Abstract
Salinity is one of the principal abiotic stresses that occurs in the Mediterranean area, causing loss of productivity and decrease of vegetable crop quality. The effect of salinity (0, 25, 75, 150 mM NaCl) was evaluated in three Diplotaxis tenuifolia varieties (Dragon Tongue, Capriccio, Piccante), previously selected for salinity tolerance and high glucosinolates production in leaves. The aim of this research was to explore the salinity tolerance of three wild rocket varieties cultivated under optimal temperature conditions and under high temperature that typically characterized the Mediterranean greenhouse. Biometric, biomass, pigment production and physiological parameters were evaluated. Biometric, physiological, and biochemical parameters significantly varied because of variety, salt level used and environmental conditions. PCA analysis highlighted that the two cultivation systems deeply affected the wild rockets response to salt stress. In general, under optimal growing conditions, wild rocket varieties showed higher growth parameters compared to greenhouse conditions. Overall Capriccio was the most susceptible variety to salinity, while Dragon Tongue (V1) and Piccante (V3) were more tolerant to salt stress. Furthermore, in both growing conditions V1 was the less productive variety while V3 showed an opposite trend. Interestingly, gene (DtOxo and DtGst) expression analysis revealed a significant increase of the target gene expression as response of salinity levels, with a clear increase of DtOxo level in V1 and V3. The results obtained in this study can be useful to plan future breeding programs aimed to increase rocket quality grown under Mediterranean conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Wild rocket (Diplotaxis tenuifolia L.), is a leaf vegetable, belonging to Brassicaceae family, largely cultivated in Europe and North America, and more recently, with its increasing commercial value, is becoming popular also in South America (Parada et al. 2019). In Europe, it is manly growth in the Mediterranean region where favorable mild climatic conditions favored its production at farm-scale level. Wild rocket is principally used as green salad, and its success is related to the peculiar pungent and peppery taste and the high amount of health promoting compounds (Cocetta et al. 2018). Vitamin C, carotenoids, phenols, flavonoids and glucosinolates (GLS) present in leaves make rocket a functional food (Heimler et al. 2007). Several environmental conditions (temperature, salinity, light etc.) can influence the secondary metabolites production in this plant. The increasing interest in wild rocket is also associated to the potential role of GLS as health promoting compound for humans (D’Antuono et al. 2009). D. tenuifolia is cultivated almost all year and is well adapted to Mediterranean areas, characterized by harsh and poor growing conditions. Furthermore, wild rocket is characterized by a fast-growing cycle and is traditionally cultivated as a winter crop mainly in dry areas. Winter cultivation required a longer cycle compared to spring–summer cultivation, where the optimal temperature of 25 °C and the long day light favor the growth rate of the species reducing its life cycle (Hall et al. 2012a). As reported by Purty et al. (2008), wild rocket is considered a promising crop adapted to coastal areas where high salinity soils select salt tolerant species (Franzoni et al. 2020). Salinity is one of the principal abiotic stresses that significantly impact plant growth, quality, and productivity (Munns and Tester 2008). Salinity (NaCl) caused several plant disorders, associated with hyperosmotic stress, ions imbalance and oxidative damage (Massa and Melito 2019). Commonly plants present a short-term (minutes, hours, the day of salinity stress occurrence), and a long-term (days, weeks, months) salinity effect. In the first case, roots present a reduced ability to absorb water because of the soil salinity. This condition of root-salt contact is called water-deficit effect of salinity and the metabolic pathway involved partially overlaps with the drought stress response. In the second case, plant response to salt is associated to its entrance in plant tissue and cells. A reduction of growth occurs as consequence of ion toxicity and nutrient imbalance, caused by the salt accumulation in cytosol that interfere with the other ion flux. In this condition general physiologic stress symptoms occur (stomatal conductance reduction, low photosynthetic activity) (Massa and Melito 2019), growth alteration (leaves number and area, plant size, fruit decrease), and alteration in secondary metabolites pathway (signaling molecules, hormone and oxidative compounds ad secondary metabolites) (Munns and Tester 2008). Previous studies (De Vos et al. 2013; Petretto et al. 2019), have shown a limited reduction of plant growth at 100 and 150 mM of NaCl, while more stress symptoms and reduction of plant biomass occurs at 200 mM. Several adaptation strategies can be used by tolerant plants to survive high salinity conditions including morphological, physiological, metabolic, and genetic variation as response to salt stress. In addition to the plant's response to salinity, various environmental factors may interact with salinity-induced stress conditions, further influencing the overall impact of these stressors on plants. Temperature, wind, radiation, soil composition etc., are some of the co-factors that can deeply alter the plant mechanism response to NaCl stress. Salt tolerance includes adaptations to secondary effects, such as oxidative damage and changes in the quantities and content of secondary metabolites in the roots and leaves of a variety of plants. As an example, under salt stress, there have been reports of changes in the ratio of saturated to unsaturated fatty acids, as well as a decrease in the concentration of triacylglycerols (Purty et al. 2008). Furthermore, the K+ or Na+ homeostasis plays a key role in regulating the transmembrane flux of cations, indeed, Na+ likely enters cells by nonselective cation channels, and at high salinities, it may do so through K+ channels or K+-insufficiently selective transporters (Munns 2005). Several genes are important in maintaining the K+/Na+ homeostasis, among these Na+ (NHX) and K+ (HKT) antiporter have been reported in higher plants (Munns 2005), while gene regulating the change in abscisic acid level are known to enhance survival under salinity (Sagervanshi et al. 2021). Furthermore, specific genes involved in the ascorbate–glutathione and oxalic acid pathways have been identified in D. tenuifolia (Cavaiuolo et al. 2017). Previous research studies have shown a significant different content of GLS among rocket species (wild and cultivated) (Petretto et al. 2019) cultivated with saline irrigation water. Based on these findings Petretto et al. (2019) indicated a pick of GLS production in three more tolerant wild rocket species at 150 mM of NaCl. To provide novel insights into the performance of wild rocket varieties under salt stress conditions, a multidisciplinary approach was adopted, specifically aiming to examine the variability in compound levels underlying quality and explore the RNA expression levels of selected target genes associated with salinity resistance. For this purpose, two experiments were designed to assess the response of three wild rocket varieties to different salt stress treatments under optimal (growth chamber) and high-temperature (Mediterranean greenhouse) conditions.
