinetobacter baumannii and P. aeruginosa coexisting in human lungs was observed to be also regulated by indole [33]. Specifically, indole from A. baumannii reduced the competitive fitness of P. aeruginosa by inhibiting its QS systems and type III secretion system. Notably, deletion of indole synthase, homolog of methyl indole-3-acetate methyltransferase, impaired biofilm formation, motility and virulence of A. baumannii, a clinically significant finding since A. baumannii has been listed in the World Health Organization’s first-ever list of priority antimicrobial-resistant pathogens [34]. Another pathogen of particular clinical relevance in both developed and developing countries is the enteric pathogen Salmonella enterica serovar Typhimurium, the leading cause of foodborne illness worldwide [35]. It has been shown that HeLa intestinal epithelial cells treated with indole prior to infection with Salmonella decreased motility and invasion of the pathogen to the HeLa intestinal cells [36]. In addition, microarray analysis revealed that indole repressed various genes related to bacterial motility and virulence but induced expression of genes related to efflux-mediated multidrug resistance [37]. Since Salmonella does not produce indole and does not harbour tnaA [37], these results suggest that the indole produced by tryptophanase-expressing bacteria in the gut may provide an advantage to the indole-producing commensal bacteria themselves as well as the host through QS. However, the indole that offers protection to the indole-producing commensal bacteria is the same indole that promotes the propagation of the non-indole-producing pathogen Clostridium difficile, leading to reduced colonic microbial diversity and dysbiosis that sustain C. difficile infection [38]. Through the Agr1 quorum signalling system of C. difficile, the pathogen induces indole biosynthesis at transcription level in E. coli. Increased indole concentration creates redox imbalances that influence cell viability of beneficial commensal bacteria such as Bacteroides species, which tended to have lower indole tolerance than C. difficile [38]. Due to considerable variation in experimental results and observations, care is needed when drawing broad conclusions on whether indole has beneficial or detrimental end effects (Fig. 2).
Another aspect in which indole signalling is relevant is on biological wastewater treatment plant since there is a significant concentration of indole in coal wastewater and in faeces from healthy adults ranging from 10 mg/L to 18.6 mg/L and 35.14 mg/L to 773.18 mg/L, respectively [39, 40]. Treatment of indole-containing wastewater is always accompanied by indigoids formation, hence indigoid-degrading bacteria may be used in waste treatment process [41]. The next section will tackle the role of indole and its metabolites in signalling in the human gut.
Impact of indole on intestinal function
The gastrointestinal tract of humans is home to a high density and diversity of bacterial cells, with the majority living in the colon (1011–1012 bacterial cells/mL) [42]. Indole concentrations are in the order of 0.25–1.1 mM and up to 0.2 mM in the human gut and blood, respectively [18]. Signalling by indole and its derivatives that are produced by microorganisms in the colon influences the digestive and immune systems in humans. Aside from the small portion of the l-Trp derived from the diet used for protein biosynthesis, l-Trp is also catabolized either by endogenous host cells via the kynurenine and serotonin pathway [43] or by intestinal microorganisms via indole and its derivatives pathway (Table 1).
Indoles generated from l-Trp catabolism exhibit diverse biological activities and play important role in the intestinal tract [50]. Typically, tryptophanase (TnaA)-expressing Gram-negative E. coli and Gram-positive bacteria such as Clostridium spp., and Bacteroides spp. catalyse the direct conversion of l-Trp to indole [51]. Commensal E. coli produce as much as 600 μM indole in suspension cultures [28] and human faeces contain indole at comparable concentrations (about 0.25 to 1.1 mM) [52]. Indoles play a crucial role in controlling intestinal barrier efficacy and can either decrease the early immune response to bacterial elicitors or trigger induced systemic resistance [50]. This section summarises the known and well-described mutual crosstalk between the cytokines involved in the immune response and indole metabolites derived from the gut.
In a murine model of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) enteropathy, it has been shown that co-administration of indole with indomethacin attenuated multiple deleterious effects of NSAID enteropathy by modulating inflammation mediated by innate immune responses and altering indomethacin-induced shift of the microbiota [44]. This indicates that indole has a potential immunomodulatory effect on NSAID enteropathy. In addition, indole was also found to induce the secretion of the anti-inflammatory cytokine interleukin-10 (IL-10), reduced the synthesis of the pro-inflammatory IL-8 and decreased tumour necrosis factor (TNF)-α-mediated activation of nuclear factor (NF-κB) [45]. The same study also showed indole to elicit a response on the intestinal epithelial cells by increasing the expression of genes involved in strengthening the mucosal barrier and mucin production.
Besides indole, indole derivatives have also been demonstrated to have positive effects on enhancing immune barrier of the colon. The aromatic indole-3-pyruvic acid was shown to have substantial anti-inflammatory impact on the colon in the inflammatory bowel disease (IBD) mouse model by inducing T regulatory cell 1 (Tr1) differentiation involving IL-10 secretion [46], which is important for intestinal immunological homeostasis [53]. In individuals with IBD, epithelial regeneration and inflammation reduction is stimulated by the reparative cytokine IL-22 [54]. IL-22 production is induced by ligand-activated transcription factor aryl hydrocarbon receptor (AhR) and is in turn influenced by the AhR ligand indole-3-acetic acid [55]. Indole-3-acetic acid produced by Bacteroides ovatus ATCC 8387 promoted IL-22 production by immune cells in a gnobiotic mouse model and trinitrobenzene sulfonic acid model of murine colitis [47]. Another ligand of AhR indole-3-lactic acid decreased production of the pro-inflammatory cytokine IL-8 in human intestinal epithelial cell lines when exposed to lipopolysaccharide (LPS), the most abundant component within the cell wall of Gram-negative bacteria [48]. LPS is a potent stimulus for the release of TNF-α and the latter in turn induces the expression of IL-8 [56]. Just like in IBD, bacterial infections through LPS cause imbalance in the intestinal immunological homeostasis and can stimulate the release of inflammatory cytokines [57]. In vitro LPS stimulation of human peripheral mononuclear blood cells (PBMCs) treated with the indole metabolite indole-3-acrylate not only significantly reduced the secretion of pro-inflammatory IL-1β and IL-6 but also downregulated the genes involved in inflammation, oxidative stress, innate immune cell activation and differentiation [49].
Applications of indoles
The diversity of biological effects mediated by indole and indole derivatives described in the above two sections opens new horizons for its biotechnological applications (Fig. 3). For instance, developing indole-based anti-virulence agents may be promising to combat recalcitrant bacterial infections as indole plays functional role in quorum sensing, the bacterial communication system pivotal for survival, adaptation and pathogenesis [58]. Indole has been demonstrated to alter pathogenicity and invasiveness of several enteric pathogens. The first step in enterohemorrhagic E. coli (EHEC) O157:H7 infection involves adhesion of bacteria to host cells and the formation of microcolonies [59, 60] and in vitro adherence experiment with EHEC and indole attenuated EHEC adherence to epithelial cells [61]. The enteric pathogen Salmonella enterica serovar Typhimurium was shown to have repressed expression of genes related to host cell invasion encoded in the Salmonella pathogenicity island 1 and decreased phenotypic invasive ability in the presence of indole [37]. The Gram-positive pathogenic Listeria monocytogenes bacterium is highly tolerant to changing environments and stresses primarily resulting from the biofilm-forming ability in food processing lines posing a major health concern [62]. Exposing L. monocytogenes to synthetic indole and indole-rich conditioned medium substantially downregulated transcript levels of virulence associated (pssE, dltA, flaA, fliI, motB, agrA and hly) and regulatory genes (codY, sigB, prfA and gmaR), as well as significantly diminished biofilm formation and related virulence including motility, cell aggregation and exopolysaccharide production [63]. Additionally, several substituted indigoids were demonstrated to be potent in killing persister cells. Halogenated indole derivatives 5-iodoindole, 4-fluoroindole, 7-chloroindole and 7-bromoindole eradicated persister formation by E. coli and the multidrug resistant pathogen Staphylococcus aureus with 5-iodoindole most potently inhibiting biofilm formation by the two bacteria [17]. Recently, the substituted indole 5-nitro-3-phenyl-1H-indol-2-yl-methylamine hydrochloride (NPIMA) was discovered to be effective in destroying persister cells of E. coli, P. aeruginosa and S. aureus by damaging their membranes [64]. Besides, 5-methylindole potentiates the efficacy of the traditional antibiotic tobramycin in killing methicillin-resistant S. aureus and Staphylococcus epidermidis persisters [65]. Remarkably, indole obliterates not only bacterial persisters but was also demonstrated to kill the persister cells of the archaeal strain Haloferax volcanii [66]. Hence, a new class of effective anti-persister compounds based on indole that eradicate both Gram-negative and Gram-positive bacteria, as well as an obligate halophilic archaeon has been discovered [19].
