Abstract
Maternal nutrient availability and its transport through the placenta are crucial for fetal development. Nutrients are transported to the fetus via specific transporters present on the microvillous (MVM) and basal membrane (BM) of the placenta. Glucose is the most abundant nutrient transferred to the fetus and plays a key role in the fetal growth and development. The transfer of glucose across the human placenta is directly proportional to maternal glucose concentrations, and is mediated by glucose transporter family proteins (GLUTs). Maternal glucose concentration influences expression and activity of GLUTs in the MVM (glucose uptake) and BM (glucose delivery). Alteration in the number and function of these transporters may affect the growth and body composition of the fetus. The thin-fat phenotype of the Indian baby (low ponderal index, high adiposity) is proposed as a harbinger of future metabolic risk. We propose that placental function mediated through nutrient transporters contributes to the phenotype of the baby, specifically that glucose transporters will influence neonatal fat. This review discusses the role of various glucose transporters in the placenta in determining fetal growth and body composition, in light of the above hypothesis.

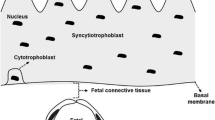
Source: Modified from Illsley et al. 2000

Similar content being viewed by others
Data availability
Not applicable.
Code Availability
Not applicable.
References
Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I--Folate, Vitamin B12, Vitamin B6. J Matern Fetal Neonatal Med. 2010; https://doi.org/10.3109/14767051003678234
Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, et al. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J Nutr. 2003. https://doi.org/10.1093/jn/133.5.1597S.
Camm EJ, Botting KJ, Sferruzzi-Perri AN. Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front Physiol. 2018. https://doi.org/10.3389/fphys.2018.00629.
Burton GJ, Jauniaux E. What is the placenta? Am J Obstet Gynecol. 2015. https://doi.org/10.1016/j.ajog.2015.07.050.
Roberts RM, Green JA, Schulz LC. The evolution of the placenta. Reproduction. 2016. https://doi.org/10.1530/REP-16-0325.
Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc B Biol Sci. 2015. https://doi.org/10.1098/rstb.2014.0066.
Eaton BM, Leach L, Firth JA. Permeability of the fetal villous microvasculature in the isolated perfused term human placenta. J Physiol. 1993. https://doi.org/10.1113/jphysiol.1993.sp019588.
Firth JA, Leach L. Not trophoblast alone: a review of the contribution of the fetal microvasculature to transplacental exchange. Placenta. 1996. https://doi.org/10.1016/s0143-4004(96)80001-4.
Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal–fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014. https://doi.org/10.3390/ijms150916153.
Castillo-Castrejon M, Powell TL. Placental nutrient transport in gestational diabetic pregnancies. Front Endocrinol (Lausanne). 2017. https://doi.org/10.3389/fendo.2017.00306.
Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012. https://doi.org/10.1155/2012/179827.
Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol. 2000. https://doi.org/10.1053/sp.2000.6360.
Kulkarni SR, Kumaran K, Rao SR, Chougule SD, Deokar TM, Bhalerao AJ, et al. Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care. 2013. https://doi.org/10.2337/dc12-2445.
Hill JC, Krishnaveni GV, Annamma I, Leary SD, Fall CHD. Glucose tolerance in pregnancy in South India: relationships to neonatal anthropometry. Acta Obstet Gynecol Scand. 2005. https://doi.org/10.1111/j.0001-6349.2005.00670.x.
Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine. 2002. https://doi.org/10.1385/ENDO:19:1:13.
Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport - a review. Placenta. 2007. https://doi.org/10.1016/j.placenta.2007.05.002.
Yajnik CS. The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev. 2002. https://doi.org/10.1046/j.1467-789x.2002.00072.x.
Yajnik CS, Fall CHD, Coyaji KJ, Hirve SS, Rao S, Barker DJP, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003; https://doi.org/10.1038/sj.ijo.802219
Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011. https://doi.org/10.1152/physrev.00055.2009.
Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med. 2013. https://doi.org/10.1016/j.mam.2012.11.002.
Jia B, Zhu XF, Pu ZJ, Duan YX, Hao LJ, Zhang J, et al. Integrative view of the diversity and evolution of SWEET and SemiSWEET sugar transporters. Front Plant Sci. 2017. https://doi.org/10.3389/fpls.2017.02178.
Nicholas P, Illsley MUB. Human placental glucose transport in fetoplacental growth and metabolism. Biochim Biophys Acta - Mol Basis Dis. 2020. https://doi.org/10.1016/j.bbadis.2018.12.010.