2 Material and Methods
2.1 Plant material, Growth Condition and Salinity Treatments
Three wild rocket (Diplotaxis tenuifolia L.) varieties widely distributed in the European market were used in this study (Table 1). Two experiments were performed: an experiment was set up in a Mediterranean greenhouse (Exp. 1) while the second one was established under controlled environmental conditions (growth chamber) (Exp. 2). Wild rocket seeds were germinated in growth chambers at University of Sassari, Italy, and at Research Centre for Vegetable and Ornamental Crops, Council for Agricultural Research and Economics (Pescia, PT, Italy, lat. 43°54′ N, long. 10°42′ E). Four/five seeds for hole were placed in rockwool seedling trays (120 holes) with the following environmental conditions: 25 °C, 14 h photoperiod and under sodium vapor lamps, SOX-lamps (Philips®) by ensuring 250 μmol m−2 s−1 PAR, reducing to one plant per hole after first true leaves emergence. After 20 days, at the stage of 2 true leaves, young plants were transplanted in the greenhouse and in growth chamber, respectively. The two experiments were arranged using a randomized block design with four treatments, three varieties and three replicates for a total of 12 experimental units. Each experimental unit consisted of a ventilated plastic crate (30 × 50 × 22 cm) covered with anti-algae mulch sheet opportunely holed to avoid the water stagnation and filled with a mixture peat and perlite 50:50 v v−1. Each experimental unit was divided into three sections, each one contained eight plants for variety. Nutrient solution was supplied from the transplant to the end of the experiment every two days with a volume of irrigation of 0.5 L and was combined to the saline solutions. This amount was deemed sufficient to avoid excessive NaCl accumulation into the root zone. Treatments consisted in four salinity (NaCl) levels (0, 25, 75, 150 mM, respectively S0, S1, S2, S3). The base nutrient solution was described in Table S1.
- Experiment 1 (Exp. 1) (Mediterranean greenhouse): the greenhouse experiment was conducted from June to July 2022, in unheated glasshouse at Ottava experimental station of the University of Sassari, Sardinia, Italy (lat. 40°46′ N, long. 8°29′ E). Air temperatures during the experiment were 30.1 °C (average minimum temperature) and 44 °C (average maximum temperature), and the average heliophany was ~ 627.1 min/day.
- Experiment 2 (Exp. 2) (Growth chamber): the experiment was conducted from June to July 2022 in a growth chamber located in CREA Research Centre for Vegetable and Ornamental Crops in Pescia. The growth chamber was set at 25 ± 1 °C both during daily and night hours with a night/day turn of 10/14 h, and 75–80% relative humidity. Light was supplied by SOX-lamps (Philips®) providing 1050 µmol m−2 s−1 PAR, on average, at canopy level.
