Abstract
Purpose of Review
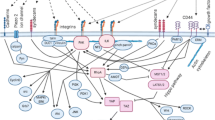
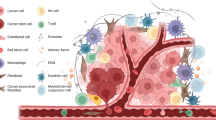
Several cancer stem cells (CSCs) niches have been identified in the tumor microenvironment. Hence, it is important to review the underlying mechanisms that allow communication between the CSCs and the tumor microenvironment.
Recent Findings
Biofabricated extracellular matrix scaffolds could also be used to study the relationship between the tumor microenvironment and CSCs. Cancer tumor microenvironment harbors cleaved extracellular components that play a role in cancer pathogenesis. Although relatively few of them have been associated with CSCs. Hence, further studies are required to establish their correlation.
Summary
This review highlights the important cascades as well as the factors released by the components of tumor microenvironment. Additionally, their ability to regulate CSCs proliferation, survival, invasion and migration has also been taken into consideration.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mohiuddin IS, Wei SJ, Kang MH. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165432. https://doi.org/10.1016/j.bbadis.2019.03.005.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. https://doi.org/10.1038/s41392-020-0110-5.
Ju F, Atyah MM, Horstmann N, Gul S, Vago R, Bruns CJ, et al. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res Ther. 2022;13(1):233. https://doi.org/10.1186/s13287-022-02904-1.
Zamfirescu AM, Yatsenko AS, Shcherbata HR. Notch signaling sculpts the stem cell niche. Front Cell Dev Biol. 2022;10:1027222. https://doi.org/10.3389/fcell.2022.1027222. This review highlights the central role of Notch signaling in niche formation.
Akindona FA, Frederico SC, Hancock JC, Gilbert MR. Exploring the origin of the cancer stem cell niche and its role in anti-angiogenic treatment for glioblastoma. Front Oncol. 2022;12:947634. https://doi.org/10.3389/fonc.2022.947634.
Habic A, Novak M, Majc B, Lah Turnšek T, Breznik B. Proteases regulate cancer stem cell properties and remodel their microenvironment. J Histochem Cytochem. 2021;69(12):775–94. https://doi.org/10.1369/00221554211035192.
Hewitt SM. Defining a (Cancer Stem Cell) Niche. J Histochem Cytochem. 2021;69(12):747. https://doi.org/10.1369/002215542110613.
Sphyris N, Hodder MC, Sansom OJ. Subversion of niche-signalling pathways in colorectal cancer: what makes and breaks the intestinal stem cell. Cancers (Basel). 2021;13(5):1000. https://doi.org/10.3390/cancers13051000.
Chen PC, Kuo YC, Chuong CM, Huang YH. Niche modulation of IGF-1R signaling: its role in stem cell pluripotency, cancer reprogramming, and therapeutic applications. Front Cell Dev Biol. 2021;8:625943. https://doi.org/10.3389/fcell.2020.625943.
Grassi ES, Pietras A. Emerging roles of DLK1 in the stem cell Niche and Cancer Stemness. J Histochem Cytochem. 2022;70(1):17–28. https://doi.org/10.1369/002215542110489.
Madan E, Peixoto ML, Dimitrion P, Eubank TD, Yekelchyk M, Talukdar S, et al. Cell competition boosts clonal evolution and hypoxic selection in cancer. Trends Cell Biol. 2020;30(12):967–78. https://doi.org/10.1016/j.tcb.2020.10.002.
Parker TM, Gupta K, Palma AM, Yekelchyk M, Fisher PB, Grossman SR, et al. Cell competition in intratumoral and tumor microenvironment interactions. EMBO J. 2021;40(17):e107271. https://doi.org/10.15252/embj.2020107271.
Popov A, Kozlovskaya E, Rutckova T, Styshova O, Vakhrushev A, Kupera E, et al. Antitumor properties of matrikines of different origins: prospects and problems of their application. Int J Mol Sci. 2023;24(11):9502. https://doi.org/10.3390/ijms24119502. The role of matrikines in the induction of stemness is less well understood, hence this article gives a summary while going into depth regarding its function in cancer.
