Abstract
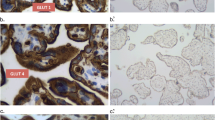
Human placenta regulates the transport of maternal molecules to the fetus. It is known that glucose transport occurs via glucose transporters (GLUTs) in the feto–placental unit. Data on the expression of GLUTs during implantation are very scarce. Moreover, the question of how the decidual leukocytes obtain the energy for their activation during implantation mechanism is still under investigation. We studied the distributions of GLUT1, GLUT3, and GLUT4 in tissue sections of first trimester pregnancies the human maternal–fetal interface. GLUT1 was present in apical microvilli of the syncytiotrophoblast, in cytotrophoblast, and in vascular patterns of the villous core, whereas GLUT3 was localized in cytotrophoblasts of placental villi and in some fetal endothelial cells. Moreover, the proliferating cells of the proximal cell columns were also immunopositive for GLUT1 and GLUT3. We did not observe any positive immunoreactivity for GLUT4 in placental and decidual tissues. Essentially, GLUT3 and also to some extent GLUT1 was present in maternal leukocytes and platelets. In conclusion, our results suggest that the glucose taken up via GLUT1 and GLUT3 from the maternal circulation might not only be needed for placental functions but also for successful implantation by trophoblast invasion, proliferation and also by having a role to support energy for maternal leukocytes.




Similar content being viewed by others
References
Barros LF, Yudilevich DL, Jarvis SM, Beaumont N, Baldwin SA (1995) Quantitation and immunolocalization of glucose transporters in the human placenta. Placenta 16:623–633
Bissonnette JM, Hohimer AR, Cronan JZ, Black JA (1979) Glucose transfer across the intact guinea-pig placenta. J Dev Physiol 1:415–426
Clarson LH, Glazier JD, Sides MK, Sibley CP (1997) Expression of the facilitated glucose transporters (GLUT1 and GLUT3) by a choriocarcinoma cell line (JAr) and cytotrophoblast cells in culture. Placenta 18:333–340
Cross JC, Werb Z, Fisher SJ (1994) Implantation and the placenta: key pieces of the development puzzle. Science 266:1508–1518
Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, Huppertz B (2004) Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta 25:560–572
Haber RS, Weinstein SP, O’Boyle E, Morgello S (1993) Tissue distribution of the human GLUT3 glucose transporter. Endocrinology 132:2538–2543
Hahn T, Hartmann M, Blaschitz A, Skofitsch G, Graf R, Dohr G, Desoye G (1995) Localisation of the high affinity facilitative glucose transporter protein GLUT 1 in the placenta of human, marmoset monkey (Callithrix jacchus) and rat at different developmental stages. Cell Tissue Res 280:49–57
Hahn D, Blaschitz A, Korgun ET, Lang I, Desoye G, Skofitsch G, Dohr G (2001) From maternal glucose to fetal glycogen: expression of key regulators in the human placenta. Mol Hum Reprod 7:1173–1178
Hauguel-de Mouzon S, Challier JC, Kacemi A, Cauzac M, Malek A, Girard J (1997) The GLUT3 glucose transporter isoform is differentially expressed within human placental cell types. J Clin Endocrinol Metab 82:2689–2694
Hay WW (1991) The placenta. Not just a conduit for maternal fuels. Diabetes 40(2):44–50
Illsley NP (2000) Glucose transporters in the human placenta. Placenta 21:14–22
Illsley NP, Sellers MC, Wright RL (1998) Glycaemic regulation of glucose transporter expression and activity in the human placenta. Placenta 19:517–524
Jansson T, Wennergren M, Illsley NP (1993) Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 77:1554–1562
Johnson LW, Smith CH (1980) Monosaccharide transport across microvillous membrane of human placenta. Am J Physiol 238:C160–168
Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B (2002) Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282:E974–E976
Korgun ET, Demir R, Sedlmayr P, Desoye G, Arikan G, Puerstner P, Haeusler M, Dohr G, Skofitsch G, Hahn T (2002) Physiological leukocytosis during pregnancy is associated with changes in glucose transporter expression of maternal peripheral blood granulocytes and monocytes. Am J Reprod Immunol 48:110–116
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Malide D, Davies-Hill TM, Levine M, Simpson IA (1998) Distinct localization of GLUT-1, −3, and −5 in human monocyte-derived macrophages: effects of cell activation. Am J Physiol 274:E516–E526
Ogura K, Sakata M, Okamoto Y, Yasui Y, Tadokoro C, Yoshimoto Y, Yamaguchi M, Kurachi H, Maeda T, Murata Y (2000) 8-Bromo-cyclicAMP stimulates glucose transporter-1 expression in a human choriocarcinoma cell line. J Endocrinol 164:171–178
Shepherd PR, Gould GW, Colville CA, McCoid SC, Gibbs EM, Kahn BB (1992) Distribution of GLUT3 glucose transporter protein in human tissues. Biochem Biophys Res Commun 188:149–154
von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T (2003) Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab 88:3885–3892
Xing AY, Challier JC, Leperq L, Caüzac M, Charron MJ, Girard J, Hauguel-de Mouzon S (1998)Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab 83:4097–4101
Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ (1993) Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 91:950–960
Acknowledgements
This study is supported by the Research Fund of Akdeniz University, (21.01.0122.02) Antalya, Turkey. We would like to thank Sibel Ozer for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korgun, E.T., Celik-Ozenci, C., Seval, Y. et al. Do glucose transporters have other roles in addition to placental glucose transport during early pregnancy?. Histochem Cell Biol 123, 621–629 (2005). https://doi.org/10.1007/s00418-005-0792-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0792-3