Pao SS, Paulsen IT, Saier Jr MH. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998; 9529885
Joost H-G, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, et al. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Metab. 2002. https://doi.org/10.1152/ajpendo.00407.2001.
Holman GD. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflugers Arch Eur J Physiol. 2020. https://doi.org/10.1007/s00424-020-02411-3.
Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2010. https://doi.org/10.1152/ajpendo.00712.2009/20009031.
Illsley NP. Glucose transporters in the human placenta. Placenta. 2000. https://doi.org/10.1053/plac.1999.0448.
Ohta T. Molecular biology of mammalian glucose transporters. Trends Glycosci Glycotechnol. 1992. https://doi.org/10.4052/tigg.4.99.
Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993. https://doi.org/10.1210/jcem.77.6.8263141.
Baumann MU, Schneider H, Malek A, Palta V, Surbek DV, Sager R, et al. Regulation of human trophoblast GLUT1 glucose transporter by insulin-like growth factor I (IGF-I). PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0106037.
Larque E, Ruiz-Palacios M, Koletzko B. Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr Metab Care. 2013. https://doi.org/10.1097/MCO.0b013e32835e3674.
Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008. https://doi.org/10.1152/ajpendo.90388.2008.
Shepherd PR, Gould GW, Colville CA, McCoid SC, Gibbs EM, Kahn BB. Distribution of GLUT3 glucose transporter protein in human tissues. Biochem Biophys Res Commun. 1992. https://doi.org/10.1016/0006-291x(92)92362-2.
Haber RS, Weinstein SP, O’Boyle E, Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993. https://doi.org/10.1210/endo.132.6.8504756.
Hauguel-de Mouzon S, Challier JC, Kacemi A, Caüzac M, Malek A, Girard J. The GLUT3 glucose transporter isoform is differentially expressed within human placental cell types. J Clin Endocrinol Metab. 1997. https://doi.org/10.1210/jcem.82.8.4147.
Korgun ET, Celik-Ozenci C, Seval Y, Desoye G, Demir R. Do glucose transporters have other roles in addition to placental glucose transport during early pregnancy? Histochem Cell Biol. 2005. https://doi.org/10.1007/s00418-005-0792-3.
Ogura K, Sakata M, Okamoto Y, Yasui Y, Tadokoro C, Yoshimoto Y, et al. 8-bromo-cyclicAMP stimulates glucose transporter-1 expression in a human choriocarcinoma cell line. J Endocrinol. 2000. https://doi.org/10.1677/joe.0.1640171.
Brown K, Heller DS, Zamudio S, Illsley NP. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta. 2011. https://doi.org/10.1016/j.placenta.2011.09.014.
James-Allan LB, Teal S, Powell TL, Jansson T. Changes in placental nutrient transporter protein expression and activity across gestation in normal and obese women. Reprod Sci. 2020. https://doi.org/10.1007/s43032-020-00173-y.
Esterman A, Greco MA, Mitani Y, Finlay TH, Ismail-Beigi F, Dancis J. The effect of hypoxia on human trophoblast in culture: morphology, glucose transport and metabolism. Placenta. 1997. https://doi.org/10.1016/s0143-4004(97)90084-9.
Hahn D, Blaschitz A, Korgun ET, Lang I, Desoye G, Skofitsch G, et al. From maternal glucose to fetal glycogen: expression of key regulators in the human placenta. Mol Hum Reprod [Internet]. 2001;7(12):1173–8. https://doi.org/10.1093/molehr/7.12.1173.
Ferré-Dolcet L, Yeste M, Vendrell M, Rigau T, Rodríguez-Gil JE, Rivera del Álamo MM. Placental and uterine expression of GLUT3, but not GLUT1, is related with serum progesterone levels during the first stages of pregnancy in queens. Theriogenology. 2018; https://doi.org/10.1016/j.theriogenology.2018.08.002
Xing AY, Challier JC, Lepercq J, Caüzac M, Charron MJ, Girard J, et al. Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab. 1998. https://doi.org/10.1210/jcem.83.11.5290.
Barrosa LF, Yudilevich DL, Jarvis SM, Beaumont N, Baldwin SA. Quantitation and immunolocalization of glucose transporters in the human placenta. Placenta. 1995. https://doi.org/10.1016/0143-4004(95)90031-4.
Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005. https://doi.org/10.1093/humrep/deh596.