In addition to the potential of indole to be used as anti-virulence agents, its derivatives also find application in agriculture. For example, indole-3-acetic acid (IAA) is a plant growth regulating hormone (auxin) as well as some of its derivatives, such as indole-3-succinic acid or 4-Cl-indole-3-acetic acid [67]. IAA and its mimetics can be used as herbicides and this class of herbicides ranks third after glyphosate and acetolactate synthase inhibitors regarding global herbicide use [68]. The cellular concentration of IAA is controlled in several ways such as biosynthesis, transport, localization, derivatization, and degradation [69]. Regulation of IAA biosynthesis and transport involves melatonin that contains an indole core derived from l-Trp and functions as hormone governing the sleep–wake cycle of animals and as antioxidant in plants, e.g. to mitigate herbicide-induced oxidative stress [67]. Due to transport, degradation and side effects, IAA auxin mimetic herbicides are structurally different from IAA. They may even lack the indole core such as in long-time established (R)-2-(2,4-dichlorophenoxy)-propanoic acid (dichlorprop) or 3,6-dichlor-2-methoxybenzoesäure (dicamba) or in benzyl 4-amino-3-chloro-6-(4-chloro-2-fluoro-3-methoxyphenyl)-5-fluoropyridine-2-carboxylate (florpyrauxifen-benzyl), the most recently developed IAA mimetic herbicide [68, 70, 71].
Antiviral activity has been shown for indole derivatives such as the β-carboline alkaloid harmine or the diketopiperazine alkaloids brevianamide F regarding plant viruses [72]. Indole derivatives have also shown potential as antifungal compounds [73] and on the other hand okaramines, indole alkaloids isolated from the fungal species of the genera Penicillium and Aspergillus, are highly potent as insecticides due to their selective activation of glutamate-gated chloride channels in invertebrates, but not in humans. Although many of the highly potent indole derivative share the mode of action of indole itself, their chemical structures may be very different. Currently, a trend in the search for indole-based bioactives focusses on natural indole products as ideal precursor compounds since these are advantageous regarding biodegradation, environmental friendliness, structural diversity and target specificity as compared to traditional synthetic drugs [72].
Indole was first isolated from reduction of indigo, one of the oldest natural dyes that mankind uses with analytical proof dating back 3500 years. Substitution of selected atoms of indigo characterise variants of this dark blue dye: the reddish-purple 6,6’-dibromoindigo also known as tyrian or royal purple, 5,5′-indigo with sulfuric acid yields the food colourant indigo carmine (E132), whilst the reddish-blue 5,5′,7,7′-tetrabromindigo is used as colourant for cotton and plastics [74]. While indigo, its glycosylated precursor and indigo carmine can be extracted either from the plants Indigofera tinctoria (true indigo), Polygonum tinctorum (dyer’s knotweed) or Isatis tinctoria (woad), their biotechnological production is independent of climate and seasons, provides high yields and sulfonation of indigo differs from chemical synthesis by providing natural indigo.
Indole also accumulates in various essential oils, especially in the oils of flowers such as jasmine, orange blossom and gardenia [75, 76]. Pure indole exhibits a pungent, floral character, and is often added to fragrance compositions in trace amounts to create a floral and intriguing impression. The special character of indole makes it an important aroma compound for the flavour and fragrance industry.
In 2022, the global market value of indole was estimated at USD 35.7 million [77]. The market is expected to grow at 6.5% compound annual growth rate in 2022–2028 due to increasing demand for pharmaceutical, fragrance, biotechnological and other applications. Bio-based indole makes up a small part of the global indole market and is mainly in demand from the flavour and fragrance industry.
Chemical synthesis of indoles
Indole occurs at about 0.2% in the fractions of hard coal tar that boil at 240 °C to 260 °C. Isolation from coal tar is a main industrial source of indole. Chemical synthesis of this aromatic heterocycle can proceed via three routes (Fig. 1B). Both rings of indole can either be constructed simultaneously via the Kanematsu strategy starting from an allene precursor and proceeding via an intramolecular Diels–Alder cyclization product [78] or from pre-existing rings. Several named reactions, e.g. Fischer, Mori, Hemetsberger or Madelung indole synthesis reactions, have been developed and they have in common to construct the pyrrole ring on a benzene core. The Fischer indole synthesis, for example, starts with enolisable N-arylhydrazones and indolisation occurs upon heating the ketone or aldehyde and the arylhydrazine in the presence of an acid (catalyst) [1]. This approach works better for the synthesis of substituted indoles than for indole itself [79]. Besides the Fischer indole synthesis, the Larock indole synthesis using ortho-iodoaniline and disubstituted alkynes is the most practicable and broadly applicable for chemical synthesis of indoles [80]. As a third strategy, benzannulation reactions construct the benzene ring on a pyrrole core [81]. Benzannulation can proceed by metal (Rh, Pd, Ru, Cu)-catalysed cyclization or by Brønsted and Lewis acid catalysis [81]. For example, in Rh(II)-catalysed benzannulation enaldiazo esters can be reacted with pyrroles to generate an electrophilic rhodium enalcarbenoid in situ [81]. Chemical indole synthesis suffers from, i. a., the need for toxic solvents, the need of transition-metal catalysts, and the large amount of waste solvents [82] (Fig. 4)
Production of indoles by biocatalysis
Enzymes for production, biodegradation and biotransformation of indole have been reviewed [41]. Enzyme catalysis to synthesize indole has been applied in colorimetric assays for l-Trp [83]. Bacterial bioconversion of indole is initiated by oxygenation of indole to 3-hydroxyindole, 2,3-hydroxyindole, 4,5-dihydroxyindole. The authors provide an overview of all identified enzymes with the ability to convert indole to indigo, which is the most researched application. This oxygenation reaction can be catalysed by three different enzyme classes: non-heme iron oxygenases, heme-containing oxygenases, and flavin-dependent monooxygenases [84]. The different enzymes can be part of multi-component systems or self-sufficient and differ in the production of the initial oxygenated product and the formation of side products. However, more research is needed to determine which enzyme system will prove most robust at industrial scale.
Production of halogenated indole by biocatalysis using halogenation enzymes was shown for 7-chloroindole and 5-bromoindole. While 7-chloroindole was produced only by Streptomyces cetonii, halogenation to 5-bromoindole was observed at higher conversion rates and by preparation of biocatalyst from several bacteria e.g. S. cetonii, Pseudomonas putida, Aeromonas hydrophila and, Citrobacter koseri [85]. Flavin-dependent halogenases catalyse halogenation of mainly aromatic compounds and require halide salts, oxygen and reduced flavin FADH2 as cofactor. The most prominent group are l-Trp halogenases, but also enzymes that can halogenate both l-Trp and indole [86] or preferably halogenate free indole were described [87]. Indole specific flavin-dependent halogenase BrvH identified in marine metagenomes converts indole to 3-bromoindole, with bromination being highly preferred above chlorination [2]. Similarly, three flavin-dependent halogenases from Xanthomonas campestris that were initially annotated as l-Trp halogenases were shown not to accept l-Trp but indole and substituted indoles and prefer bromination above chlorination [88]. Alternatively, when studying the biosynthetic cluster leading to the formation of a highly brominated cyanobacterial toxin aetokthonotoxin formed by Aetokthonos hydrillicola, 5-bromoindole was shown to be synthesised in vitro by purified tryptophanase AetE from 5-bromo-l-Trp [89].