Brassart-Pasco S, Brezillon S, Brassart B, Ramont L, Oudart JB, Monboisse JC. Tumor microenvironment: extracellular matrix alterations influence tumor progression. Front Oncol. 2020;10:397. https://doi.org/10.3389/fonc.2020.00397.
Gu YH, Shen YC, Ou-Yang Y, Rao XM, Fu DD, Wen FQ. Combined BRM270 and endostatin inhibit relapse of NSCLC while suppressing lung cancer stem cell proliferation induced by endostatin. Mol Ther Oncolytics. 2021;22:565–73. https://doi.org/10.1016/j.omto.2021.05.011.
Wu T, Duan X, Hu T, Mu X, Jiang G, Cui S. Effect of endostatin on Wnt pathway of stem-like cells in bladder cancer in tumor microenvironment. Mol Biol Rep. 2020;47(5):3937–48. https://doi.org/10.1007/s11033-020-05487-3.
Tian W, Li J, Wang Z, Zhang T, Han Y, Liu Y, et al. HYD-PEP06 suppresses hepatocellular carcinoma metastasis, epithelial-mesenchymal transition and cancer stem cell-like properties by inhibiting PI3K/AKT and WNT/β-catenin signaling activation. Acta Pharm Sin B. 2021;11(6):1592–606. https://doi.org/10.1016/j.apsb.2021.03.040.
Yu W, Hu J, Le H, Lu Y, Xu W, Yu W, et al. Tumstatin attenuates the promotion effect of IL-17 secreted by Th17 cells on the stemness maintenance of glioma cells. Pathol Res Pract. 2021;223:153463. https://doi.org/10.1016/j.prp.2021.153463.
Yazici SE, Gedik ME, Leblebici CB, Kosemehmetoglu K, Gunaydin G, Dogrul AB. Can endocan serve as a molecular “hepatostat” in liver regeneration? Mol Med. 2023;29(1):29. https://doi.org/10.1186/s10020-023-00622-9.
Milazzo M, Jung GS, Danti S, Buehler MJ. Wave propagation and energy dissipation in collagen molecules. ACS Biomater Sci Eng. 2020;6(3):1367–74. https://doi.org/10.1021/acsbiomaterials.9b01742.
Izzi V, Heljasvaara R, Heikkinen A, Karppinen SM, Koivunen J, Pihlajaniemi T. Exploring the roles of MACIT and multiplexin collagens in stem cells and cancer. Semin Cancer Biol. 2020;62:134–48. https://doi.org/10.1016/j.semcancer.2019.08.033. Excellent review that addressed essentially every collagen type that has the potential abilities to regulate cancer cells' stemness.
Bojin F, Robu A, Bejenariu MI, Ordodi V, Olteanu E, Cean A, et al. 3D bioprinting of model tissues that mimic the tumor microenvironment. Micromachines (Basel). 2021;12(5):535. https://doi.org/10.3390/mi12050535.
Fang L, Liu Y, Qiu J, Wan W. Bioprinting and its use in tumor-on-a-chip technology for cancer drug screening: a review. Int J Bioprint. 2022;8(4):603. https://doi.org/10.18063/ijb.v8i4.603.
Maji S, Lee H. Engineering hydrogels for the development of three-dimensional in vitro models. Int J Mol Sci. 2022;23(5):2662. https://doi.org/10.3390/ijms23052662.
Prieto EI, Mojares EBA, Cortez JJM, Vasquez MR Jr. Electrospun nanofiber scaffolds for the propagation and analysis of breast cancer stem cells in vitro. Biomed Mater. 2021;16(3):035004. https://doi.org/10.1088/1748-605x/abc3dd.
Qiu Y, Qiu S, Deng L, Nie L, Gong L, Liao X, et al. Biomaterial 3D collagen I gel culture model: A novel approach to investigate tumorigenesis and dormancy of bladder cancer cells induced by tumor microenvironment. Biomaterials. 2020;256:120217. https://doi.org/10.1016/j.biomaterials.2020.120217.