2.2 Biometric Measurements, Biomass Evaluation and Mineral Analysis
Disruptive analyses of all plants were conducted 26 days after the beginning of saline irrigation. Leaf number (LN) and leaf area (LA) were recorded for each plant. Leaf area was measured using a planimeter (LI-COR, model 3100 area meter) and the WinDIAS Image Analysis System (Delta-T Devices, U.K.) in Exp. 1 and Exp. 2, respectively. Roots, stems and leaves were separated and weighted for fresh weight (FW) and dry weight (DW) measurement. DW was obtained after drying plant tissues in a forced-air oven (at 60 °C), until constant weight. Shoot and root DW were used to calculate the relative ratio; leaf area and leaf DW were used for specific leaf area (SLA) calculation. Plant tissue dry matter was used for mineral analysis, after acid digestion. In detail, after nitric digestion, K, Ca, Mg, Na, Fe, Mn, Zn and Cu were quantified by ICP-OES analysis (Optima 7000, PerkinElmer, Waltham, MA, USA). Total N (Ntot) was calculated as the sum of the organic N, determined by an organic elemental analyser (Flash 2000 CHNS/O, Thermo Fisher Scientific Inc., MA, USA) after dry matter digestion with H2SO4, and the N-NO3, analysed on the water extract by the spectrophotometric method (Evolution(tm) 300 UV–Vis Spectrophotometer, Thermo Fisher Scientific Inc., MA, USA) as described by Cataldo et al. (1975). Chloride was determined by potentiometric detection with ion-selective electrodes (ISEs) (AMEL 338, AMEL S.r.l., Italy with ISEs Radiometer ISE/HS25Cl, Hach Company/Hach Lange GmbH, Switzerland).
2.3 Pigment Determination, SPAD Index and Leaf Physiological Parameters
Total chlorophyll (Chl tot), chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids (CAR), Chl a/b ratio and Chl tot/car ratio were determined by leaf extraction (100 mg FW) with MeOH (0.1 ml mg−1 FW), maintaining samples 2 days at − 20 °C and renewing the solution after 1 day. Then measures were performed by the spectrophotometer and expressing data on fresh weight basis. SPAD index (502 Plus, Konica Minolta) was measured as non-destructive parameter using the middle portion of five fully expanded leaves per plant. Plant physiological status (transpiration rate, Tr, stomatal conductance, gs, net photosynthesis, Pn, and intercellular CO2 concentration, CI) was recorded by a portable infrared gas analyser (CIRAS-2, PP-Systems, Amesbury, MA 01913 USA) at the end of the experiment. Measurements were performed directly in greenhouse condition (Exp. 1) and in the growth chamber (Exp. 2) on the first mature leaf of three different plants per replicate for a total of 12 measurements per treatment, by setting CO2 concentration at 400 ppm and PAR at 1,000 µmol m−2 s−1. Instantaneous Water Use Efficiency (IWUE; expressed as μmol CO2 mmol−1 H2O) was calculated as the ratio of CO2-uptake (Pn) relative to water loss (Tr).
2.4 RNA Isolation and qRT-PCR Analysis
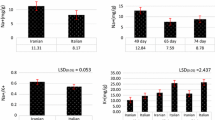
For RNA isolation, samples were collected from leaves of each variety grown in the four different salt treatments. We considered three biological replicates for a total of 36 samples. Tissues were ground into fine powder in liquid nitrogen, then, the RNA was isolated using the Norgen RNA Purification Kit (Norgen Biotek Corp, Ontario, Canada) following manufacturer’s instructions. Total RNA was quantified using a Qubit 3.0 (Thermo Scientific) and its integrity was assayed on 2% agarose gel electrophoresis. Reverse transcription reaction was performed using the iScript RT Supermix for RT-qPCR (Biorad, Hercules, CA, United States) following manufacturer’s instructions. The resulting cDNA samples were used as template for qRT-PCR. Each reaction was performed in a total volume of 20 μl including 10 μl 2 × SsoAdvanced Univ SYBR Green Supermix (Biorad, Hercules, CA, United States), 6.25 ng cDNA and 300 nM of each primer (final concentration), nuclease free water to final volume. qRT-PCRwas carried out on a CFX96 TouchTM Real-Time PCR Detection System (Biorad, Hercules, CA, United States). Thermocycling conditions were as follows: 95 °C for 2 min, 40 cycles of 95 °C for 10 s and 60 °C for 30 s. A final ramping stage 65–95 °C, + 0.5 °C each 5 s was performed to confirm the absence of dimers and multiple products. Relative expression values were determined using the 2ΔΔCt method implemented in the Gene Expression Module of the CFX Manager Software (Biorad). We selected five markers from the literature, for gene expression analysis considering the Arabidopsis thaliana RT-PCR primer pair database (Han and Kim 2006) and D. tenuifolia transcriptome (Cavaiuolo et al. 2017) (Table 2). For gene expression normalization we considered two reference genes: the A. thaliana actin gene (ACT-for 5′-CTCCTGCCATGTATGTCGCTATCC-3′ and ACT-rev 5′-AAGGTCCAAACGCAGAATAGCATGT-3′) retrieved for Pandey and Penna (2017) and Actin 2 retrieved for the AT3G18780 sequence (TAIR database). For the latter, primer pairs were designed by using the online tool available at https://eu.idtdna.com/scitools/Applications/RealTimePCR. Forward and reverse sequence were as following: ACT2_For 5'-TCC CTC AGC ACA TTC CAG CAG AT-3', ACT2_Rev 5'-AAC GAT TCC TGG ACC TGC CTC ATC-3'. Prior to the gene expression analysis, the standard curve for amplicons of each marker was established using fourfold cDNA dilution series and three replicates (Fig. S1). Actin 2 was selected as best candidate reference gene given its better efficiency (130.5%) and higher R2 (0.96) in the standard curve compared to actin (Fig. S1).