James-Allan LB, Arbet J, Teal SB, Powell TL, Jansson T. Insulin stimulates GLUT4 trafficking to the syncytiotrophoblast basal plasma membrane in the human placenta. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/jc.2018-02778.
Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, et al. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000. https://doi.org/10.1073/pnas.97.13.7313.
Pinto AB, Carayannopoulos MO, Hoehn A, Dowd L, Moley KH. Glucose transporter 8 expression and translocation are critical for murine blastocyst survival. Biol Reprod. 2002. https://doi.org/10.1095/biolreprod66.6.1729.
Adastra KL, Frolova AI, Chi MM, Cusumano D, Bade M, Carayannopoulos MO, et al. Slc2a8 deficiency in mice results in reproductive and growth impairments. Biol Reprod. 2012. https://doi.org/10.1095/biolreprod.111.097675.
Limesand SW, Regnault TRH, Hay WW. Characterization of glucose transporter 8 (GLUT8) in the ovine placenta of normal and growth restricted fetuses. Placenta. 2004. https://doi.org/10.1016/j.placenta.2003.08.012.
Janzen C, Lei MYY, Jeong ISD, Ganguly A, Sullivan P, Paharkova V, et al. Humanin (HN) and glucose transporter 8 (GLUT8) in pregnancies complicated by intrauterine growth restriction. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0193583.
Preitner F, Bonny O, Laverrière A, Rotman S, Firsov D, Da Costa A, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009. https://doi.org/10.1073/pnas.0904411106.
Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007. https://doi.org/10.1080/09687680701298143.
Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004. https://doi.org/10.1074/jbc.M312226200.
Bibee KP, Illsley NP, Moley KH. Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies. Reprod Sci. 2011. https://doi.org/10.1177/1933719110380276.
McVie-Wylie AJ, Lamson DR, Chen YT. Molecular cloning of a novel member of the GLUT family of transporters, SLC2a10 (GLUT10), localized on chromosome 20q13.1: a candidate gene for NIDDM susceptibility. Genomics. 2001; https://doi.org/10.1006/geno.2000.6457
Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, et al. GLUT12 expression in human placenta in first trimester and term. Placenta. 2003. https://doi.org/10.1053/plac.2002.0925.
Gude NM, Stevenson JL, Murthi P, Rogers S, Best JD, Kalionis B, et al. Expression of GLUT12 in the fetal membranes of the human placenta. Placenta. 2005. https://doi.org/10.1016/j.placenta.2004.04.006.
Stanirowski PJ, Lipa M, Bomba-Opoń D, Wielgoś M. Expression of placental glucose transporter proteins in pregnancies complicated by fetal growth disorders. Adv Protein Chem Struct Biol. 2021. https://doi.org/10.1016/bs.apcsb.2019.12.003.
Li H, Gu Y, Zhang Y, Lucas MJ, Wang Y. High glucose levels down-regulate glucose transporter expression that correlates with increased oxidative stress in placental trophoblast cells in vitro. J Soc Gynecol Investig. 2004. https://doi.org/10.1016/j.jsgi.2003.08.002.
Jones HN, Crombleholme T, Habli M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0074632.
Mateos RM, Jiménez G, Álvarez-Gil C, Visiedo F, Rivera-Rodríguez F, Santos-Rosendo C, et al. Excess hydrocortisone hampers placental nutrient uptake disrupting cellular metabolism. BioMed Res Int. 2018. https://doi.org/10.1155/2018/5106174.
Kipmen-Korgun D, Ozmen A, Unek G, Simsek M, Demir R, Korgun ET. Triamcinolone up-regulates GLUT 1 and GLUT 3 expression in cultured human placental endothelial cells. Cell Biochem Funct. 2012. https://doi.org/10.1002/cbf.1817.
Gao L, Lv C, Xu C, Li Y, Cui X, Gu H, et al. Differential regulation of glucose transporters mediated by CRH receptor type 1 and type 2 in human placental trophoblasts endocrinology. 2012; https://doi.org/10.1210/en.2011-1673
Duval F, Santos ED, Poidatz D, Sérazin V, Gronier H, Vialard F, et al. Adiponectin inhibits nutrient transporters and promotes apoptosis in human villous cytotrophoblasts: involvement in the control of fetal growth. Biol Reprod. 2016. https://doi.org/10.1095/biolreprod.115.134544.