Zhong C, Tao B, Tang F, Yang X, Peng T, You J, et al. Remodeling cancer stemness by collagen/fibronectin via the AKT and CDC42 signaling pathway crosstalk in glioma. Theranostics. 2021;11(4):1991–2005. https://www.thno.org/v11p1991.htm. Accessed 06/09/23
Shi R, Zhang Z, Zhu A, Xiong X, Zhang J, Xu J, et al. Targeting type I collagen for cancer treatment. Int J Cancer. 2022;151(5):665–83. https://doi.org/10.1002/ijc.33985.
Hsu HS, Liu CC, Lin JH, Hsu TW, Hsu JW, Li AF, et al. Involvement of collagen XVII in pluripotency gene expression and metabolic reprogramming of lung cancer stem cells. J Biomed Sci. 2020;27(1):5. https://doi.org/10.1186/s12929-019-0593-y.
Zhang J, Zhang J, Wang F, Xu X, Li X, Guan W, et al. Overexpressed COL5A1 is correlated with tumor progression, paclitaxel resistance, and tumor-infiltrating immune cells in ovarian cancer. J Cell Physiol. 2021;236(10):6907–19. https://doi.org/10.1002/jcp.30350.
Rodrigues FS, Ciccarelli FD, Malanchi I. Reflected stemness as a potential driver of the tumour microenvironment. Trends Cell Biol. 2022;32(12):979–87. https://doi.org/10.1016/j.tcb.2022.04.007.
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):218. https://doi.org/10.1038/s41392-021-00641-0.
Nallasamy P, Nimmakayala RK, Karmakar S, Leon F, Seshacharyulu P, Lakshmanan I, et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis. Gastroenterology. 2021;161(6):1998-2013.e7. https://doi.org/10.1053/j.gastro.2021.08.023.
Tan HX, Xiao ZG, Huang T, Fang ZX, Liu Y, Huang ZC. CXCR4/TGF-β1 mediated self-differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and promoted colorectal carcinoma development. Cancer Biol Ther. 2020;21(3):248–57. https://doi.org/10.1080/15384047.2019.1685156.
López de Andrés J, Griñán-Lisón C, Jiménez G, Marchal JA. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. J Hematol Oncol. 2020;13(1):136. https://doi.org/10.1186/s13045-020-00966-3. Excellent review highlighting the importance of the cytokines, growth factors, and angiogeneic factors released by cancer stem cells for invasion, metastasis, and survival.
Domvri K, Petanidis S, Anestakis D, Porpodis K, Bai C, Zarogoulidis P, et al. Exosomal lncRNA PCAT-1 promotes Kras-associated chemoresistance via immunosuppressive miR-182/miR-217 signaling and p27/CDK6 regulation. Oncotarget. 2020;11(29):2847–62. https://doi.org/10.18632/oncotarget.27675.
Osman A, Afify SM, Hassan G, Fu X, Seno A, Seno M. Revisiting cancer stem cells as the origin of cancer-associated cells in the tumor microenvironment: a hypothetical view from the potential of iPSCs. Cancers (Basel). 2020;12(4):879. https://doi.org/10.3390/cancers12040879.
Yang Y, Meng WJ, Wang ZQ. Cancer stem cells and the tumor microenvironment in gastric cancer. Front Oncol. 2022;11:803974. https://doi.org/10.3389/fonc.2021.803974.
Vaziri N, Shariati L, Zarrabi A, Farazmand A, Haghjooy JS. Cancer-associated fibroblasts regulate the plasticity of breast cancer stemness through the production of leukemia inhibitory factor. Life (Basel). 2021;11(12):1298. https://doi.org/10.3390/life11121298.
Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21(1):62. https://doi.org/10.1186/s12935-020-01719-5.
Davis L, Recktenwald M, Hutt E, Fuller S, Briggs M, Goel A, et al. Targeting HIF-2α in the tumor microenvironment: redefining the role of HIF-2α for solid cancer therapy. Cancers (Basel). 2022;14(5):1259. https://doi.org/10.3390/cancers14051259.
