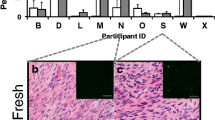
Abstract
Purpose
To improve human primordial follicle culture.
Methods
Thin or thick ovarian slices were cultured on alginate scaffolds or in PEG-fibrinogen hydrogels with or without bpV (pic), which prevents the conversion of phosphatidylinositol-trisphosphate (PIP3) to phosphatidylinositol-bisphosphate (PIP2) or 740Y-P which converts PIP2 to PIP3. Follicular growth was evaluated by follicular counts, Ki67 immunohistochemistry, and 17β-estradiol (E2) levels.
Results
BpV (pic) had a destructive effect on cultured follicles. Thawed-uncultured samples had more primordial follicles than samples cultured in basic medium and fewer developing follicles than samples cultured in PEG-fibrinogen hydrogels with 740Y-P. There were more atretic follicles in samples cultured on alginate scaffolds than in PEG-fibrinogen hydrogels, and in samples cultured in PEG-fibrinogen hydrogels with 740Y-P than in PEG-fibrinogen hydrogels with basic medium. Ki67 staining was higher in PEG-fibrinogen hydrogels than on alginate scaffolds. E2 levels were higher in thick than in thin slices.
Conclusions
PEG-fibrinogen hydrogels appear to have an advantage over alginate scaffolds for culturing human primordial follicles. Folliculogenesis is not increased in the presence of substances that enhance PIP3 production or with thin rather than thick sectioning.



Similar content being viewed by others
References
Feigin E, Freud E, Fisch B, Orvieto R, Kravarusic D, Avrahami G, et al. Fertility preservation in female adolescents with malignancies. In: Moorland MT, editor. Cancer in female adolescents. Hauppauge: Nova Science Publishers; 2008. p. 38–103.
Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67.
Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Mirco-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1033–97.
Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–98.
Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, et al. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod. 2010;25:1708–12.
Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8.
Garor R, Abir R, Erman A, Felz C, Nitke S, Fisch B. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91:1967–75.
Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96:1246–54.
Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S. Alginate scaffold for organ culture of cryopresereved-thawed human ovarian cortical follicles. J Assist Reprod Genet. 2011;28:761–9.
Li J, Kawarmura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant follicle to generate mature eggs. PNAS. 2010;107:10280–4.
Kim SR, Lee YC. PTEN as a unique promising therapeutic target for occupational asthma. Immunotoxicology. 2008;30:793–814.
John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204.
Adhikari D, Gorre N, Risal S, Zhao Z, Zhang H, Shen Y, et al. The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs. PLoS One. 2012;7:e39034.
Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94.
Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–8.
Dikovskey D, Bianco-Peled H, Seliktar D. Investigating the molecular structure and physical properties of PEG-Fibrinogen hydrogels. Adv Eng Mater. 2010;12:B200–9.
Peled E, Boss J, Bejar J, Zinman C, Seliktar D. A novel poly(ethylene glycol)-fibrinogen hydrogel for tibial segmental defect repair in a rat model. J Biomed Mater Res A. 2007;80:874–84.
Federovich NE, Oudshoorn MH, Van Geemen D, Hennink WE, Alblas J. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344–53.
Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–57.
Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpentrating networks. J Biomed Mater Res. 2000;51:164–71.
Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–6.
Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–86.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:55–121.
Gerdes J, Lemke H, Baisch J. Cell cycle analysis of cell proliferation associated human nuclear antigen defined by the monoclonal antibody Ki67. J Immunol. 1984;133:1710–5.
Scholzen T, Geredes J. The Ki67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22.
Rivera OE, Varayoud J, Rodrigues HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32:304–12.
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–9.
Fabbri R, Venturoli S, D'Errico A, Iannascoli C, Gabusi E, Valeri B, et al. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89:259–66.
Rahmanzadeh R, Hüttmann G, Gerdes J, Scholzen T. Chromophore-assisted light inactivation of pKi67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 2007;40:422–30.
David A, Van Langendonckt A, Gilliaux S, Dolmans MM, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod. 2012;27:1088–95.
Kleinman HK. Preparation of basement membrane components from EHS tumors. Curr Protoc Cell Biol. 2001;10:10.2.1–10.
Seliktar D, Zisch AH, Lutolf MP, Wrana IJL, Hubbell JA. MMP-2 sensitive, VEGF bearing bioactive hydrogels for promotion of vascular healing. Biomed Mater Res A. 2004;68:704–16.
Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46.
Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85.
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8.
Kagawa N, Sliber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568–77.
Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–9.
O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–6.
Acknowledgments
The authors are grateful to Prof. Smadar Cohen from The Avram and Stella Goldstein-Goren Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel for the production of alginate scaffolds in her laboratory. The authors are greatly indebted to Ms. Gloria Ganzach from the Editorial Board of Rabin Medical Center, Beilinson Hospital for the English editing. We are also indebted to our laboratory technician, Ms. Carmela Felz for the histological sectioning. The study was partially funded by a research grant from Tel Aviv University, Leo Minz Fund (to R. A and B. F) and the Israel Cancer Association (to R. A and B. F).
Disclosure summary
RA and BF received grant support from Leo Minz Fund, Tel Aviv University and from the Israel Cancer Association, GL-S, NS, MS, OK, DS, SC and AB-H have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Galit Lerer-Serfaty and Dr. Nivin Samara contributed equally to this study as first authors.
Capsule
PEG-fibrinogen hydrogels seem to have an advantage over alginate scaffolds for culturing human primordial follicles. Folliculogenesis is not enhanced with the addition of PIP3-inducing substances or use of thin-slice sectioning.
Rights and permissions
About this article
Cite this article
Lerer-Serfaty, G., Samara, N., Fisch, B. et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet 30, 1279–1288 (2013). https://doi.org/10.1007/s10815-013-0052-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-013-0052-8