Balachandiran M, Bobby Z, Dorairajan G, Gladwin V, Vinayagam V, Packirisamy RM. Decreased maternal serum adiponectin and increased insulin-like growth factor-1 levels along with increased placental glucose transporter-1 expression in gestational diabetes mellitus: Possible role in fetal overgrowth. Placenta. 2021. https://doi.org/10.1016/j.placenta.2020.11.008.
Lager S, Ramirez VI, Acosta O, Meireles C, Miller E, Gaccioli F, et al. Docosahexaenoic acid supplementation in pregnancy modulates placental cellular signaling and nutrient transport capacity in obese women. J Clin Endocrinol Metab. 2017. https://doi.org/10.1210/jc.2017-01384.
Michelsen TM, Holme AM, Holm MB, Roland MC, Haugen G, Powell TL, et al. Uteroplacental glucose uptake and fetal glucose consumption: a quantitative study in human pregnancies. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/jc.2018-01154.
Kramer AC, Steinhauser CB, Gao H, Seo H, McLendon BA, Burghardt RC, et al. Steroids regulate SLC2A1 and SLC2A3 to deliver glucose into trophectoderm for metabolism via glycolysis. Endocrinology. 2020. https://doi.org/10.1210/endocr/bqaa098.
Tung E, Roberts CT, Heinemann GK, De Blasio MJ, Kind KL, van Wettere WH, et al. Increased placental nutrient transporter expression at midgestation after maternal growth hormone treatment in pigs: a placental mechanism for increased fetal growth. Biol Reprod. 2012. https://doi.org/10.1095/biolreprod.112.100222.
Halloran KM, Hoskins EC, Stenhouse C, Moses RM, Dunlap KA, Satterfield MC, et al. Pre-implantation exogenous progesterone and pregnancy in sheep. II. Effects on fetal-placental development and nutrient transporters in late pregnancy. J Anim Sci Biotechnol. 2021; https://doi.org/10.1186/s40104-021-00567-1
Aye ILMH, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015. https://doi.org/10.1073/pnas.1515484112.
Duval F, Dos Santos E, Maury B, Serazin V, Fathallah K, Vialard F, et al. Adiponectin regulates glycogen metabolism at the human fetal–maternal interface. J Mol Endocrinol. 2018. https://doi.org/10.1530/JME-18-0013.
Mayeur S, Wattez JS, Lukaszewski MA, Lecoutre S, Butruille L, Drougard A, et al. Apelin controls fetal and neonatal glucose homeostasis and is altered by maternal undernutrition. Diabetes. 2016. https://doi.org/10.2337/db15-0228.
Liu N, Dai Z, Zhang Y, Chen J, Yang Y, Wu G, et al. Maternal L-proline supplementation enhances fetal survival, placental development, and nutrient transport in mice †. Biol Reprod. 2019. https://doi.org/10.1093/biolre/ioy240.
Shrestha N, Holland OJ, Kent NL, Perkins AV, McAinch AJ, Cuffe JSM, et al. Maternal high linoleic acid alters placental fatty acid composition. Nutrients. 2020. https://doi.org/10.3390/nu12082183.
Stanirowski PJ, Szukiewicz D, Pazura-Turowska M, Sawicki W, Cendrowski K. Placental Expression of Glucose Transporter Proteins in Pregnancies Complicated by Gestational and Pregestational Diabetes Mellitus. Can J Diabetes. 2018. https://doi.org/10.1016/j.jcjd.2017.04.008.
Devaskar SU, Devaskar UP, Schroeder RE, deMello D, Fiedorek FT, Mueckler M. Expression of genes involved in placental glucose uptake and transport in the nonobese diabetic mouse pregnancy. Am J Obstet Gynecol. 1994. https://doi.org/10.1016/0002-9378(94)90154-6.
Boileau P, Mrejen C, Girard J, Hauguel-De MS. Overexpression of GLUT3 placental glucose transporter in diabetic rats. J Clin Invest. 1995. https://doi.org/10.1172/JCI118036.
Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab. 1999. https://doi.org/10.1210/jcem.84.2.5438.
Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol. 1999. https://doi.org/10.1016/s0002-9378(99)70169-9.
Borges MH, Pullockaran J, Catalano PM, Baumann MU, Zamudio S, Illsley NP. Human placental GLUT1 glucose transporter expression and the fetal insulin-like growth factor axis in pregnancies complicated by diabetes. Biochim Biophys Acta - Mol Basis Dis. 2019. https://doi.org/10.1016/j.bbadis.2019.06.002.