Microbial production of indole
Recently, different strategies for fermentative indole production have been described, using either a bacterial tryptophanase (TnaA) that converts l-Trp to indole in a β-elimination reaction, or plant or bacterial indole-3-glycerol-phosphate lyases (IGLs), that support indole production from indole-3-glycerol-phosphate (IGP) in a retroaldol cleavage.
The first fermentative indole production was subject to a study designed to understand the relevance of exogeneous l-Trp for native indole production by Escherichia coli. The authors described indole secretion upon l-Trp supplementation up to almost 6 mM (corresponds to 0.7 g/L), a concentration so far known to have a toxic physiological effect on the microbial host [90]. A higher indole titre was achieved several years later in a process using the indole-negative Corynebacterium glutamicum [91]. C. glutamicum is known for its natural ability to secret high amounts of glutamate and its product portfolio was expanded to several value-added molecules such as aromatic compounds [92, 93]. Prospecting of bacterial genomes retrieved hundreds of tryptophanase gene (tnaA) candidates and upon in vivo testing in C. glutamicum a tnaA from Providencia rettgeri showed highest indole accumulation [91]. The bioconversion of supplemented l-Trp was most successful upon co-expression of the tryptophanase gene tnaA in combination with a native aromatic amino acid permease gene (aroP) facilitating l-Trp uptake. Yet, the product toxicity proved to be the major limiting factor at a concentration of 0.9 g/L. Sequestering indole into dibutyl sebacate, a water-immiscible organic solvent, circumvented accumulation of indole to toxic concentrations in the medium and enabled a final production titre of 5.7 g/L indole. Shortly thereafter, de novo production of indole by a tnaA-expressing C. glutamicum strain, initially optimised for l-Trp secretion, was reported by Kerbs et al. [94]. This study aimed at the production of halogenated indole and indole alkaloids, however, indole itself accumulated as byproduct to concentrations of 0.1 g/L in culture supernatants. An even higher de novo indole production titre was reached by introduction of the P. rettgeri tryptophanase into another engineered C. glutamicum strain [95]. First, a shikimate producing strain was metabolically engineered to produce l-Trp up to 2 g/L. This strain produced indole upon expression of tnaA, yet, also to low concentrations of 0.1 g/L. Application of in situ product removal by addition of the water-immiscible tributyrin resulted in de novo indole production up to 1.4 g/L.
A different route for de novo production of indole from glucose and ammonium made use of IGL activity [96]. It is commonly accepted that IGLs evolved from tryptophan synthase α-subunit (TSA). The tryptophan synthase complex catalyses the conversion of IGP to l-Trp in a two-step reaction, in which indole is channelled from the α-subunit to the β-subunit (TSB) without its release [97]. Plant derived IGLs function as stand-alone enzymes whilst their ancestors TSAs are tightly regulated by interaction with TSBs and as consequence have very low activity in the absence of TSB. Surprisingly, the TSA from C. glutamicum functions as bona-fide IGL and converts IGP efficiently to indole also in the absence of TSB. Mining of bacterial TSAs with high similarity to C. glutamicum TSA and plant IGLs resulted in identification of new IGL enzymes with the ability to support indole production, whilst all selected and tested bacterial TSAs did not show indole release. A shikimate producing C. glutamicum strain was metabolically engineered to produce IGP by deletion of trpB (encoding TSB), channelling chorismate to anthranilate by deletion of chorismite mutase gene csm, prevention of glutamate secretion by yggB deletion and deregulation of the native trp operon by trpL knock-out. Upon expression of the endogenous trpA (encoding TSA) or an IGL from wheat and subsequent addition of tributyrin for in situ product removal, a final indole concentration of 0.7 g/L was reported.
The importance of the shikimate pathway for biosynthesis of indoles is long known and very well researched in model bacteria and plants. Metabolic engineering of the shikimate pathway to boost overproduction of indoles helped to identify bottlenecks even regarding enzymes of well-characterised pathways. On the other hand, metabolic engineering screens enzymes of this pathway from many different microbial and plant sources leading to new discoveries. This may be exemplified by the surprising finding that l-Trp synthase subunit A of C. glutamicum functions as a bona fide IGL [96].
Metabolic engineering for the production of indole derivatives in microbial cell factories
Hydroxylation of indole to 2-hydroxyindole or 3-hydroxyindole (indoxyl) in microbial cell factories is employed to produce indigo and other indigoids [98]. Several successful engineering strategies were reported to produce indigo in g/L scale in recombinant microorganisms, based on the conversion of indole to indoxyl by naphthalene dioxygenases (NDO) [99,100,101] or flavin-containing monooxygenases (FMO) [3, 102] followed by spontaneous conversion of indoxyl to indigo. Indigo production from glucose was achieved in recombinant E. coli where indole was converted to indoxyl by the naphthalene dioxygenase (NDO) from P. putida. The strain was further engineered to overexpress the feedback deregulated DAHP synthase gene aroGfbr and the transketolase whilst both isoenzymes of the pyruvate kinase were inactivated. This strategy led to the production of 18 g/L indigo in fed-batch fermenters (Table 2). The formation of indirubin side product that has an undesirable red cast was observed in finished denim garments. To reduce indirubin content an isatin hydrolase identified in an indole-degrading P. putida strain WW2 was expressed and resulted in a 50% indirubin reduction [101]. A recombinant E. coli strain expressing the flavin-containing monooxygenase gene from Methylophaga aminisulfidivorans resulted in 911 mg/L of indigo from 2 g/L l-Trp in a 3000 L fermenter [102]. The production of indigo by biotransformation in E. coli cells by a fusion enzyme of flavin-containing monooxygenase fused to tryptophanase resulted in production of 1.7 g/L indigo from 2 g l-Trp [103]. An alternative strategy to produce indigo in microbial cell factories was devised employing the FMO from M. aminisulfidivorans in combination with P. tinctorium glucosyltransferase UGT which adds a glucose moiety as a protective group to indoxyl to form indican, thus preventing formation of crystalline indigo during fermentation. During the dying process indoxyl is released using beta-glucosidase, avoiding the need for reducing agents during denim dying. Formation of 2.9 g/L indican was achieved from l-Trp [3].
Indirubin is formed by condensation of 2-hydroxyindole and 3-hydroxyindole. In recombinant E. coli cells expressing the FMO from M. aminisulfidivorans production of 5 mg/L indirubin and 950 mg/L indigo was reported. Upon supplementation of cysteine which increased the regioselectivity towards 2-hydroxyindole the titre of indirubin of 233 mg/L was obtained from l-Trp, together with 7 mg/L indole [104]. Extensive metabolic engineering of E. coli including the inactivation of the repressor gene trpR and removing feedback inhibitions on AroG and TrpE, identifying the rate-controlling step (AroL) in the shikimate pathway and increasing shikimate pathway precursor availability resulted in successful production of indirubin de novo from glucose at a titre of 56 mg/L together with coproduction of 640 mg/L indigo [105].
Production of halogenated indoles de novo was demonstrated in C. glutamicum. Firstly, production of 7-halo-l-Trp was engineered by expression of the FAD-dependent tryptophan 7-halogenase RebH and the NADH-dependent flavin reductase RebF in a l-Trp overproducing strain of C. glutamicum in a fermentative process based on glucose, ammonium and bromide or chloride salts [108, 109]. The production of halogenated indoles was next achieved by expressing tryptophanases from E. coli and Proteus vulgaris [94]. Both TnaA enzymes were able to accept halogenated l-Trp as substrate, however, with reduced activity compared to the natural substrate l-Trp. Upon expression of the tnaA enzymes in the 7-halo-l-Trp producing strains, the final titres of 16 mg/L 7-Cl-indole and 23 mg/L 7-Br-indole were obtained.
