Abstract
Introduction
Posterior spinal fusion (PSF) represents a large physiologic challenge for children with neuromuscular scoliosis (NMS). Perioperative complications are numerous with many occurring in the post-operative period due to pain and relative immobilization. This study assessed the impact of steroids on patients undergoing PSF for NMS.
Methods
A retrospective review of consecutive patients managed at a single center with PSF for NMS was reviewed. Clinical and radiographic analysis was used to evaluate baseline demographics, curve characteristics, and post-operative course.
Results
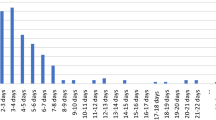
Eighty-nine patients who underwent PSF for NMS were included. Fifty-seven of these patients did not receive post-operative steroids (NS) while 32 patients were treated with post-operative steroids (dexamethasone, WS) for a median of 3 doses (median 6.0 mg/dose every 8 h after surgery). The demographic variables of the cohorts were similar with no difference in curve magnitude, number of vertebrae fused, number of osteotomies, or EBL between groups. A 70% decrease in the median post-operative morphine equivalents was observed in the steroid cohort (0.50 mg/kg WS vs 1.65 mg/kg NS, p value < 0.001). There was an association between post-operative morphine equivalents and length of stay (Spearman’s rho = 0.22, p value = 0.04). There was no difference in wound healing, infection, and pulmonary or gastrointestinal complications between groups. No difference was found in pain at discharge, 30-day ED returns, or 30-day OR returns between groups.
Conclusions
Post-operative dexamethasone resulted in a 70% decrease in morphine equivalent use after PSF for NMS without any increase in perioperative wound infections.
Level of evidence
Level 3: case–control series.
Similar content being viewed by others
Data availability
Deidentified data are available on request.
Code availability
Not applicable.
References
Kang GR, Suh SW, Lee IO (2011) Preoperative predictors of postoperative pulmonary complications in neuromuscular scoliosis. J Orthop Sci 16(2):139–147. https://doi.org/10.1007/s00776-011-0028-4
Murphy NA, Firth S, Jorgensen T et al (2006) Spinal surgery in children with idiopathic and neuromuscular scoliosis. What’s the difference? J Pediatr Orthop 26(2):216–220. https://doi.org/10.1097/01.bpo.0000206516.61706.6e
Rumalla K, Yarbrough CK, Pugely AJ et al (2016) Spinal fusion for pediatric neuromuscular scoliosis: national trends, complications, and in-hospital outcomes. J Neurosurg Spine 25(4):500–508. https://doi.org/10.3171/2016.2.SPINE151377
Yuan N, Skaggs DL, Dorey F et al (2005) Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol 40(5):414–419. https://doi.org/10.1002/ppul.20291
Mohamad F, Parent S, Pawelek J et al (2007) Perioperative complications after surgical correction in neuromuscular scoliosis. J Pediatr Orthop 27(4):392–397. https://doi.org/10.1097/01.bpb.0000271321.10869.98
Perrin C, Unterborn JN, Ambrosio CD et al (2004) Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 29(1):5–27. https://doi.org/10.1002/mus.10487
Moore RP, Wester T, Sunder R et al (2013) Peri-operative pain management in children with cerebral palsy: comparative efficacy of epidural vs systemic analgesia protocols. Paediatr Anaesth 23(8):720–725. https://doi.org/10.1111/pan.12187
Yiu CH, Vitharana N, Gnjidic D et al (2022) Patient risk factors for opioid-related adverse drug events in hospitalized patients: a systematic review. Pharmacotherapy. https://doi.org/10.1002/phar.2666
Fletcher ND, Ruska T, Austin TM et al (2020) Postoperative dexamethasone following posterior spinal fusion for adolescent idiopathic scoliosis. J Bone Joint Surg Am 102(20):1807–1813. https://doi.org/10.2106/JBJS.20.00259