Wang X, Mao J, Zhou T, Chen X, Tu H, Ma J, et al. Hypoxia-induced myeloid derived growth factor promotes hepatocellular carcinoma progression through remodeling tumor microenvironment. Theranostics. 2021;11(1):209–21. https://www.thno.org/v11p0209.htm. Accessed 09/09/23
Dhanota N, Bal A, Singh G, Arora SK. Evaluation of breast cancer stem cells in human primary breast carcinoma and their role in aggressive behavior of the disease. J Clin Transl Res. 2021;7(5):687–700. https://doi.org/10.18053/jctres.07.202105.008.
Cui G, Li G, Pang Z, Florholmen J, Goll R. The presentation and regulation of the IL-8 network in the epithelial cancer stem-like cell niche in patients with colorectal cancer. Biomed Pharmacother. 2022;152:113252. https://doi.org/10.1016/j.biopha.2022.113252.
Persson E, Gregersson P, Gustafsson A, Fitzpatrick P, Rhost S, Ståhlberg A, et al. Patient-derived scaffolds influence secretion profiles in cancer cells mirroring clinical features and breast cancer subtypes. Cell Commun Signal. 2021;19(1):66. https://doi.org/10.1186/s12964-021-00746-7.
Kim B, Seo Y, Kwon JH, Shin Y, Kim S, Park SJ, et al. IL-6 and IL-8, secreted by myofibroblasts in the tumor microenvironment, activate HES1 to expand the cancer stem cell population in early colorectal tumor. Mol Carcinog. 2021;60(3):188–200. https://doi.org/10.1002/mc.23283.
Lu CS, Shiau AL, Su BH, Hsu TS, Wang CT, Su YC, et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J Hematol Oncol. 2020;13(1):62. https://doi.org/10.1186/s13045-020-00887-1.
Gao W, Wen H, Liang L, Dong X, Du R, Zhou W, et al. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics. 2021;11(6):2564–80. https://www.thno.org/v11p2564.htm. Accessed 09/09/23
Sorrentino C, Ciummo SL, D’Antonio L, Fieni C, Lanuti P, Turdo A, et al. Interleukin-30 feeds breast cancer stem cells via CXCL10 and IL23 autocrine loops and shapes immune contexture and host outcome. J Immunother Cancer. 2021;9(10):e002966. https://doi.org/10.1136/jitc-2021-002966.
Zhou C, Wang D, Li J, Wang Q, Wo L, Zhang X, et al. TGFB2-AS1 inhibits triple-negative breast cancer progression via interaction with SMARCA4 and regulating its targets TGFB2 and SOX2. Proc Natl Acad Sci U S A. 2022;119(39):e2117988119. https://doi.org/10.1073/pnas.2117988119.
Yuan S, Stewart KS, Yang Y, Abdusselamoglu MD, Parigi SM, Feinberg TY, et al. Ras drives malignancy through stem cell crosstalk with the microenvironment. Nature. 2022;612(7940):555–63. https://doi.org/10.1038/s41586-022-05475-6.
Liu Q, Wu H, Li Y, Zhang R, Kleeff J, Zhang X, et al. Combined blockade of TGf-β1 and GM-CSF improves chemotherapeutic effects for pancreatic cancer by modulating tumor microenvironment. Cancer Immunol Immunother. 2020;69(8):1477–92. https://doi.org/10.1007/s00262-020-02542-7.
Yan R, Li J, Xiao Z, Fan X, Liu H, Xu Y, et al. DCLK1 suppresses tumor-specific cytotoxic T lymphocyte function through recruitment of MDSCs via the CXCL1-CXCR2 axis. Cell Mol Gastroenterol Hepatol. 2023;15(2):463–85. https://doi.org/10.1016/j.jcmgh.2022.10.013.
Abou Shousha S, Baheeg S, Ghoneim H, Zoheir M, Hemida M, Shahine Y. The effect of Fas/FasL pathway blocking on apoptosis and stemness within breast cancer tumor microenvironment (preclinical study). Breast Dis. 2023;42(1):163–76. https://doi.org/10.3233/bd-220077.