2.5 Data Analysis
To analyze the effects of variety, salinity treatment and their interaction on biometric traits, mineral contents, and physiological parameters, a linear mixed-effect model was constructed using the lmer function in the lmer4 package. The model was constructed using variety, and salinity treatment as fixed factors and block as random factor. The lmer model was followed by Tukey’s post-hoc test to determine pairwise statistical significance. When normality and homoscedasticity assumptions were not respected, data were log10- or square root-transformed prior to analysis. If even after transformation, the collected data did not respect parametric assumptions (root/shoot DW, Chl b, gs, Pn, Mg, Mn, Na, Ntot, P) the Scheirer Ray Hare test was used for treatment comparisons and their interactions along with a Dunn post-hoc test for multiple nonparametric comparisons. The correlations among traits scored in the two locations and for each treatment were calculated from accession means using the psych and corrplot R packages. The Spearman linear coefficients of correlation (r) were calculated between pairs of traits and the significance of correlations was evaluated at p < 0.01. A principal component analysis (PCA) was carried out among varieties means considering the three treatments and two experiments to determine which are the most effective traits in discriminating among varieties. PCA loading and score plots were drawn using the FactoMineR and factoexta R libraries. The prediction ellipses with a 95% level of confidence were added to the PCA score plot.
3 Results
3.1 Effect of Environmental Conditions
Plants were grown under stress salinity in two different conditions with the aim to highlight their responses in both optimal (growth chamber) and stressful (high temperature in a Mediterranean greenhouse in spring–summer) growth conditions.
The PCA in the first two dimensions explained 66.1% of the total variation observed, with the first (PC1) and the second (PC2) components accounting for 42.9 and 23.2% of the total variation, respectively (Fig. 1). The PCA clearly distinguished the two experimental sites, thus showing how the cultivating conditions (i.e., Mediterranean greenhouse and growth chamber) were preponderant in determining the differences between the varieties tested. Furthermore, the distribution of the varieties in the biplot highlighted a greater variation for the varieties grown in Exp. 2. Vector analysis related to the PCA is showed in Fig. S2.
3.2 Effect on Plant Growth and Biomass
Data referred to measured fresh weight are shown in supplementary material (Tables S2 and S3) for both experiments. For the most part, the effects of individual factors, when statistically significant, were commented upon only when the effect of the interaction did not prove to be significant (Shadish et al. 2002).
In Exp. 1, all the biomass data (Leaf, root, stem, total shoot, and total dry weight) were found to be statistically influenced by varieties, where the V3 showed from 62.8% (Root DW) to 73% (Stem DW) higher biomass compared with V1 (Table 3). Additionally, V3 showed the highest LN and LA compared with V1, indicating increases of 48.3 and 69.6%, respectively. Among the analyzed morphological traits, only root/shoot DW and SLA were significantly affected by salt treatments. The former parameter showed increasing values with increasing stress levels, while the latter exhibited the lowest value under S3 (Table 3). Data obtained from Exp. 2 showed a significant effect of the variety in almost all biometric and biomass parameters (Table 4). Under optimal growing conditions, plants showed higher values for most of the biometric and biomass parameters compared to the greenhouse conditions. V3 had the highest LN and LA with increases of 38.6 and 77.9%, respectively, compared to the lowest value observed for V1. This trend was also confirmed by the biomass data where the V3 variety showed an increase in biomass ranging from 75% (Root DW) to 79.2% (Stem DW) compared to V1. Similarly to Exp. 1, even in the optimal growth chamber, salt treatments significantly influenced SLA and root/shoot dry weight ratio. Specifically, SLA showed the highest value in the Control, progressively decreasing with increasing saline stress. Conversely, root/shoot DW (Table 4) showed the lowest value in the S2 treatment, statistically different from S1 and S3 (Table 4).
3.3 Effect on Pigment Content and Leaf Physiological Parameters
For each variety, SPAD index was significantly higher in S0 treatment. Within S0, S1, S2 treatment, V1 and V2 had significantly higher values of SPAD index compared to V3 (Table 5 and Fig. 2a). Pigment analysis revealed different results in the two experiments. In Exp. 1, Chl a and Chl tot decreased overall with increasing salinity levels, revealing the highest values in V1 and V3 under S0 and S1 treatments (Table 5; Fig. 2b and c). Chlorophyll b was significantly affected only by the saline treatment, showing a significant reduction in content with increasing salinity levels. Overall chlorophyll data are coherent with SPAD trend observed in Table 5. Similarly, carotenoids content decreased significantly with increasing salt levels, with the highest value observed in the S0 treatment for V1 and V2 varieties (Table 5; Fig. 2d). Under growth chamber conditions in Exp. 2, no significant differences were observed for pigment parameters among varieties, treatments, and variety × treatment, except for Chl a/b and Chl tot/car ratio, which were affected by variety and treatment as single effects (Table 6). In Exp. 1, both saline treatments and varieties significantly influenced stomatal conductance and photosynthesis (Table 7). Specifically, V2 and V3 showed higher stomatal conductance which, as expected, was coupled with a higher photosynthetic activity. A significant interaction was observed between variety and salt treatment for CI (Fig. S3). Varieties and salinity had significant effects on both transpiration and net assimilation rate in growth chamber conditions (Exp. 2) (Table 8). V1 had a significantly lower photosynthesis rate compared to V2 and V3. Additionally, transpiration and net assimilation rate were significantly impacted by saline treatments, with control treatment S0 showing higher values for both parameters. Instantaneous Water Use Efficiency showed the same trend as stomatal conductance being affected by variety factor with lower values for V1.