Polderman JA, Farhang-Razi V, Van Dieren S et al (2018) Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev 11:CD011940. https://doi.org/10.1002/14651858.CD011940.pub3
Polderman JA, Farhang-Razi V, Van Dieren S et al (2018) Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev 8:11940. https://doi.org/10.1002/14651858.CD011940.pub2
Polderman JAW, Farhang-Razi V, van Dieren S et al (2019) Adverse side-effects of dexamethasone in surgical patients - an abridged Cochrane systematic review. Anaesthesia 74(7):929–939. https://doi.org/10.1111/anae.14610
Vuorinen MA, Palanne RA, Makinen TJ et al (2019) Infection safety of dexamethasone in total hip and total knee arthroplasty: a study of eighteen thousand, eight hundred and seventy two operations. Int Orthop 43(8):1787–1792. https://doi.org/10.1007/s00264-018-4156-8
Jain A, Sponseller PD, Shah SA et al (2016) Subclassification of GMFCS level-5 cerebral palsy as a predictor of complications and health-related quality of life after spinal arthrodesis. J Bone Jt Surg Am 98(21):1821–1828. https://doi.org/10.2106/JBJS.15.01359
Bellaire LL, Bruce RW Jr, Ward LA et al (2019) Use of an accelerated discharge pathway in patients with severe cerebral palsy undergoing posterior spinal fusion for neuromuscular scoliosis. Spine Deform 7(5):804–811. https://doi.org/10.1016/j.jspd.2019.02.002
Sucato DJ, Lovejoy JF, Agrawal S et al (2008) Postoperative ketorolac does not predispose to pseudoarthrosis following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 33(10):1119–1124. https://doi.org/10.1097/BRS.0b013e31816f6a2a
Vitale MG, Choe JC, Hwang MW et al (2003) Use of ketorolac tromethamine in children undergoing scoliosis surgery. An analysis of complications. Spine J 3(1):55–62. https://doi.org/10.1016/s1529-9430(02)00446-1
Mayell A, Srinivasan I, Campbell F et al (2014) Analgesic effects of gabapentin after scoliosis surgery in children: a randomized controlled trial. Paediatr Anaesth 24(12):1239–1244. https://doi.org/10.1111/pan.12524
Prevention CfDCa (2019) CDC guideline for prescribing opioids for chronic pain. Centers for Disease Control and Prevention. https://www.cdc.gov/drugoverdose/prescribing/guideline.html. Accessed 28 May 2022
Yaszay B, Bartley CE, Sponseller PD et al (2020) Major complications following surgical correction of spine deformity in 257 patients with cerebral palsy. Spine Deform 8(6):1305–1312. https://doi.org/10.1007/s43390-020-00165-7
Fletcher NDBL, Bowman CA, Ward LA, Bruce RW (2017) Is early discharge possible following posterior spinal fusion for neuromuscular scoliosis? Pediatric Orthopaedic Society of North America, Barcelona, Spain
Almenrader N, Patel D (2006) Spinal fusion surgery in children with non-idiopathic scoliosis: is there a need for routine postoperative ventilation? Br J Anaesth 97(6):851–857. https://doi.org/10.1093/bja/ael273
Crowther MA, Webb PJ, Eyre-Brook IA (2002) Superior mesenteric artery syndrome following surgery for scoliosis. Spine (Phila Pa 1976) 27(24):E528–E533. https://doi.org/10.1097/01.BRS.0000035725.29012.A0
Tsirikos AI, Lipton G, Chang WN et al (2008) Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine (Phila Pa 1976) 33(10):1133–1140. https://doi.org/10.1097/BRS.0b013e31816f63cf
Zhu Z, Qiu Y, Wang B et al (2006) Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. Stud Health Technol Inform 123:610–614
Jalanko T, Helenius I, Pakarinen M et al (2018) Gastrointestinal complications after surgical correction of neuromuscular scoliosis: a retrospective cohort study. Scand J Surg 107(3):252–259. https://doi.org/10.1177/1457496917748223
Long LS, Ved S, Koh JL (2009) Intraoperative opioid dosing in children with and without cerebral palsy. Paediatr Anaesth 19(5):513–520. https://doi.org/10.1111/j.1460-9592.2009.02980.x