Xie C, Liang C, Wang R, Yi K, Zhou X, Li X, et al. Resveratrol suppresses lung cancer by targeting cancer stem-like cells and regulating tumor microenvironment. J Nutr Biochem. 2023;112:109211. https://doi.org/10.1016/j.jnutbio.2022.109211.
Zhang H, Steed A, Co M, Chen X. Cancer stem cells, epithelial-mesenchymal transition, ATP and their roles in drug resistance in cancer. Cancer Drug Resist. 2021;4(3):684–709. https://doi.org/10.20517/cdr.2021.32.
Aggarwal V, Montoya CA, Donnenberg VS, Sant S. Interplay between tumor microenvironment and partial EMT as the driver of tumor progression. iScience. 2021;24(2):102113. https://doi.org/10.1016/j.isci.2021.102113.
Jonckheere S, Adams J, De Groote D, Campbell K, Berx G, Goossens S. Epithelial-mesenchymal transition (EMT) as a therapeutic target. Cells Tissues Organs. 2022;211(2):157–82. https://doi.org/10.1159/000512218.
Chen X, Yang M, Yin J, Li P, Zeng S, Zheng G, et al. Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun Signal. 2022;20(1):92. https://doi.org/10.1186/s12964-022-00888-2.
Yang P, Hu Y, Zhou Q. The CXCL12-CXCR4 signaling axis plays a key role in cancer metastasis and is a potential target for developing novel therapeutics against metastatic cancer. Curr Med Chem. 2020;27(33):5543–61. https://doi.org/10.2174/0929867326666191113113110.
Dong G, Wang Q, Wen M, Xia Z, Zhang S, Gao W, et al. DDX18 drives tumor immune escape through transcription-activated STAT1 expression in pancreatic cancer. Oncogene. 2023. https://doi.org/10.1038/s41388-023-02817-0.
Zhang Z, Xu Y. FZD7 accelerates hepatic metastases in pancreatic cancer by strengthening EMT and stemness associated with TGF-β/SMAD3 signaling. Mol Med. 2022;28(1):82. https://doi.org/10.1186/s10020-022-00509-1.
Zhang R, Xia J, Wang Y, Cao M, Jin D, Xue W, et al. Co-expression of stem cell and epithelial mesenchymal transition markers in circulating tumor cells of bladder cancer patients. Onco Targets Ther. 2020;13:10739–48. https://doi.org/10.2147/OTT.S259240.
Wu R, Guo W, Qiu X, Wang S, Sui C, Lian Q, et al. Comprehensive analysis of spatial architecture in primary liver cancer. Sci Adv. 2021;7(51):eabg3750. https://doi.org/10.1126/sciadv.abg3750.
Xia S, Pan Y, Liang Y, Xu J, Cai X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2020;51:102610. https://doi.org/10.1016/j.ebiom.2019.102610.
Caputo S, Grioni M, Brambillasca CS, Monno A, Brevi A, Freschi M, et al. Galectin-3 in prostate cancer stem-like cells is immunosuppressive and drives early metastasis. Front Immunol. 2020;11:1820. https://doi.org/10.3389/fimmu.2020.01820.
Zhao R, Li B, Zhang S, He Z, Pan Z, Guo Q, et al. The N6-methyladenosine-modified pseudogene HSPA7 correlates with the tumor microenvironment and predicts the response to immune checkpoint therapy in glioblastoma. Front Immunol. 2021;12:653711. https://doi.org/10.3389/fimmu.2021.653711.
Ko YS, Rugira T, Jin H, Joo YN, Kim HJ. Radiotherapy-resistant breast cancer cells enhance tumor progression by enhancing premetastatic niche formation through the HIF-1α-LOX axis. Int J Mol Sci. 2020;21(21):8027. https://doi.org/10.3390/ijms21218027.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqui, Z., Equbal, Z., Muhammad, N. et al. Cancer Stem Cells Niche Regulation Within the Tumor Microenvironment. Curr. Tissue Microenviron. Rep. (2024). https://doi.org/10.1007/s43152-023-00051-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s43152-023-00051-0