Brominated indigiods can be produced by combined halogenation and hydroxylation of the indole backbone. Indigoid dye tyrian purple (6,6′-dibromoindigo) originating from Mediterranean Sea snail Murex brandaris was reported to be produced in E. coli [106]. Firstly, the tryptophan-6-halogenase from Streptomyces toxytricini was fused with the flavin reductase Fre from E. coli (Fre-L3-SttH) and used for conversion of l-Trp to 6-bromo-l-Trp. Subsequently, TnaA was used to cleave 6-bromo-l-Trp into 6-bromoindole and a flavin-containing monooxygenase FMO from M. aminisulfidivorans was used to produce 6-bromoindoxyl from which 6,6′-dibromoindigo was formed via an auto-oxidation reaction. The reaction was optimised in a two-cell reaction system where l-Trp bromination was temporally and spatially separated from 6-bromo-l-Trp degradation and oxygenation. Murex sea snails also produce 6‐bromoindirubin which can be used as a precursor of anti-cancer drugs. To produce this compound in E. coli a three-cell reaction system was set up. In the first strain the tryptophan-6-halogenase fused with the flavin reductase (Fre-L3-SttH) was used for conversion of l-Trp to 6-bromotryptophan followed by conversion to 6-bromoindole by TnaA. The second E. coli strain was engineered to hydroxylate 6-bromoindole to form 6-bromo-2-hydroxy-indole/6-bromo-2-oxoindole by toluene 4‐monooxygenase T4MO from Pseudomonas mendocina KR1. The third E. coli strain was engineered to express tnaA in combination with the FMO gene from M. aminisulfidivorans to produce 3-hydroxyindole. L-Cys was added to prevent indigo formation of 2‐cysteinyl indoleninone. Spontaneous oxidative condensation of 3-hydroxyindole/2-Cys-indoleninone and 6-bromo-2-oxoindole to 6-bromoindirubin was enhanced by a shift from pH 7 to 9. In the optimised process, where the three strains were sequentially added in the fermentation process, a titre of 34 mg/L was achieved [107]. Very recently, halogenated monoterpene indole alkaloids such as bromoalstonine, the brominated, more active form of the antipsychotic drug alstonine could be produced by an engineered yeast [110].
Research needs and future directions
Indoles and their derivatives have many functions relevant for health and for technical applications. However, further research is needed into the role of indoles in biological systems and the environment. Several indole derivatives are used as anti-cancer agents. They exert their therapeutic benefit through several mechanisms including arresting cell cycle, regulating aromatase inhibitor oestrogen receptor, inhibiting tubulin, inhibiting tyrosine kinase, inhibiting topoisomerase and inhibiting the adaptive immune pathway NFkB/PI3/Akt/mTOR [111]. However, cells in the body are vastly heterogenous and molecular difference between individual cells, even of the same cell type, can lead to dramatic differences in cell response to a drug treatment such as death or survival of cancer cells [112]. Applying new omics technology such as single-cell RNA sequencing may unmask rare cells within an isogenic population and may lead to better understanding on the signalling and molecular pathways that indoles regulate or affect especially in the context of cancer therapy.
The different (bio)synthesis routes to indoles and their derivatives ease the access to specifically isotope-labelled versions that may be conducive to trace their fate in the cell, tissue, organ, body or the environment as well as their degradation. For example, exchanging the medium nitrogen source from NH4Cl to 14NH4Cl will allow access to 14N-labelled indoles such as to 14N-indole or twice 14N-labelled 6‐bromoindirubin. Similarly, uniformly 13C-labelled glucose-13C6 will allow access to indoles that only carry 13C-labelled carbon atoms and the use of position-13C-labelled glucose will yield indoles with certain carbon atoms fully 13C-labelled, whilst others remain unlabelled. Thus, the fate of indole or of the indole core of l-Trp can be followed in subsequent biosynthesis reactions such as gramine biosynthesis in barley [113], the auxin network in plants [114] or in the anoxygenic photosynthetic bacterium Rubrivivax benzoatilyticus [115]. Since the fermentative production of brominated indoles was established [94], it is also possible to prepare radiolabelled 77Br-indoles, e.g. to follow their fate in healthy tissues and tumours of mice as demonstrated for Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor approved for treating non-small-cell lung cancer that contains an indole group [116]. It is not always required to use isotopically labelled indoles since one property of indole, namely its indole (-NH) proton, can be monitored relatively easily by proton nuclear magnetic resonance (1H-NMR). This property was used in NMR imaging focussing on the downfield NMR spectrum, where the indole (-NH) proton can be detected, to detect l-Trp, the lowest concentration amino acid present in the brain, in the brains of healthy human volunteers. Potentially, this property may be used as a new biomarker in the study of neuropsychiatric disorders associated with serotonin receptors in the human brain such as anxiety, depression or autism [117].
About two-thirds of all known agrochemicals contain halogenated aromatic ring systems, e.g. the pro-insecticide indoxacarb [118]. Halogenation affects the compound’s lipophilicity, size, polarity, and capacity for hydrogen bonding [118]. Consequently, e.g. the metabolism of the halogenated compound, the stability in soil or water, membrane permeability or ligand-target binding interactions may have been changed. The halogenation of indole-3-acetic acid results in a stronger inhibitory activity for root growth [119]. Fermentative production of indole and derivatives will provide not only biologically active compounds, but biosynthetic intermediates or degradation products may be synthesised to study these processes [118]. However, hitherto, biotechnological chlorination and bromination of indoles have been achieved, but iodation and fluorination not yet, thus, biotechnological access to nematocidal and/or insecticidal indoles such 7-fluoro-5-iodoindole [120] still must be developed.
For efficient biotechnological production of indoles and their derivatives improved enzyme discovery and characterisation pipelines are needed. The discovery of enzymes may be greatly accelerated due to increased sequencing efforts, also discovering the functional diversity in the so-called metagenome dark matter, by annotating the protein families with no similarity to the current reference genomes or the Pfam database [121]. The enzyme-based approaches will also benefit from the increasing number of protein structures predicted by AlphaFold [122]. Machine and deep learning can provide insight in the small molecule substrates of enzymes facilitating in silico testing of substrates and accelerating bioprospecting of new enzymes for production of natural products [123]. On the other hand, directed evolution approaches may provide improved enzymes compatible with industrial fermentation processes, as shown for the tryptophan synthase β-subunit which was successfully evolved as a stand-alone enzyme having high activity with indole analogues [124]. The biosensor-guided expression of rate-limiting enzymes may be used, as was shown for the tryptophan synthase β-subunit trpB gene which was expressed under the L-serine-responsive transcriptional activator SerR in the C. glutamicum cells for production of l-Trp derivatives [125]. The biotechnological production of indoles may be further accelerated in the next years due to advances in development of non-model microorganisms as microbial cell factories for the production of chemicals [126], acceleration of high-throughput strain construction [127], the automation of the cultivation platforms [128, 129] and new cell cultivation approaches, such as the use of a segregostat in which in contrast to the chemostat cultivation, phenotypic diversification of microbial populations is better controlled [130]. Segregostat application is especially interesting in synthetic co-culture process of indole bioproduction since indole as previously described affects bacteria through signalling, i.e. altering of cell membrane permeability and inducing stress response, which may trigger phenotypic diversification. This approach may lead to several potential applications ranging from homogenizing bioprocess population during indole fermentation to expanding our knowledge about the dynamics of phenotypic diversification of microbial populations along with the potential link of indole in the functionality of this diversification process [130].
In conclusion, the development of a plethora of fermentation, whole-cell biotransformation, enzyme catalysis and chemical approaches for the synthesis of indoles offers the potential to deepen knowledge on the biological role of indoles and their impact in biotechnological application. This includes established markets such as for the fragrance and flavour industry, but it may also lay the foundation to new applications, such as engineering of more complex pathways, e. g., from plants to produce more complex compounds with sought-after bioactivity as achieved for the anti-cancer drug (+)-vinblastine.
References
Taber DF, Tirunahari PK. Indole synthesis: a review and proposed classification. Tetrahedron. 2011;67:7195–210. https://doi.org/10.1016/j.tet.2011.06.040.
Neubauer PR, Widmann C, Wibberg D, Schroder L, Frese M, Kottke T, Kalinowski J, Niemann HH, Sewald N. A flavin-dependent halogenase from metagenomic analysis prefers bromination over chlorination. PLoS ONE. 2018;13:e0196797. https://doi.org/10.1371/journal.pone.0196797.
