Abstract
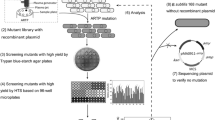
As an important industrial enzyme, protease is widely used in feed, food and other fields. At present, the insufficient protease activity obtained from microorganisms cannot meet the purpose of industrial production. In this study, Bacillus amyloliquefaciens with high protease production was screened from animal feces by plate transparent circle method. To improve the production of protease, atmospheric room temperature plasma (ARTP) mutagenesis was used in the first round, protease activity reached 315.0 U/mL. Then, to enhance production of protease, 60Co-γ irradiation was used for combined mutagenesis, leading to protease activity of B. amyloliquefaciens FMME ZK003 up to 355.0 U/mL. Furthermore, to realize the efficient production of protease, after optimization of fermentation conditions, protease activity was increased to 456.9 U/mL. Finally, protease activity of B. amyloliquefaciens FMME ZK003 reached 823.0 U/mL in a 5 L fermenter. These results indicate that B. amyloliquefaciens can efficiently produce protease, which provides a good foundation for the industrial production of protease.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Razzaq A, Shamsi S, Ali A, Ali Q, Sajjad M, Malik A, Ashraf M. Microbial proteases applications. front bioeng. Biotechnol. 2019;7:110.
Contesini FJ, de Melo RR, Sato HH. An overview of Bacillus proteases: from production to application. Crit Rev Biotechnol. 2018;38:321–34.
Wang L, Wang Y-J. Rice starch isolation by neutral protease and high-intensity ultrasound. J Cereal Sci. 2004;39:291–6.
Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124:1354–62.
Hejdysz M, Kaczmarek SA, Kubiś M, Wiśniewska Z, Peris S, Budnik S, Rutkowski A. The effect of protease and Bacillus licheniformis on nutritional value of pea, faba bean, yellow lupin and narrow-leaved lupin in broiler chicken diets. Br Poult Sci. 2020;61:287–93.
Naveed M, Nadeem F, Mehmood T, Bilal M, Anwar Z, Amjad F. Protease—a versatile and ecofriendly biocatalyst with multi-industrial applications: an updated review. Catal Lett. 2021;151:307–23.
Vojcic L, Pitzler C, Körfer G, Jakob F, Ronny M, Maurer K-H, Schwaneberg U. Advances in protease engineering for laundry detergents. New Biotechnol. 2015;32:629–34.
Guleria S, Walia A, Chauhan A, Shirkot CK. Purification and characterization of detergent stable alkaline protease from Bacillus amyloliquefaciens SP1 isolated from apple rhizosphere. J Basic Microbiol. 2016;56:138–52.
Dayanandan A, Kanagaraj J, Sounderraj L, Govindaraju R, Rajkumar GS. Application of an alkaline protease in leather processing: an ecofriendly approach. J Cleaner Prod. 2003;11:533–6.
Rehman R, Ahmed M, Siddique A, Hasan F, Hameed A, Jamal A. Catalytic role of thermostable metalloproteases from bacillus subtilis KT004404 as dehairing and destaining agent. Appl Biochem Biotechnol. 2017;181:434–50.
Gupta R, Beg QK, Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol. 2002;59:15–32.
Calik P, Ozdamar TH. Carbon sources affect metabolic capacities of Bacillus species for the production of industrial enzymes: theoretical analyses for serine and neutral proteases and alpha-amylase. Biochem Eng J. 2001;8:61–81.
Ahmed Z, Donkor O, Street WA, Vasiljevic T. Proteolytic activities in fillets of selected underutilized Australian fish species. Food Chem. 2013;140:238–44.
Chalamaiah M, Dineshkumar B, Hemalatha R, Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012;135:3020–38.
Mala BR, Aparna MT, Mohini SG, Vasanti VD. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev. 1998;62:597–635.
Tufvesson P, Lima-Ramos J, Nordblad M, Woodley JM. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org Process Res Dev. 2011;15:266–74.
Sn N, D J. Optimization of alkaline protease production from Bacillus subtilis NS isolated from sea water. Afr J Biotechnol. 2014;13:1707–13.
Ali N, Ullah N, Qasim M, Rahman H, Khan SN, Sadiq A, Adnan M. Molecular characterization and growth optimization of halo-tolerant protease producing Bacillus Subtilis Strain BLK-1.5 isolated from salt mines of Karak. Pakistan Extremophiles. 2016;20:395–402.
Karray A, Alonazi M, Horchani H, Ben BA. A novel thermostable and alkaline protease produced from Bacillus stearothermophilus isolated from olive oil mill sols suitable to industrial biotechnology. Molecules. 2021. https://doi.org/10.3390/molecules26041139.
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol. 2017. https://doi.org/10.3389/fmicb.2017.01490.
Soares VF, Castilho LR, Bon EPS, Freire DMG. High-yield bacillus subtilis protease production by solid-state fermentation. Appl Biochem Biotechnol. 2005;121:311–9.
Elyasi Far B, Yari Khosroushahi A, Dilmaghani A. In silico study of alkaline serine protease and production optimization in Bacillus sp. Khoz1 closed bacillus safensis isolated from honey. Int J Pept Res Ther. 2020;26:2241–51.
Lario LD, Chaud L, Almeida MdG, Converti A, Durães Sette L, Pessoa A. Production, purification, and characterization of an extracellular acid protease from the marine Antarctic yeast Rhodotorula mucilaginosa L7. Fungal Biol. 2015;119:1129–36.
Yegin S, Fernandez-Lahore M, JoseGamaSalgado A, Guvenc U, Goksungur Y, Tari C. Aspartic proteinases from Mucor spp in cheese manufacturing. Appl Microbiol Biotechnol. 2011;89:949–60.