Kafer ER, Marsh HM (1977) The effects of anesthetic drugs and disease on the chemical regulation of ventilation. Int Anesthesiol Clin 15(2):1–38. https://doi.org/10.1097/00004311-197715020-00001
Fernandez-Bustamante A, Frendl G, Sprung J et al (2017) Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg 152(2):157–166. https://doi.org/10.1001/jamasurg.2016.4065
Sandur S, Stoller JK (1999) Pulmonary complications of mechanical ventilation. Clin Chest Med 20(2):223–247. https://doi.org/10.1016/s0272-5231(05)70139-8
Al-Iede MM, Al-Zayadneh E, Bridge C et al (2020) Risk factors for postoperative pulmonary complications in children with severely compromised pulmonary function secondary to severe scoliosis. Pediatr Pulmonol 55(10):2782–2790. https://doi.org/10.1002/ppul.24997
Park SY, Kim SH, Lee AR et al (2010) Prophylactic effect of dexamethasone in reducing postoperative sore throat. Korean J Anesthesiol 58(1):15–19. https://doi.org/10.4097/kjae.2010.58.1.15
Subedi A, Tripathi M, Pokharel K et al (2019) Effect of intravenous lidocaine, dexamethasone, and their combination on postoperative sore throat: a randomized controlled trial. Anesth Analg 129(1):220–225. https://doi.org/10.1213/ANE.0000000000003842
Thomas S, Beevi S (2007) Dexamethasone reduces the severity of postoperative sore throat. Can J Anaesth 54(11):897–901. https://doi.org/10.1007/BF03026793
Zhao X, Cao X, Li Q (2015) Dexamethasone for the prevention of postoperative sore throat: a systematic review and meta-analysis. J Clin Anesth 27(1):45–50. https://doi.org/10.1016/j.jclinane.2014.06.014
Dodwell ER, Pathy R, Widmann RF et al (2018) Reliability of the modified Clavien-Dindo-sink complication classification system in pediatric orthopaedic surgery. JB JS Open Access 3(4):e0020. https://doi.org/10.2106/JBJS.OA.18.00020
Guisse NF, Stone JD, Keil LG et al (2022) Modified Clavien-Dindo-sink classification system for adolescent idiopathic scoliosis. Spine Deform 10(1):87–95. https://doi.org/10.1007/s43390-021-00394-4
Keil LG, Himmelberg SM, Guisse NF et al (2022) Complications following posterior spinal fusion for adolescent idiopathic scoliosis: a retrospective cohort study using the modified Clavien-Dindo-Sink system. Spine Deform. https://doi.org/10.1007/s43390-021-00468-3
Khirani S, Bersanini C, Aubertin G et al (2014) Non-invasive positive pressure ventilation to facilitate the post-operative respiratory outcome of spine surgery in neuromuscular children. Eur Spine J 23(Suppl 4):S406–S411. https://doi.org/10.1007/s00586-014-3335-6
Vianello A, Carraro E, Pipitone E et al (2015) Clinical and pulmonary function markers of respiratory exacerbation risk in subjects with quadriplegic cerebral palsy. Respir Care 60(10):1431–1437. https://doi.org/10.4187/respcare.04024
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335(1):2–13. https://doi.org/10.1016/j.mce.2010.04.005
Ismael H, Horst M, Farooq M et al (2011) Adverse effects of preoperative steroid use on surgical outcomes. Am J Surg 201(3):305–358. https://doi.org/10.1016/j.amjsurg.2010.09.018
Kenig J, Richter P, Zurawska S et al (2012) Risk factors for wound dehiscence after laparotomy - clinical control trial. Pol Przegl Chir 84(11):565–573. https://doi.org/10.2478/v10035-012-0094-0
Makela JT, Kiviniemi H, Juvonen T et al (1995) Factors influencing wound dehiscence after midline laparotomy. Am J Surg 170(4):387–390. https://doi.org/10.1016/s0002-9610(99)80309-2
Singla A, Qureshi R, Chen DQ et al (2019) Risk of surgical site infection and mortality following lumbar fusion surgery in patients with chronic steroid usage and chronic methicillin-resistant Staphylococcus aureus infection. Spine (Phila Pa 1976) 44(7):E408–E13. https://doi.org/10.1097/BRS.0000000000002864