Hsu TM, Welner DH, Russ ZN, Cervantes B, Prathuri RL, Adams PD, Dueber JE. Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat Chem Biol. 2018;14:256–61. https://doi.org/10.1038/nchembio.2552.
Zhang J, Hansen LG, Gudich O, Viehrig K, Lassen LMM, Schrubbers L, Adhikari KB, Rubaszka P, Carrasquer-Alvarez E, Chen L, D’Ambrosio V, Lehka B, Haidar AK, Nallapareddy S, Giannakou K, Laloux M, Arsovska D, Jorgensen MAK, Chan LJG, Kristensen M, Christensen HB, Sudarsan S, Stander EA, Baidoo E, Petzold CJ, Wulff T, O’Connor SE, Courdavault V, Jensen MK, Keasling JD. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature. 2022;609:341–7. https://doi.org/10.1038/s41586-022-05157-3.
Weisskopf L, Schulz S, Garbeva P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat Rev Microbiol. 2021;19:391–404. https://doi.org/10.1038/s41579-020-00508-1.
Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34:426–44. https://doi.org/10.1111/j.1574-6976.2009.00204.x.
Zarkan A, Liu J, Matuszewska M, Gaimster H, Summers DK. Local and universal action: the paradoxes of indole signalling in bacteria. Trends Microbiol. 2020;28:566–77. https://doi.org/10.1016/j.tim.2020.02.007.
Baca-DeLancey RR, South MM, Ding X, Rather PN. Escherichia coli genes regulated by cell-to-cell signaling. Proc Natl Acad Sci USA. 1999;96:4610–4. https://doi.org/10.1073/pnas.96.8.4610.
Wang DD, Ding XD, Rather PN. Indole can act as an extracellular signal in Escherichia coli. J Bacteriol. 2001;183:4210–6. https://doi.org/10.1128/Jb.183.14.4210-4216.2001.
Chattoraj DK. Tryptophanase in sRNA control of the Escherichia coli cell cycle. Mol Microbiol. 2007;63:1–3. https://doi.org/10.1111/j.1365-2958.2006.05517.x.
Chant EL, Summers DK. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol. 2007;63:35–43. https://doi.org/10.1111/j.1365-2958.2006.05481.x.
Misra HS, Maurya GK, Chaudhary R, Misra CS. Interdependence of bacterial cell division and genome segregation and its potential in drug development. Microbiol Res. 2018;208:12–24. https://doi.org/10.1016/j.micres.2017.12.013.
Chimerel C, Field CM, Pinero-Fernandez S, Keyser UF, Summers DK. Indole prevents Escherichia coli cell division by modulating membrane potential. Biochim Biophys Acta. 2012;1818:1590–4. https://doi.org/10.1016/j.bbamem.2012.02.022.
Sakai M, Tohyama K, Mutai M. Effect of indole on adenylate energy charge and mitochondrial phosphorylative activity of rat liver. Int J Biochem. 1982;14:569–72. https://doi.org/10.1016/0020-711x(82)90037-4.
Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nat Chem Biol. 2012;8:431–3. https://doi.org/10.1038/nchembio.915.
Hu Y, Kwan BW, Osbourne DO, Benedik MJ, Wood TK. Toxin YafQ increases persister cell formation by reducing indole signalling. Environ Microbiol. 2015;17:1275–85. https://doi.org/10.1111/1462-2920.12567.
Lee JH, Kim YG, Gwon G, Wood TK, Lee J. Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express. 2016;6:123. https://doi.org/10.1186/s13568-016-0297-6.
Lang M, Krin E, Korlowski C, Sismeiro O, Varet H, Coppee JY, Mazel D, Baharoglu Z. Sleeping ribosomes: bacterial signaling triggers RaiA mediated persistence to aminoglycosides. iScience. 2021;24:103128. https://doi.org/10.1016/j.isci.2021.103128.
Song S, Wood TK. Combatting persister cells with substituted indoles. Front Microbiol. 2020;11:1565. https://doi.org/10.3389/fmicb.2020.01565.
Zhang W, Yamasaki R, Song S, Wood TK. Interkingdom signal indole inhibits Pseudomonas aeruginosa persister cell waking. J Appl Microbiol. 2019;127:1768–75. https://doi.org/10.1111/jam.14434.
Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The “undrained abscess” of multiple organ failure. Ann Surg. 1993;218:111–9. https://doi.org/10.1097/00000658-199308000-00001.
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–75. https://doi.org/10.1038/nrmicro.2016.94.
Schulze A, Mitterer F, Pombo JP, Schild S. Biofilms by bacterial human pathogens: clinical relevance—development, composition and regulation—therapeutical strategies. Microb Cell. 2021;8:28–56. https://doi.org/10.15698/mic2021.02.741.
Lee JT, Jayaraman A, Wood TK. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 2007;7:42. https://doi.org/10.1186/1471-2180-7-42.
Lee JH, Wood TK, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23:707–18. https://doi.org/10.1016/j.tim.2015.08.001.
Hu M, Zhang C, Mu Y, Shen Q, Feng Y. Indole affects biofilm formation in bacteria. Indian J Microbiol. 2010;50:362–8. https://doi.org/10.1007/s12088-011-0142-1.
Di Martino P, Fursy R, Bret L, Sundararaju B, Phillips RS. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can J Microbiol. 2003;49:443–9. https://doi.org/10.1139/W03-056.
Domka J, Lee J, Wood TK. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl Environ Microbiol. 2006;72:2449–59. https://doi.org/10.1128/AEM.72.4.2449-2459.2006.
Zhang XS, Garcia-Contreras R, Wood TK. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J Bacteriol. 2007;189:3051–62. https://doi.org/10.1128/Jb.01832-06.
Lee J, Bansal T, Jayaraman A, Bentley WE, Wood TK. Enterohemorrhagic Escherichia coli biofilms are inhibited by 7-hydroxyindole and stimulated by isatin. Appl Environ Microbiol. 2007;73:4100–9. https://doi.org/10.1128/AEM.00360-07.
Lee J, Zhang XS, Hegde M, Bentley WE, Jayaraman A, Wood TK. Indole cell signaling occurs primarily at low temperatures in Escherichia coli. Isme J. 2008;2:1007–23. https://doi.org/10.1038/ismej.2008.54.
Hashidoko Y, Kim D. Bidirectional cell-cell communication via indole and cyclo(Pro-Tyr) modulates interspecies biofilm formation. Appl Environ Microbiol. 2021;87:e0127721. https://doi.org/10.1128/AEM.01277-21.
Cui B, Chen X, Guo Q, Song S, Wang M, Liu J, Deng Y. The cell-cell communication signal indole controls the physiology and interspecies communication of Acinetobacter baumannii. Microbiol Spectr. 2022;10:e0102722. https://doi.org/10.1128/spectrum.01027-22.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Group WHOPPLW. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27. https://doi.org/10.1016/S1473-3099(17)30753-3.
Bawn M, Alikhan NF, Thilliez G, Kirkwood M, Wheeler NE, Petrovska L, Dallman TJ, Adriaenssens EM, Hall N, Kingsley RA. Evolution of Salmonella enterica serotype typhimurium driven by anthropogenic selection and niche adaptation. PLoS Genet. 2020;16:e1008850. https://doi.org/10.1371/journal.pgen.1008850.
Kohli N, Crisp Z, Riordan R, Li M, Alaniz RC, Jayaraman A. The microbiota metabolite indole inhibits Salmonella virulence: involvement of the PhoPQ two-component system. PLoS ONE. 2018;13:e0190613. https://doi.org/10.1371/journal.pone.0190613.
Nikaido E, Giraud E, Baucheron S, Yamasaki S, Wiedemann A, Okamoto K, Takagi T, Yamaguchi A, Cloeckaert A, Nishino K. Effects of indole on drug resistance and virulence of Salmonella enterica serovar typhimurium revealed by genome-wide analyses. Gut Pathog. 2012;4:5. https://doi.org/10.1186/1757-4749-4-5.