SPAD index (a), chlorophyll a (b), total chlorophyll (c), and carotenoids (d) content affected by variety × treatment interaction at Exp. 1. Different lower-case letters within each variety indicate significant difference among treatments according to Tukey's test, different upper-case letters within treatment indicate significant difference among varieties according to Tukey's test. Bars indicate standard errors of the mean (Number of replicates = 3). V1 = Dragon Tongue, V2 = Capriccio, V3 = Piccante. S0 = 0 mM NaCl, S1 = 25 mM NaCl, S2 = 75 mM NaCl, S3 = 150 nM NaCl. The base nutrient solution was described in Table S1
3.4 Effect on Mineral Nutrient Tissue Concentrations
Mineral contents in dry leaves exhibited different trends based on growing conditions and varieties in both experiments (Tables 9 and 10). In Exp. 1 (Table 9), minerals presented four main trends: 1) No significant effects of treatments and varieties, as it was observed for Ca and Fe; 2) Significant effects associated to the variety factor were observed for Zn, with the highest content found in V1, followed by V3 and V2; 3) Significant effects were associated to the saline treatments for Na and K, with an increase in Na content in leaves from S0 to S3, reaching a maximum value of 45.8 g kg−1 DW. Conversely, K exhibited an opposite trend; 4) Both variety and saline treatment had significant impact on mineral content. Magnesium, and Mn showed similar trends in terms of variety, but opposite trends based on the salt levels, as also Ntot and P. Magnesium decreased with increasing NaCl levels, reaching the highest values in V1 and the lowest in V2. Similarly, Mn presented a significant effect associated with variety revealing the highest value for V1. Conversely, total N was significantly higher in V2 and V3, while among the saline treatments, it was higher in both the control and S1. A similar trend was observed within the different salt stress levels for P (Table 9).
In Exp. 2, mineral content showed four distinct trends: 1) Significant variation of mineral elements associated only to the variety factor, as in the cases of Ca Mg, Mn, and P (Table 10). Calcium and P showed similar trend, with the highest values in V2, and the lowest recorded for V1. Also, Mg tissue concentration revealed V2 as the variety with the highest content, and V1 exhibiting the lowest, similar to Ca and P trends. A different trend was observed for Mn, which presented a peak value in V1, although like V2, and a reduced content in V3 (Table 10); 2) Significant effect related to the treatment. Sodium increased in wild rocket tissues due to salt treatment, reaching the highest value in S3, as observed throughout Exp. 1; 3) Significant effect of variety and treatment. Potassium and total N showed similar trends with higher concentrations observed for V2 and V3 and minimum in V1. In addition, both minerals had their highest accumulation in S0 and in S1 and the lowest in S3 treatment, with S2 showing an intermediate accumulation (Table 10). Even Fe was significantly influenced by both the individual effects of variety and treatment, particularly showing significantly higher values in V2. Among treatments, the control recorded the highest value in Fe; 4) Mineral element significantly affected by variety × treatment interaction, such as in the case of Zn (Table 10; Fig. S4). Within varieties, Zn content appeared systematically higher in S3 (Fig. S4). However, at the treatment level, V2 exhibited the highest values across all treatments except for S3, where V1 showed significantly higher Zn content (Table 10; Fig. S4).
3.5 Multivariate Analysis
The Spearman rank correlation considering a significance threshold p < 0.01 using the Spearman coefficient revealed how some traits were rather independent, whereas a group of traits clustered together because of a reciprocal tight correlation for the same category of measures (Fig. 3). At both locations traits exhibited the same sign of correlation although a higher number of correlations were found in Exp. 1. For instance, Mn content was negatively correlated with plant traits.