Asehnoune K, Vourc’h M, Roquilly A (2018) Corticosteroids administration to improve outcome in high-risk surgical patients. Curr Opin Crit Care 24(6):575–580. https://doi.org/10.1097/MCC.0000000000000553
De Oliveira GS, Almeida MD, Benzon HT et al (2011) Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 115(3):575–588. https://doi.org/10.1097/ALN.0b013e31822a24c2
Lei Y, Huang Q, Xu B et al (2018) Multiple low-dose dexamethasone further improves clinical outcomes following total hip arthroplasty. J Arthroplast 33(5):1426–1431. https://doi.org/10.1016/j.arth.2017.11.057
Lei YT, Xu B, Xie XW et al (2018) The efficacy and safety of two low-dose peri-operative dexamethasone on pain and recovery following total hip arthroplasty: a randomized controlled trial. Int Orthop 42(3):499–505. https://doi.org/10.1007/s00264-017-3537-8
Lunn TH, Andersen LO, Kristensen BB et al (2013) Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth 110(1):66–73. https://doi.org/10.1093/bja/aes345
Lunn TH, Kehlet H (2013) Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand 57(7):823–834. https://doi.org/10.1111/aas.12115
Vidal PM, Ulndreaj A, Badner A et al (2018) Methylprednisolone treatment enhances early recovery following surgical decompression for degenerative cervical myelopathy without compromise to the systemic immune system. J Neuroinflammation 15(1):222. https://doi.org/10.1186/s12974-018-1257-7
Waldron NH, Jones CA, Gan TJ et al (2013) Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 110(2):191–200. https://doi.org/10.1093/bja/aes431
Ostojic K, Paget S, Kyriagis M et al (2020) Acute and chronic pain in children and adolescents with cerebral palsy: prevalence, interference, and management. Arch Phys Med Rehabil 101(2):213–219. https://doi.org/10.1016/j.apmr.2019.08.475
Ostojic K, Paget SP, Morrow AM (2019) Management of pain in children and adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol 61(3):315–321. https://doi.org/10.1111/dmcn.14088
Ozcan F, UnsalDelialioglu S, Ozel S et al (2021) Perception of pain in patients with adolescent cerebral palsy: self report or parent’s report. Somatosens Mot Res. https://doi.org/10.1080/08990220.2021.2014805
Funding
Funding was provided through the Harrison Foundation.
Author information
Authors and Affiliations
Contributions
NDF: study design, data collection, writing original draft preparation, approval of final version of manuscript, and agreed to be accountable for the work. TA: conducted statistical analyses, wrote “Data Analysis” section of the manuscript and provided revisions to the manuscript, and agreed to be accountable for the work. RWB: contributed to the research design, edited and approved final version of manuscript, and agreed to be accountable for the work. TR: study design, data collection, approval of final version of manuscript, and agreed to be accountable for the work.
Corresponding author
Ethics declarations
Conflict of interest
NF: reports consulting fees from Orthopaediatrics, Nuvasive, and Medtronic; speakers fees from Orthopaediatrics, Nuvasive, and Zimmer Biomet; grant support from the Harrison Foundation and POSNA; Board Membership with the Children’s Healthcare of Atlanta. TR, RB, and TA: declare no conflicts of interest.
Ethical approval
This study received full approval from the institutional review board (IRB) including review by the IRB ethics committee.
Consent to participate
As this study was retrospective, need for consent was waived.
IRB statement
IRB approval was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruska, T., Austin, T.M., Bruce, R.W. et al. Post-operative steroids in patients with patients with severe cerebral palsy undergoing posterior spinal fusion. Spine Deform 11, 415–422 (2023). https://doi.org/10.1007/s43390-022-00603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-022-00603-8