Darkoh C, Plants-Paris K, Bishoff D, DuPont HL. Clostridium difficile modulates the gut microbiota by inducing the production of indole, an interkingdom signaling and antimicrobial molecule. Systems. 2019. https://doi.org/10.1128/mSystems.00346-18.
Darkoh C, Chappell C, Gonzales C, Okhuysen P. A rapid and specific method for the detection of indole in complex biological samples. Appl Environ Microbiol. 2015;81:8093–7. https://doi.org/10.1128/AEM.02787-15.
Wu D, Yi X, Tang R, Feng C, Wei C. Single microbial fuel cell reactor for coking wastewater treatment: simultaneous carbon and nitrogen removal with zero alkaline consumption. Sci Total Environ. 2018;621:497–506. https://doi.org/10.1016/j.scitotenv.2017.11.262.
Ma Q, Zhang X, Qu Y. Biodegradation and biotransformation of indole: advances and perspectives. Front Microbiol. 2018;9:2625. https://doi.org/10.3389/fmicb.2018.02625.
Walters M, Sperandio V. Quorum sensing in Escherichia coli and Salmonella. Int J Med Microbiol. 2006;296:125–31. https://doi.org/10.1016/j.ijmm.2006.01.041.
Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D. Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology. 2020;79:89–99. https://doi.org/10.1159/000496293.
Whitfield-Cargile CM, Cohen ND, Chapkin RS, Weeks BR, Davidson LA, Goldsby JS, Hunt CL, Steinmeyer SH, Menon R, Suchodolski JS, Jayaraman A, Alaniz RC. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7:246–61. https://doi.org/10.1080/19490976.2016.1156827.
Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107:228–33. https://doi.org/10.1073/pnas.0906112107.
Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J Immunol. 2018;201:3683–93. https://doi.org/10.4049/jimmunol.1701734.
Ihekweazu FD, Engevik MA, Ruan W, Shi Z, Fultz R, Engevik KA, Chang-Graham AL, Freeborn J, Park ES, Venable S, Horvath TD, Haidacher SJ, Haag AM, Goodwin A, Schady DA, Hyser JM, Spinler JK, Liu Y, Versalovic J. Bacteroides ovatus promotes IL-22 production and reduces trinitrobenzene sulfonic acid-driven colonic inflammation. Am J Pathol. 2021;191:704–19. https://doi.org/10.1016/j.ajpath.2021.01.009.
Ehrlich AM, Pacheco AR, Henrick BM, Taft D, Xu GG, Huda MN, Mishchuk D, Goodson ML, Slupsky C, Barile D, Lebrilla CB, Stephensen CB, Mills DA, Raybould HE. Indole-3-lactic acid associated with bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020;20:357. https://doi.org/10.1186/s12866-020-02023-y.
Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O’Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22:e6. https://doi.org/10.1016/j.chom.2017.06.007.
Ye X, Li H, Anjum K, Zhong X, Miao S, Zheng G, Liu W, Li L. Dual role of indoles derived from intestinal microbiota on human health. Front Immunol. 2022;13:903526. https://doi.org/10.3389/fimmu.2022.903526.
Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294. https://doi.org/10.1038/s41467-018-05470-4.
Karlin DA, Mastromarino AJ, Jones RD, Stroehlein JR, Lorentz O. Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J Cancer Res Clin Oncol. 1985;109:135–41. https://doi.org/10.1007/BF00391888.
Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–11. https://doi.org/10.1016/j.immuni.2009.08.011.
Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–74. https://doi.org/10.1007/s00535-017-1401-7.
Mar JS, Ota N, Pokorzynski ND, Peng Y, Jaochico A, Sangaraju D, Skippington E, Lekkerkerker AN, Rothenberg ME, Tan MW, Yi T, Keir ME. IL-22 alters gut microbiota composition and function to increase aryl hydrocarbon receptor activity in mice and humans. Microbiome. 2023;11:47. https://doi.org/10.1186/s40168-023-01486-1.
van der Bruggen T, Nijenhuis S, van Raaij E, Verhoef J, van Asbeck BS. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the RAF1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun. 1999;67:3824–9. https://doi.org/10.1128/IAI.67.8.3824-3829.1999.
Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. https://doi.org/10.1002/jlb.60.1.8.
Kalia VC, Patel SKS, Kang YC, Lee JK. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2019;37:68–90. https://doi.org/10.1016/j.biotechadv.2018.11.006.
Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–40. https://doi.org/10.1038/nrmicro818.
Torres AG, Kaper JB. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect Immun. 2003;71:4985–95. https://doi.org/10.1128/IAI.71.9.4985-4995.2003.
Bansal T, Englert D, Lee J, Hegde M, Wood TK, Jayaraman A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 2007;75:4597–607. https://doi.org/10.1128/IAI.00630-07.
NicAogain K, O’Byrne CP. The role of stress and stress adaptations in determining the fate of the bacterial pathogen listeria monocytogenes in the food chain. Front Microbiol. 2016;7:1865. https://doi.org/10.3389/fmicb.2016.01865.
Rattanaphan P, Mittraparp-Arthorn P, Srinoun K, Vuddhakul V, Tansila N. Indole signaling decreases biofilm formation and related virulence of Listeria monocytogenes. FEMS Microbiol Lett. 2020. https://doi.org/10.1093/femsle/fnaa116.
Song S, Gong T, Yamasaki R, Kim JS, Wood TK. Identification of a potent indigoid persister antimicrobial by screening dormant cells. Biotechnol Bioeng. 2019;116:2263–74. https://doi.org/10.1002/bit.27078.
Sun FQ, Bian MM, Li ZY, Lv BY, Gao YY, Wang Y, Fu XM. 5-Methylindole potentiates aminoglycoside against gram-positive bacteria including Staphylococcus aureus persisters under hypoionic conditions. Front Cell Infect Mi. 2020;10:84. https://doi.org/10.3389/fcimb.2020.00084.
Megaw J, Gilmore BF. Archaeal persisters: persister cell formation as a stress response in Haloferax volcanii. Front Microbiol. 2017;8:1589. https://doi.org/10.3389/fmicb.2017.01589.
Sun P, Huang Y, Yang X, Liao A, Wu J. The role of indole derivative in the growth of plants: a review. Front Plant Sci. 2022;13:1120613. https://doi.org/10.3389/fpls.2022.1120613.
Busi R, Goggin DE, Heap IM, Horak MJ, Jugulam M, Masters RA, Napier RM, Riar DS, Satchivi NM, Torra J, Westra P, Wright TR. Weed resistance to synthetic auxin herbicides. Pest Manag Sci. 2018;74:2265–76. https://doi.org/10.1002/ps.4823.
Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49–64. https://doi.org/10.1146/annurev-arplant-042809-112308.
Wang X, Luo MJ, Wang YX, Han WQ, Miu JX, Luo XP, Zhang AD, Kuang Y. Design, synthesis, and herbicidal activity of indole-3-carboxylic acid derivatives as potential transport inhibitor response 1 antagonists. Front Chem. 2022;10:975267. https://doi.org/10.3389/fchem.2022.975267.
Grossmann K. Mediation of herbicide effects by hormone interactions. J Plant Growth Regul. 2003;22:109–22. https://doi.org/10.1007/s00344-003-0020-0.
Xie J, Xu W, Song H, Liu Y, Zhang J, Wang Q. Synthesis and antiviral/fungicidal/insecticidal activities study of novel chiral indole diketopiperazine derivatives containing acylhydrazone moiety. J Agric Food Chem. 2020;68:5555–71. https://doi.org/10.1021/acs.jafc.0c00875.
Zheng SJ, Jiang QJ, Massande GN, Wu WB, Lin CS, Fang Y, Tan Y, Zhu R. Synthesis and antifungal activity of indole derivatives. Chem Nat Compd. 2023;59:111–8. https://doi.org/10.1007/s10600-023-03929-5.
Böckler F, Dill B, Eisenbrand G, Faupel F, Fugmann B, Gamse T, Matissek R, Pohnert G, Rühling A, Schmidt S, Sprenger G. RÖMPP. Stuttgart: Georg Thieme Verlag; 2023.