Jansson T, Ekstrand Y, Wennergren M, Powell TL. Placental glucose transport in gestational diabetes mellitus. Am J Obstet Gynecol. 2001;184(2):111–6. https://doi.org/10.1067/mob.2001.108075.
Colomiere M, Permezel M, Riley C, Desoye G, Lappas M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol. 2009. https://doi.org/10.1530/EJE-09-0031.
Zhang B, Jin Z, Sun L, Zheng Y, Jiang J, Feng C, et al. Expression and correlation of sex hormone-binding globulin and insulin signal transduction and glucose transporter proteins in gestational diabetes mellitus placental tissue. Diabetes Res Clin Pract. 2016. https://doi.org/10.1016/j.diabres.2016.07.003.
Szukiewicz D, Abdalla N, Cendrowski K. Impact of pre-gestational and gestational diabetes mellitus on the expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 in human term placenta. Endocrine. 2017. https://doi.org/10.1007/s12020-016-1202-4.
Korgun ET, Acar N, Sati L, Kipmen-Korgun D, Ozen A, Unek G, et al. Expression of glucocorticoid receptor and glucose transporter-1 during placental development in the diabetic rat. Folia Histochem Cytobiol. 2011. https://doi.org/10.5603/fhc.2011.0045.
Kappen C, Kruger C, Jones S, Herion NJ, Salbaum JM. Maternal diet modulates placental nutrient transporter gene expression in a mouse model of diabetic pregnancy. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0224754.
Díaz P, Dimasuay KG, Koele-Schmidt L, Jang B, Barbour LA, Jansson T, et al. Glyburide treatment in gestational diabetes is associated with increased placental glucose transporter 1 expression and higher birth weight. Placenta. 2017. https://doi.org/10.1016/j.placenta.2017.05.016.
Yao G, Zhang Y, Wang D, Yang R, Sang H, Han L, et al. GDM-induced macrosomia is reversed by Cav-1 via AMPK-mediated fatty acid transport and GLUT1-mediated glucose transport in placenta. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0170490.
Stanirowski PJ, Szukiewicz D, Pyzlak M, Abdalla N, Sawicki W, Cendrowski K. Analysis of correlations between the placental expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 and selected maternal and fetal parameters in pregnancies complicated by diabetes mellitus. J Matern Neonatal Med. 2019; https://doi.org/10.1080/14767058.2017.1387897
Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA. Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta. 2011. https://doi.org/10.1016/j.placenta.2010.09.019.
Stang J, Huffman LG. Position of the academy of nutrition and dietetics: obesity, reproduction, and pregnancy outcomes. J Acad Nutr Diet. 2016. https://doi.org/10.1016/j.jand.2016.01.008.
Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009. https://doi.org/10.1096/fj.08-116889.
Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring). 2015. https://doi.org/10.1002/oby.21165.
Sferruzzi-Perri AN, Vaughan OR, Haro M, Cooper WN, Musial B, Charalambous M, et al. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J. 2013. https://doi.org/10.1096/fj.13-234823.
Reynolds CM, Vickers MH, Harrison CJ, Segovia SA, Gray C. Maternal high fat and/or salt consumption induces sex-specific inflammatory and nutrient transport in the rat placenta. Physiol Rep. 2015; https://doi.org/10.14814/phy2.12399
Nam J, Greenwald E, Jack-Roberts C, Ajeeb TT, Malysheva OV, Caudill MA, et al. Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J Nutr Biochem. 2017. https://doi.org/10.1016/j.jnutbio.2017.08.004.
Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, et al. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol. 2015. https://doi.org/10.1016/j.ajog.2014.08.009.
Ganguly A, Devaskar SU. High-fat diet affects pregestational adiposity and glucose tolerance perturbing gestational placental macronutrient transporters culminating in an obese offspring in wild-type and glucose transporter isoform 3 heterozygous null mice. J Nutr Biochem. 2018. https://doi.org/10.1016/j.jnutbio.2018.09.001.
Appel S, Grothe J, Storck S, Janoschek R, Bae-Gartz I, Wohlfarth M, et al. A potential role for GSK3b in glucose-driven intrauterine catch-up growth in maternal obesity. Endocrinology. 2019. https://doi.org/10.1210/en.2018-00899.
Qiao L, Wattez JS, Lim L, Rozance PJ, Hay WW, Shao J. Prolonged prepregnant maternal high-fat feeding reduces fetal and neonatal blood glucose concentrations by enhancing fetal β-cell development in C57BL/6 mice. Diabetes. 2019. https://doi.org/10.2337/db18-1308.