The Spearman’s rank significant correlations between pairs of traits evaluated in three Diplotaxis tenuifolia varieties with four stress conditions. Correlation coefficients are reported in Table S3. Coloured cells are those with p < 0.01. Colour intensity is directly proportional to the coefficients. According to the scale on the right, blue and red colours correspond to positive and negative correlations, respectively. Correlations among traits in Mediterranean greenhouse condition (Exp. 1) are shown above the diagonal; correlations in growth chamber condition (Exp. 2) below the diagonal. SPAD index (SPAD), leaf number (LN), dry weight (DW), fresh weight (FW), leaf area (LA), specific leaf area (SLA), chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl tot), carotenoids (CAR), chlorophyll a to chlorophyll b ratio (Chl a/b), total chlorophyll to carotenoids ratio (Chl tot/car), transpiration (Tr), stomatal conductance (gs), net assimilation (Pn), intercellular CO2 concentration (CI), Instantaneous Water Use Efficiency (IWUE)
3.6 Gene Expression Analysis
Prior to gene expression analysis, candidate genes were tested for their efficiency. Only marker specific for DtOxo and DtGst were assayed on all samples considering their accuracy in the standard curve (Fig. S1). Results of the gene expression in the three studied varieties grown under diverse salt concentrations are reported in Fig. 4. DtOxo expression was up regulated in both V1 and V3 when the salt concentration increased from S0 to S3, with the highest expression in V1 at S3 (Fig. 4a). Instead, V2 showed similar expression levels in the four treatments, being slightly higher only in S3. The expression of DtGst confirmed the trend in the accession V1 increasing its expression from the lowest to the highest salt concentration (Fig. 4b). Contrariwise, V2 and V3 showed contrasting results. While results for V2 agreed with DtOxo with similar gene expression among treatments, for V3 we observed an increase of the expression from S0 to S1 and then the gene was downregulated up to S3.
Expression of DtOxo and DtGst genes in leaves of three D. tenuifolia varieties (V1, V2, V3) grown at different salt concentration (increasing from S0 to S3). The average and confidence interval of three replicates is reported for each accession and treatment. The different letters above each bar indicate significant differences (p ≤ 0.05) among treatments within each variety. V1 = Dragon Tongue, V2 = Capriccio, V3 = Piccante. S0 = 0 mM NaCl, S1 = 25 mM NaCl, S2 = 75 mM NaCl, S3 = 150 nM NaCl. The base nutrient solution was described in Table S1
4 Discussion
Several studies have reported reductions in vegetative plant growth caused by salinity in rocket (e.g., Bonasia et al. 2017). However, understanding the responses of plants to salinity requires considering environmental conditions. Salinity adversely affected the growth of rocket varieties, leading to reductions in all biometric and biomass parameters observed in both experiments. However, one of the intents of this experiment was to test the different varieties, grown under salinity stress, in the optimal growing conditions of growth chamber and in the extreme (high temperature) growing conditions of greenhouse during the hot season. The growth chamber resembled the ideal conditions for wild rocket growth described by Hall et al. (2012b) more closely than the greenhouse conditions, which was reflected in the production values. Although the two growing environments influenced biomass showing similar trends among varieties and treatments, the values obtained in the growth chamber were consistently higher than those in the greenhouse, and the greatest yield reductions were observed in the latter one. However, the tested varieties exhibited differences in total DW, suggesting that each variety has distinct patterns of assimilate partitioning among sink organs when photosynthesis and growth are limited due to salt stress. In rocket, both cell division and enlargement within leaf tissues are negatively affected even at low Na concentrations (D’Anna et al. 2003), although leaf elongation is reported to be more sensitive to salinity (Urlić et al. 2017). In our Exp. 1, under more stressful growing conditions, varieties V2 and V3 exhibited the highest leaf surface area per plant as a function of salinity with values decreased with increasing salinity stress level (even if without significant differences), consistently with results by Shariatinia et al. (2021). Variety V1 consistently showed the lowest leaf area at any given salinity level. The different trends between Exp.1 and Exp. 2 underline the importance of testing plant varieties under suboptimal growing conditions in breeding programs, especially for those variables that can exacerbate the effects of the studied abiotic stress like high temperature in greenhouse in combination with salinity. The highest DW values were indeed obtained at lower levels of salt stress, consistent with findings in other Brassica species such as canola, where higher NaCl concentrations led to lower DW (Byobordi 2010). Specific leaf area was significantly affected by salt stress levels in both experiments. These findings align with the results of de Vos et al. (2013), who found that salt stressed Diplotaxis tenuifolia leaves were smaller but thicker with a maximum SLA decrease of 54% at 300 mM NaCl. Increased leaf thickness (reduced SLA) due to enlarged leaf mesophyll area has been reported as an adaptive response to salinity (Vile et al. 2005). In our experiment, SLA was greater at S0 and S1 in both experiments, indicating that it may serve as a tolerance mechanism in salt-stressed plants to allocate more biomass to leaf expansion, but only when sufficient water is available, as suggested by Munns and Tester (2008). These findings agreed with the lower total biomass observed at increasing salinity since plants at higher SLA may presents reduced photosynthesis efficiency as later discussed. Multivariate analysis and gene expression analysis confirmed the trends showed in the two experiments for the observed traits. Among the tested genes, only DtOxo and DtGst exhibited variable expression across the different salinity stress. This agrees with Cavaiuolo et al. (2017) confirming that specific genes for Diplotaxis better explain the response of rocket salad to saline stress.