Edris AE, Chizzola R, Franz C. Isolation and characterization of the volatile aroma compounds from the concrete headspace and the absolute of Jasminum sambac (L.) Ait. (Oleaceae) flowers grown in Egypt. Eur Food Res Technol. 2008;226:621–6. https://doi.org/10.1007/s00217-007-0623-y.
Mookherjee BD, Trenkle RW, Wilson RA. Live vs. dead. Part II. A comparative analysis of the headspace volatiles of some important fragrance and flavor raw materials. J Essent Oil Res. 1989;1:85–90. https://doi.org/10.1080/10412905.1989.9697755.
https://precisionbusinessinsights.com/market-reports/indole-market/
Feierfeil J, Magauer T. De novo synthesis of benzannelated heterocycles. Chemistry. 2018;24:1455–8. https://doi.org/10.1002/chem.201705662.
Humphrey GR, Kuethe JT. Practical methodologies for the synthesis of indoles. Chem Rev. 2006;106:2875–911. https://doi.org/10.1021/cr0505270.
Larock RC, Yum EK, Refvik MD. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J Org Chem. 1998;63:7652–62. https://doi.org/10.1021/jo9803277.
Sahu S, Banerjee A, Kundu S, Bhattacharyya A, Maji MS. Synthesis of functionalized indoles via cascade benzannulation strategies: a decade’s overview. Org Biomol Chem. 2022;20:3029–42. https://doi.org/10.1039/d2ob00187j.
Nasri S, Bayat M, Miankooshki FR, Samet NH. Recent developments in green approaches for sustainable synthesis of indole-derived scaffolds. Mol Divers. 2022;26:3411–45. https://doi.org/10.1007/s11030-021-10376-3.
Wu Y, Wang T, Zhang C, Xing XH. A rapid and specific colorimetric method for free tryptophan quantification. Talanta. 2018;176:604–9. https://doi.org/10.1016/j.talanta.2017.08.002.
Fabara AN, Fraaije MW. An overview of microbial indigo-forming enzymes. Appl Microbiol Biotechnol. 2020;104:925–33. https://doi.org/10.1007/s00253-019-10292-5.
Medici R, Garaycoechea JI, Dettorre LA, Iribarren AM, Lewkowicz ES. Biocatalysed halogenation of nucleobase analogues. Biotechnol Lett. 2011;33:1999–2003. https://doi.org/10.1007/s10529-011-0655-z.
Domergue J, Erdmann D, Fossey-Jouenne A, Petit JL, Debard A, de Berardinis V, Vergne-Vaxelaire C, Zaparucha A. XszenFHal, a novel tryptophan 5-halogenase from Xenorhabdus szentirmaii. AMB Express. 2019;9:175. https://doi.org/10.1186/s13568-019-0898-y.
Bradley SA, Zhang J, Jensen MK. Deploying microbial synthesis for halogenating and diversifying medicinal alkaloid scaffolds. Front Bioeng Biotechnol. 2020;8:594126. https://doi.org/10.3389/fbioe.2020.594126.
Ismail M, Frese M, Patschkowski T, Ortseifen V, Niehaus K, Sewald N. Flavin-dependent halogenases from Xanthomonas campestris pv. campestris B100 prefer bromination over chlorination. Adv Synth Catal. 2019;361:2475–86. https://doi.org/10.1002/adsc.201801591.
Adak S, Lukowski AL, Schafer RJB, Moore BS. From tryptophan to toxin: nature’s convergent biosynthetic strategy to aetokthonotoxin. J Am Chem Soc. 2022;144:2861–6. https://doi.org/10.1021/jacs.1c12778.
Li G, Young KD. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology (Reading). 2013;159:402–10. https://doi.org/10.1099/mic.0.064139-0.
Mindt M, Beyraghdar Kashkooli A, Suarez-Diez M, Ferrer L, Jilg T, Bosch D, Martins Dos Santos V, Wendisch VF, Cankar K. Production of indole by Corynebacterium glutamicum microbial cell factories for flavor and fragrance applications. Microb Cell Fact. 2022;21:45. https://doi.org/10.1186/s12934-022-01771-y.
Wolf S, Becker J, Tsuge Y, Kawaguchi H, Kondo A, Marienhagen J, Bott M, Wendisch VF, Wittmann C. Advances in metabolic engineering of Corynebacterium glutamicum to produce high-value active ingredients for food, feed, human health, and well-being. Essays Biochem. 2021;65:197–212. https://doi.org/10.1042/EBC20200134.
Cankar K, Henke NA, Wendisch VF. Functional food additives/ingredients production by engineered Corynebacterium glutamicum. SMAB. 2022;3:110–21.
Kerbs A, Burgardt A, Veldmann KH, Schaffer T, Lee JH, Wendisch VF. Fermentative production of halogenated tryptophan derivatives with Corynebacterium glutamicum overexpressing tryptophanase or decarboxylase genes. ChemBioChem. 2022;23: e202200007. https://doi.org/10.1002/cbic.202200007.
Mindt M, Ferrer L, Bosch D, Cankar K, Wendisch VF. De novo tryptophanase-based indole production by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2023;107:1621–34. https://doi.org/10.1007/s00253-023-12397-4.
Ferrer L, Mindt M, Suarez-Diez M, Jilg T, Zagorscak M, Lee JH, Gruden K, Wendisch VF, Cankar K. Fermentative indole production via bacterial tryptophan synthase alpha subunit and plant indole-3-glycerol phosphate lyase enzymes. J Agric Food Chem. 2022;70:5634–45. https://doi.org/10.1021/acs.jafc.2c01042.
Hyde CC, Ahmed SA, Padlan EA, Miles EW, Davies DR. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988;263:17857–71.
Xiao SJ, Wang Z, Wang BX, Hou B, Cheng J, Bai T, Zhang Y, Wang W, Yan LX, Zhang JM. Expanding the application of tryptophan: industrial biomanufacturing of tryptophan derivatives. Front Microbiol. 2023. https://doi.org/10.3389/fmicb.2023.1099098.
Ensley BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, Gibson DT. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983;222:167–9. https://doi.org/10.1126/science.6353574.
Murdock D, Ensley BD, Serdar C, Thalen M. Construction of metabolic operons catalyzing the de novo biosynthesis of indigo in Escherichia coli. Biotechnology (NY). 1993;11:381–6. https://doi.org/10.1038/nbt0393-381.
Berry A, Dodge TC, Pepsin M, Weyler W. Application of metabolic engineering to improve both the production and use of biotech indigo. J Ind Microbiol Biotechnol. 2002;28:127–33. https://doi.org/10.1038/sj.jim.7000228.
Han GH, Bang SE, Babu BK, Chang M, Shin HJ, Kim SW. Bio-indigo production in two different fermentation systems using recombinant Escherichia coli cells harboring a flavin-containing monooxygenase gene (fmo). Process Biochem. 2011;46:788–91. https://doi.org/10.1016/j.procbio.2010.10.015.
Fabara AN, Fraaije MW. Production of indigo through the use of a dual-function substrate and a bifunctional fusion enzyme. Enzyme Microb Technol. 2020;142:109692. https://doi.org/10.1016/j.enzmictec.2020.109692.
Han GH, Gim GH, Kim W, Seo SI, Kim SW. Enhanced indirubin production in recombinant Escherichia coli harboring a flavin-containing monooxygenase gene by cysteine supplementation. J Biotechnol. 2012;164:179–87. https://doi.org/10.1016/j.jbiotec.2012.08.015.
Du J, Yang D, Luo ZW, Lee SY. Metabolic engineering of Escherichia coli for the production of indirubin from glucose. J Biotechnol. 2018;267:19–28. https://doi.org/10.1016/j.jbiotec.2017.12.026.
Lee J, Kim J, Song JE, Song WS, Kim EJ, Kim YG, Jeong HJ, Kim HR, Choi KY, Kim BG. Production of tyrian purple indigoid dye from tryptophan in Escherichia coli. Nat Chem Biol. 2021;17:104–12. https://doi.org/10.1038/s41589-020-00684-4.
