Abstract
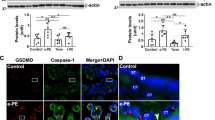
Inflammation is a well-recognized factor associated with preeclampsia (PE). Stress granules (SGs) have been shown to play an important role in regulating inflammation and immune responses. However, whether SGs are involved in the pathogenesis of PE has not been studied. Here, we evaluated the expression of SG components in placenta of pregnancies with PE. Placental samples or serum were collected from PE patients (n = 31) or healthy age-matched pregnancy (n = 17). mRNA expressions of SG-associated genes in placenta from PE or normal pregnancies were detected by real-time quantitative PCR, and protein expressions of HuR and G3BP were detected using western blot. Immunofluorescence staining was performed to evaluate SG components expression in placentas or 10% serum treated HTR-8/Svneo cells using antibodies against HuR and G3BP. Our study showed higher levels of elavl1, lsm2, lsm4, and ago1 mRNA expression and SG marker proteins expression in placental homogenates of PE patients. HuR/G3BP-positive SG structure was further observed in placental villi of PE by immunofluorescence assay. Besides, serum from PE patients could induce SG aggregation in human trophoblast cell line HTR-8/Svneo cells, suggesting the involvement of SGs in the development of PE.





Similar content being viewed by others
References
Mol B, Roberts C, Thangaratinam S, Magee L, de Groot C, Hofmeyr G. Pre-eclampsia. Lancet. 2016;387(10022):999–1011. https://doi.org/10.1016/s0140-6736(15)00070-7.
Young B, Levine R, Karumanchi S. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. https://doi.org/10.1146/annurev-pathol-121808-102149.
Sharma S. Autophagy-based diagnosis of pregnancy hypertension and pre-eclampsia. Am J Pathol. 2018;188(11):2457–60. https://doi.org/10.1016/j.ajpath.2018.09.001.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim Y, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. https://doi.org/10.1038/nm1429.
Tong M, Cheng S, Chen Q, DeSousa J, Stone P, James J, et al. Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci Rep. 2017;7(1):6694. https://doi.org/10.1038/s41598-017-07017-x.
Cheng S, Davis S, Sharma S. Maternal-fetal cross talk through cell-free fetal DNA, telomere shortening, microchimerism, and inflammation. Am J Reprod Immunol. 2018;79(5):e12851. https://doi.org/10.1111/aji.12851.
Poon L, Shennan A, Hyett J, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(suppl 1):1–33. https://doi.org/10.1002/ijgo.12802.
Schmella M, Assibey-Mensah V, Parks W, Roberts J, Jeyabalan A, Hubel C, et al. Plasma concentrations of soluble endoglin in the maternal circulation are associated with maternal vascular malperfusion lesions in the placenta of women with preeclampsia. Placenta. 2019;78:29–35. https://doi.org/10.1016/j.placenta.2019.02.014.
Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33(3):141–50. https://doi.org/10.1016/j.tibs.2007.12.003.
Mokas S, Mills J, Garreau C, Fournier M, Robert F, Arya P, et al. Uncoupling stress granule assembly and translation initiation inhibition. Mol Biol Cell. 2009;20(11):2673–83. https://doi.org/10.1091/mbc.e08-10-1061.
Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler M, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169(6):871–84. https://doi.org/10.1083/jcb.200502088.
Decker C, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. 2012;4(9):a012286. https://doi.org/10.1101/cshperspect.a012286.
Roberts J, August P, Bakris G, Barton J, Bernstein I, Druzin M, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013;122(5):1122–31. https://doi.org/10.1097/01.aog.0000437382.03963.88.
Yung H, Atkinson D, Campion-Smith T, Olovsson M, Charnock-Jones D. Bur ton G. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset preeclampsia. J. Pathol. 2014;234(2):262–76. https://doi.org/10.1002/path.4394.
Li Z, Chen Z, Zhang T, Wei C, Shi W. TGF-β and NF-κB signaling pathway crosstalk potentiates corneal epithelial senescence through an RNA stress response. Aging. 2016;8(10):2337–54. https://doi.org/10.18632/aging.101050.
Fisher S. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2004;2:53. https://doi.org/10.1186/1477-7827-2-53.
George E, Granger J. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep. 2011;13(4):269–75. https://doi.org/10.1007/s11906-011-0204-0.
Warrington J, George E, Palei A, Spradley F, Granger J. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension. 2013;62(4):666–73. https://doi.org/10.1161/hypertensionaha.113.00588.
Fan J, Ishmael F, Fang X, Myers A, Cheadle C, Huang S, et al. Chemokine transcripts as targets of the RNA-binding protein HuR in human airway epithelium. J Immunol. 2011;186(4):2482–94. https://doi.org/10.4049/jimmunol.0903634.
Katsanou V, Papadaki O, Milatos S, Blackshear P, Anderson P, Kollias G, et al. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005;19(6):777–89. https://doi.org/10.1016/j.molcel.2005.08.007.
Luppi P, Deloia J. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006;118(2-3):268–75. https://doi.org/10.1016/j.clim.2005.11.001.
Raghupathy R. Cytokines as key players in the pathophysiology of preeclampsia. Med Princ Pract. 2013;22(suppl 1):8–19. https://doi.org/10.1159/000354200.
Peraçoli J, Rudge M, Peraçoli M. Tumor necrosis factor-alpha in gestation and puerperium of women with gestational hypertension and pre-eclampsia. Am J Reprod Immunol. 2007;57(3):177–85. https://doi.org/10.1111/j.1600-0897.2006.00455.x.
Tangerås L, Silva G, Stødle G, Gierman L, Skei B, Collett K, et al. Placental inflammation by HMGB1 activation of TLR4 at the syncytium. Placenta. 2018;72:53–61. https://doi.org/10.1016/j.placenta.2018.10.011.
Hu S, Claud E, Musch M, Chang E. Stress granule formation mediates the inhibition of colonic Hsp70 translation by interferon-gamma and tumor necrosis factor-alpha. Am J Physiol Gastrointest Liver Physiol. 2010;298(4):G481–92. https://doi.org/10.1152/ajpgi.00234.2009.
Han C, Wang C, Chen Y, Wang J, Xu X, Hilton T, et al. Placenta-derived extracellular vesicles induce preeclampsia in mouse models. Haematologica. 2020;105(6):1686–94. https://doi.org/10.3324/haematol.2019.226209.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 127 kb)
Rights and permissions
About this article
Cite this article
Ma, C., Li, C., Shao, S. et al. Assembly of Cytoplasmic Stress Granules in Placentas in Women with Preeclampsia. Reprod. Sci. 28, 2869–2877 (2021). https://doi.org/10.1007/s43032-021-00592-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00592-5