Chlorophyll biosynthesis is crucial for plant photosynthetic activity, where salt stress usually negatively affect its content by reducing its synthesis and/or inducing degradation and Chl b converting into Chl a (Santos 2004). In salt tolerant species, chlorophyll content can vary depending on different adaptative mechanisms, usually increasing, but also without showing significant variations (Acosta-Motos et al. 2017), while carotenoids usually increase thus quenching ROS and protecting the photosynthetic apparatus (Gupta and Huang 2014).
As salt-tolerant species, all the observed varieties did not show significant differences regarding total chlorophyll (i.e., Chl a and Chl b and Chl tot) and carotenoids content, consistently with previous studies on saline stress adaptative mechanisms (Acosta-Motos et al. 2017), including specific works on rocket (Franzoni et al. 2020; Shariatinia et al. 2021), when tested in growth chamber condition (Exp. 2). Conversely, in greenhouse condition (Exp. 1), Chl a, Chl tot and carotenoids showed a certain interaction variety × treatment. These kinds of data recorded in Exp. 1 condition in respect to salt stress responses confirm the previous study of Petretto et al. (2019) on these varieties, however highlighting the different performances of the three varieties. The salt-tolerance can also explain the unexpected results in terms of Chl a/b ratio and Chl tot/car ratio, indeed observed only in limited light growth chamber conditions, whereas stress conditions are known to induce an increase of the Chl a/b ratio and a decrease of the greenness ratio (Santos 2004). SPAD index measurements showed the same trends of chlorophyll and, interestingly, were not positively correlated with the Chl tot/car ratio as instead observed for other leafy vegetables under salinity (Germano et al. 2022). Previous studies have shown that salt stress affects several gas exchange parameters, including gs, CI, Tr, and Pn (Mahlooji et al. 2018; Qiu et al. 2018). Stomatal closure, induced by decreased leaf turgor and atmospheric vapor pressure, as well as chemical signals from the roots, is a common response to salt stress (Arif et al. 2020). In line with the findings of Hniličková et al. (2017), we observed that salt stress treatments effectively reduced gs and Pn in both environments. Thus, maintaining control over stomatal conductance is crucial for efficient CO2 acquisition and prevention of desiccation. However, the impact of salt stress on Pn can be attributed to both stomatal and nonstomatal factors as underlined by Balasubramaniam et al. (2023). The decrease in gs is also consistent with the reduced Tr. In fact, when stomatal conductance decreases under salt stress, as observed in our study, there is an increased resistance to water diffusion from inside the leaf to the atmosphere. Concerning the intercellular CO2 concentration, previous studies have reported conflicting results. In cases where salt stress reduces stomatal conductance, an increase in intercellular CO2 concentration is typically observed. However, in other instances, CI may be reduced (Seemann et al. 1985) or remain unaffected. Similarly, we found a significant interaction between variety and salt stress treatment in Exp. 1, but under growth chamber conditions we did not observe any effect on CI. As regards the response of varieties to saline stress, studies carried out on other species suggested that the high stomatal conductance (gs) could be an effective method for identifying genotypic tolerance to saline stress (Rahnama et al. 2010). This may be attributed to the fact that tolerant varieties have a higher number of open stomata and may utilize Na+ instead of K+ for stomatal movements, or they might reduce stomatal density to conserve water under saline conditions (Zhao et al. 2020).
Despite both varieties and/or saline treatments exerted some influences on mineral tissue concentration, no evident deficiency symptoms were observed in the two environments. This trend is confirmed by recorded values, generally higher than the range of sufficiency especially considering macro-nutrients as nitrogen or phosphorus (Bozokalfa et al. 2009). Deepening inside salt stress responses, K, Ca and Mg tissue concentrations are all considered as functional markers to determine plant salt tolerance, whereas sodium usually inhibits their uptake with detrimental effects on many physiological mechanisms (e.g., osmotic regulation, gas exchange, chlorophyll biosynthesis, enzymes activity, cell walls building up), as reported in different studies (Gupta and Huang 2014; Acosta-Motos et al. 2017). As expected, in both experiments, Na tissue concentration increased only because of the saline treatments, while Ca was influenced by variety in growth chamber conditions only. Looking instead to K and Mg, only the highest saline treatment was able to induce some reduction, but only in K ascribable to a deficiency condition as well (i.e., 35.49 g kg−1 DW in greenhouse condition), as many authors reported average values of roughly 4.5% on dry matter. As expected, trends observed for these three elements confirmed the same behaviors observed in varieties V2 and V3 regarding previously discussed biometric and eco-physiological parameters.