Lee J, Kim J, Kim H, Park H, Kim JY, Kim EJ, Yang YH, Choi KY, Kim BG. Constructing multi-enzymatic cascade reactions for selective production of 6-bromoindirubin from tryptophan in Escherichia coli. Biotechnol Bioeng. 2022;119:2938–49. https://doi.org/10.1002/bit.28188.
Veldmann KH, Dachwitz S, Risse JM, Lee JH, Sewald N, Wendisch VF. Bromination of L-tryptophan in a fermentative process with Corynebacterium glutamicum. Front Bioeng Biotechnol. 2019;7:219. https://doi.org/10.3389/fbioe.2019.00219.
Veldmann KH, Minges H, Sewald N, Lee JH, Wendisch VF. Metabolic engineering of Corynebacterium glutamicum for the fermentative production of halogenated tryptophan. J Biotechnol. 2019;291:7–16. https://doi.org/10.1016/j.jbiotec.2018.12.008.
Bradley SA, Lehka BJ, Hansson FG, Adhikari KB, Rago D, Rubaszka P, Haidar AK, Chen L, Hansen LG, Gudich O, Giannakou K, Lengger B, Gill RT, Nakamura Y, de Bernonville TD, Koudounas K, Romero-Suarez D, Ding L, Qiao Y, Frimurer TM, Petersen AA, Besseau S, Kumar S, Gautron N, Melin C, Marc J, Jeanneau R, O’Connor SE, Courdavault V, Keasling JD, Zhang J, Jensen MK. Biosynthesis of natural and halogenated plant monoterpene indole alkaloids in yeast. Nat Chem Biol. 2023. https://doi.org/10.1038/s41589-023-01430-2.
Devi N, Kaur K, Biharee A, Jaitak V. Recent development in indole derivatives as anticancer agent: a mechanistic approach. Anticancer Agents Med Chem. 2021;21:1802–24. https://doi.org/10.2174/1871520621999210104192644.
Emert BL, Cote CJ, Torre EA, Dardani IP, Jiang CL, Jain N, Shaffer SM, Raj A. Variability within rare cell states enables multiple paths toward drug resistance. Nat Biotechnol. 2021;39:865–76. https://doi.org/10.1038/s41587-021-00837-3.
Ishikawa E, Kanai S, Sue M. Detection of a novel intramolecular rearrangement during gramine biosynthesis in barley using stable isotope-labeled tryptophan. Biochem Biophys Rep. 2023;34:101439. https://doi.org/10.1016/j.bbrep.2023.101439.
Cohen JD, Tang Q, Hegeman AD. Using targeted metabolomics to elucidate the indole auxin network in plants. Methods Enzymol. 2022;676:239–78. https://doi.org/10.1016/bs.mie.2022.07.038.
Ahmad S, Mohammed M, Mekala LP, Anusha R, Sasikala C, Ramana CV. Stable isotope-assisted metabolite profiling reveals new insights into L-tryptophan chemotrophic metabolism of Rubrivivax benzoatilyticus. World J Microbiol Biotechnol. 2023;39:98. https://doi.org/10.1007/s11274-023-03537-z.
Mishiro K, Nishii R, Sawazaki I, Sofuku T, Fuchigami T, Sudo H, Effendi N, Makino A, Kiyono Y, Shiba K, Taki J, Kinuya S, Ogawa K. Development of radiohalogenated osimertinib derivatives as imaging probes for companion diagnostics of osimertinib. J Med Chem. 2022;65:1835–47. https://doi.org/10.1021/acs.jmedchem.1c01211.
Nanga RPR, Elliott MA, Swain A, Wilson N, Swago S, Soni ND, Witschey WR, Reddy R. Identification of L-tryptophan by down-field (1) H MRS: a precursor for brain NAD(+) and serotonin syntheses. Magn Reson Med. 2022;88:2371–7. https://doi.org/10.1002/mrm.29414.
Jeschke P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag Sci. 2010;66:10–27. https://doi.org/10.1002/ps.1829.
Zhang N, Hasenstein KH. Halogenated auxins affect microtubules and root elongation in Lactuca sativa. J Plant Growth Regul. 2000;19:397–405. https://doi.org/10.1007/s003440000042.
Rajasekharan SK, Lee JH, Ravichandran V, Kim JC, Park JG, Lee J. Nematicidal and insecticidal activities of halogenated indoles. Sci Rep. 2019;9:2010. https://doi.org/10.1038/s41598-019-38561-3.
Pavlopoulos GA, Baltoumas FA, Liu S, Selvitopi O, Camargo AP, Nayfach S, Azad A, Roux S, Call L, Ivanova NN, Chen IM, Paez-Espino D, Karatzas E, Iliopoulos I, Konstantinidis K, Tiedje JM, Pett-Ridge J, Baker D, Visel A, Ouzounis CA, Ovchinnikov S, Buluc A, Kyrpides NC. Unraveling the functional dark matter through global metagenomics. Nature. 2023;622:594–602. https://doi.org/10.1038/s41586-023-06583-7.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. https://doi.org/10.1038/s41586-021-03819-2.
Kroll A, Ranjan S, Engqvist MKM, Lercher MJ. A general model to predict small molecule substrates of enzymes based on machine and deep learning. Nat Commun. 2023;14:2787. https://doi.org/10.1038/s41467-023-38347-2.
Buller AR, Brinkmann-Chen S, Romney DK, Herger M, Murciano-Calles J, Arnold FH. Directed evolution of the tryptophan synthase beta-subunit for stand-alone function recapitulates allosteric activation. Proc Natl Acad Sci USA. 2015;112:14599–604. https://doi.org/10.1073/pnas.1516401112.
Ferrer L, Elsaraf M, Mindt M, Wendisch VF. l-Serine biosensor-controlled fermentative production of l-tryptophan derivatives by Corynebacterium glutamicum. Biology (Basel). 2022;11:744. https://doi.org/10.3390/biology11050744.
Sun L, Alper HS. Non-conventional hosts for the production of fuels and chemicals. Curr Opin Chem Biol. 2020;59:15–22. https://doi.org/10.1016/j.cbpa.2020.03.004.
Tenhaef N, Stella R, Frunzke J, Noack S. Automated rational strain construction based on high-throughput conjugation. ACS Synth Biol. 2021;10:589–99. https://doi.org/10.1021/acssynbio.0c00599.
Janzen NH, Striedner G, Jarmer J, Voigtmann M, Abad S, Reinisch D. Implementation of a fully automated microbial cultivation platform for strain and process screening. Biotechnol J. 2019;14:e1800625. https://doi.org/10.1002/biot.201800625.
Helleckes LM, Puchta D, Czech H, Morschett H, Geinitz B, Wiechert W, Oldiges M. From frozen cell bank to product assay: high-throughput strain characterisation for autonomous Design-Build-Test-Learn cycles. Microb Cell Fact. 2023;22:130. https://doi.org/10.1186/s12934-023-02140-z.
Sassi H, Nguyen TM, Telek S, Gosset G, Grunberger A, Delvigne F. Segregostat: a novel concept to control phenotypic diversification dynamics on the example of Gram-negative bacteria. Microb Biotechnol. 2019;12:1064–75. https://doi.org/10.1111/1751-7915.13442.
Acknowledgements
The authors would like to thank members of their groups for discussions.
Funding
Open Access funding enabled and organized by Projekt DEAL. Support of the ERA CoBioTech project INDIE (European Union’s Horizon 2020 research and innovation programme under grant agreement No. 722361) with national funding is acknowledged by KC and MM (Dutch research council (NWO) grant number 053.80.732) and LF and VFW (Renewable Resources Scheme (FNR) of the Federal Ministry of Food and Agriculture, Germany, grant number 22023517).
Author information
Authors and Affiliations
Contributions
LF, MM, VFW, KC: conceptualization, literature research and manuscript writing, and manuscript revision. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
MM was employed by Axxence Aromatic GmbH. All other authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrer, L., Mindt, M., Wendisch, V.F. et al. Indoles and the advances in their biotechnological production for industrial applications. Syst Microbiol and Biomanuf 4, 511–527 (2024). https://doi.org/10.1007/s43393-023-00223-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00223-x