Kalahroudi RJ, Valizadeh V, Atyabi SM, Keramati M, Cohan RA, Aghai A, Norouzian D. Increment in protease activity of Lysobacter enzymogenes strain by ultra violet radiation. Iran J Microbiol. 2020;12:601–6.
Tuly JA, Ma H, Zabed HM, Dong Y, Janet Q, Golly MK, Feng L, Li T, Chen G. Harnessing the keratinolytic activity of bacillus licheniformis through random mutagenesis using ultraviolet and laser irradiations. Appl Biochem Biotechnol. 2022;194:1546–65.
Wang Y, Wang J, Zhang X, Tong Y, Yang R. Genomic and transcriptomic analysis of Bacillus subtilis JNFE1126 with higher nattokinase production through ultraviolet combined 60Co-γ ray mutagenesis. LWT. 2021;147:111652.
Yadav SK, Singh P, Dubey KK, Singh BP. Combined mutagenic improvement of Bacillus licheniformis SK7 for cost-effective protease production. Asian J Bio Sci. 2016;11:91–4.
Zhang X. Applying the mutation of Bacillus subtilis and the optimization of feather fermentation medium to improve Keratinase activity. Adv Biol Chem. 2012;02:64–9.
Zhang JF, Zhu BY, Li XY, Xu XJ, Li DK, Zeng F, Zhou CX, Liu YH, Li Y, Lu FP. Multiple modular engineering of Bacillus Amyloliquefaciens cell factories for enhanced production of alkaline proteases from B. Clausii Front Bioeng Biotechnol. 2022;10:866066.
Zhou CX, Yang GC, Zhang L, Zhang HT, Zhou HY, Lu FP. Construction of an alkaline protease overproducer strain based on Bacillus licheniformis 2709 using an integrative approach. Int J Biol Macromol. 2021;193:1449–56.
Zhou C, Zhou H, Li D, Zhang H, Wang H, Lu F. Optimized expression and enhanced production of alkaline protease by genetically modified Bacillus licheniformis 2709. Microb Cell Fact. 2020;19:45.
Mo QS, Tian Y, Zhang HT, Bu LJ, Lu FP. Using 16S rDNA as target site for homologous recombination to improve the alkaline protease production of Bacillus alcalophilus. Adv Mater Res. 2014;886:349–54.
Suberu Y, Akande I, Samuel T, Lawal A, Olaniran A. Optimization of protease production in indigenous Bacillus species isolated from soil samples in Lagos. Nigeria using response surface methodology: Biocatal Agric Biotechnol; 2019. https://doi.org/10.1016/j.bcab.2019.01.049.
Cai CG, Zheng XD. Medium optimization for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. J Ind Microbiol Biotechnol. 2009;36:875–83.
He F, Chao J, Yang D, Zhang X, Yang C, Xu Z, Jiewei T, Yongqiang T. Optimization of fermentation conditions for production of neutral metalloprotease by Bacillus subtilis SCK6 and its application in goatskin-dehairing. Prep Biochem Biotechnol. 2021;52:789–99.
Ding Q, Luo QL, Zhou J, Chen XL, Liu LM. Enhancing l-malate production of Aspergillus oryzae FMME218-37 by improving inorganic nitrogen utilization. Appl Microbiol Biotechnol. 2018;102:8739–51.
Liu H, Zhang Z, Hong P, Zhou C, Yang P, Chen K, Wu J. Isolation of microbial strain producing thermostable protease and characterization of the enzyme. Sci Technol Food Ind. 2017;38:133.
Shu L, Si XG, Yang XD, Ma WY, Sun JL, Zhang J, Xue XL, Wang DP, Gao Q. Enhancement of acid protease activity ofaspergillus oryzaeusing atmospheric and room temperature plasma. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.01418.
Gao X, Liu E, Yin Y, Yang L, Huang Q, Chen S, Ho C-T. Enhancing activities of salt-tolerant proteases secreted by aspergillus oryzae using atmospheric and room-temperature plasma mutagenesis. J Agric Food Chem. 2020;68:2757–64.
Xue G, Chen L, Wu B, He B. Selection of high-yield thermostable protease producing strain by ARTP and the study on its enzymological properties. Sci Technol Food Ind. 2015;36(177–180):206.
Wang S, Luo Q, Liu J, Liu L, Chen X. Mutation and fermentation optimization of Bacillus amyloliquefaciens for acetoin production. Chin J Biotechnol. 2018;34:803–11.
Liu S, Fang Y, Lv M, Wang S, Chen L. Optimization of the production of organic solvent-stable protease by Bacillus sphaericus DS11 with response surface methodology. Bioresour Technol. 2010;101:7924–9.
Singh S, Bajaj BK. Medium optimization for enhanced production of protease with industrially desirable attributes from Bacillus subtilis K-1. Chem Eng Commun. 2015;202:1051–60.
Keshavamurthy M, Vishwanatha T, Kumar SM, Gaddad SM. Enhanced production of alkaline protease from novel bacterium Bacillus cereus GVK21 under submerged fermentation. Biosci Biotechnol Res Commun. 2018;11:416–25.
Funding
This study was supported by the Provincal Outstanding Youth Foundation of Jiangsu Province (BK20211529) and the National Science Fund for Excellent Young Scholars (22122806).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, K., Liu, H., Song, W. et al. Combinatorial mutagenesis of Bacillus amyloliquefaciens for efficient production of protease. Syst Microbiol and Biomanuf 3, 457–468 (2023). https://doi.org/10.1007/s43393-022-00130-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00130-7