Wang Y, Bucher M, Myatt L. Use of glucose, glutamine, and fatty acids for trophoblast respiration in lean women, women with obesity, and women with gestational diabetes. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/jc.2019-00166.
Winterhager E, Gellhaus A. Transplacental nutrient transport mechanisms of intrauterine growth restriction in rodent models and humans. Front Physiol. 2017. https://doi.org/10.3389/fphys.2017.00951.
Janzen C, Lei MYY, Cho J, Sullivan P, Shin B-C, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta. 2013. https://doi.org/10.1016/j.placenta.2013.08.010.
Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002. https://doi.org/10.1053/plac.2002.0826.
Langdown ML, Sugden MC. Enhanced placental GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation. Mol Cell Endocrinol. 2001. https://doi.org/10.1016/s0303-7207(01)00629-3.
Nüsken E, Gellhaus A, Kühnel E, Swoboda I, Wohlfarth M, Vohlen C, et al. Increased rat placental fatty acid, but decreased amino acid and glucose transporters potentially modify intrauterine programming. J Cell Biochem. 2016. https://doi.org/10.1002/jcb.25450.
Gibbins KJ, Gibson-Corley KN, Brown AS, Wieben M, Law RC, Fung CM. Effects of excess thromboxane A2 on placental development and nutrient transporters in a Mus musculus model of fetal growth restriction. Biol Reprod. 2018. https://doi.org/10.1093/biolre/ioy006.
Cao X, Hua X, Wang X, Chen L. Exposure of pregnant mice to triclosan impairs placental development and nutrient transport. Sci Rep. 2017. https://doi.org/10.1038/srep44803.
Kainulainen H, Järvinen T, Heinonen PK. Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol Obstet Invest. 1997. https://doi.org/10.1159/000291493.
Chandrasiri UP, Chua CLL, Umbers AJ, Chaluluka E, Glazier JD, Rogerson SJ, et al. Insight into the pathogenesis of fetal growth restriction in placental malaria: decreased placental glucose transporter isoform 1 expression. J Infect Dis. 2014. https://doi.org/10.1093/infdis/jit803.
Lüscher BP, Marini C, Joerger-Messerli MS, Huang X, Hediger MA, Albrecht C, et al. Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia. Placenta. 2017. https://doi.org/10.1016/j.placenta.2017.04.023.
Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002. https://doi.org/10.1210/jc.2002-020434.
Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004. https://doi.org/10.1016/S0140-6736(03)15269-5.
Yajnik CS. Size and body composition at birth and risk of type-2 diabetes. Nestle Nutr Workshop Ser Pediatr Program. 2005. https://doi.org/10.1159/000082601.
Krishnaveni GV, Hill JC, Veena SR, Leary SD, Saperia J, Chachyamma KJ, Karat SC, Fall CH. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatr. 2005;42(6):527–38.
Lakshmi S, Metcalf B, Joglekar C, Yajnik CS, Fall CH, Wilkin TJ. Differences in body composition and metabolic status between white U.K. and Asian Indian children (EarlyBird 24 and the Pune Maternal Nutrition Study). Pediatr Obes. 2012; https://doi.org/10.1111/j.2047-6310.2012.00063.x
Anand SS, Gupta MK, Schulze KM, Desai D, Abdalla N, Wahi G, et al. What accounts for ethnic differences in newborn skinfold thickness comparing South Asians and White Caucasians? Findings from the START and FAMILY Birth Cohorts. Int J Obes (Lond). 2016. https://doi.org/10.1038/ijo.2015.171.
Wells JC, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS. The elevated susceptibility to diabetes in India: an evolutionary perspective. Front Public Health. 2016. https://doi.org/10.3389/fpubh.2016.00145.
Acknowledgements
Author AS was the recipient of “Research Associate” from Indian Council of Medical Research, Government of India.
Funding
The authors received funding from the Department of Biotechnology (DBT), India.
Author information
Authors and Affiliations
Contributions
Nikita P. Joshi, Aditi R. Mane, Akriti S. Sahay, Deepali P. Sundrani, Sadhana R. Joshi, and Chittaranjan S. Yajnik contributed to writing the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Joshi, N.P., Mane, A.R., Sahay, A.S. et al. Role of Placental Glucose Transporters in Determining Fetal Growth. Reprod. Sci. 29, 2744–2759 (2022). https://doi.org/10.1007/s43032-021-00699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00699-9