5 Conclusions
Biometric, physiological and biochemical parameters significantly varied because of variety, salt level used and environmental conditions. The two cultivation systems used in Exp. 1 (greenhouse) and Exp. 2 (growth chamber) deeply affected the variety response in presence and absence of salt stress. In general, under optimal growing conditions, the wild rocket varieties used showed higher growth (leaf number, leaf area, specific leaf area, dry weight) compared to greenhouse conditions. Overall V1 (‘Dragon Tongue’ variety) and V3 (‘Piccante’ variety) presented similar trends and response in both growing conditions: V1 was the least, while V3 was the most productive. Interestingly, gene expression analysis revealed a significant increase of two target genes for tolerance to salinity (DtOxo and DtGst) therefore to salt level used. This trend is mainly confirmed in V1 and V3 varieties under optimal growing condition, without any other environmental variable. These two genes in agreement with the morpho-physiological parameters tested, could be used as potential markers for future breeding program for salt tolerance in wild rocket.
Data Availability
The data are available upon reasonable request to the authors.
References
Acosta-Motos J, Ortuño M, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco M, Hernandez J (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. https://doi.org/10.3390/agronomy7010018
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77. https://doi.org/10.1016/j.plaphy.2020.08.042
Balasubramaniam T, Shen G, Esmaeili N, Zhang H (2023) Plants’ response mechanisms to salinity stress. Plants 12:2253. https://doi.org/10.3390/plants12122253
Bonasia A, Lazzizera C, Elia A, Conversa G (2017) Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front Plant Sci 8:300. https://doi.org/10.3389/fpls.2017.00300
Bozokalfa MK, Yagmur B, Ilbi H, Esiyok D, Kavak S (2009) Genetic variability for mineral concentration of Eruca sativa L. and Diplotaxis tenuifolia L. accessions. Crop Breed Appl Biotechnol 9. https://doi.org/10.12702/1984-7033.v09n04a12
Byobordi A (2010) The influence of salt stress on seed germination, growth and yield of canola cultivars. Not Bot Horti Agrobot Cluj-Napoca 38:128–133
Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicylic-acid. Commun Soil Sci Plant Anal 6:71–80
Cavaiuolo M, Cocetta G, Spadafora ND, Müller CT, Rogers HJ, Ferrante A (2017) Gene expression analysis of rocket salad under pre-harvest and postharvest stresses: a transcriptomic resource for Diplotaxis tenuifolia. PLoS ONE 12:e0178119. https://doi.org/10.1371/journal.pone.0178119
Cocetta G, Mishra S, Raffaelli A, Ferrante A (2018) Effect of heat root stress and high salinity on glucosinolates metabolism in wild rocket. J Plant Physiol 231:261–270. https://doi.org/10.1016/j.jplph.2018.10.003
D’Anna F, Miceli A, Vetrano F (2003) First results of floating system cultivation of Eruca sativa L. Acta Hortic 609:361–364. https://doi.org/10.17660/ActaHortic.2003.609.54
D’Antuono LF, Elementi S, Neri R (2009) Exploring new potential health-promoting vegetables: glucosinolates and sensory attributes of rocket salads and related Diplotaxis and Eruca species. J Sci Food Agric 89:713–722. https://doi.org/10.1002/jsfa.3507
de Vos AC, Broekman R, de Almeida Guerra CC, van Rijsselberghe M, Rozema J (2013) Developing and testing new halophyte crops: A case study of salt tolerance of two species of the Brassicaceae, Diplotaxis tenuifolia and Cochlearia officinalis. Environ Exp Bot 92:154–164. https://doi.org/10.1016/j.envexpbot.2012.08.003
Franzoni G, Cocetta G, Trivellini A, Ferrante A (2020) Transcriptional regulation in rocket leaves as affected by salinity. Plants 9:20. https://doi.org/10.3390/plants9010020
Germano RP, Melito S, Cacini S, Carmassi G, Leoni F, Maggini R, Montesano FF, Pardossi A, Massa D (2022) Sweet basil can be grown hydroponically at low phosphorus and high sodium chloride concentration: Effect on plant and nutrient solution management. Sci Hortic 304:111324. https://doi.org/10.1016/j.scienta.2022.111324
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 701596. https://doi.org/10.1155/2014/701596
Hall MKD, Jobing JJ, Rogers GS (2012a) Some perspectives on rocket as vegetable crop: a review. J Fruit Ornam Plant 76:21–41. https://doi.org/10.2478/v10032-012-0002-5
Hall MKD, Jobling JJ, Rogers GS (2012b) Factors affecting growth of perennial wall rocket and annual garden rocket. Int J Veg Sci 18:393–411. https://doi.org/10.1080/19315260.2012.660565
Han S, Kim D (2006) AtRTPrimer: database for Arabidopsis genome-wide homogeneous and specific RT PCR primer-pairs. BMC Bioinf 7:179. https://doi.org/10.1186/1471-2105-7-179
Heimler D, Isolani L, Vignolini P, Tombelli S, Romani A (2007) Polyphenol content and antioxidative activity in some species of freshly consumed salads. J Agric Food Chem 55:1724–1729. https://doi.org/10.1021/jf0628983
Hniličková H, Hnilička F, Martinková J, Kraus K (2017) Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ 63:362–367. https://doi.org/10.17221/398/2017-PSE
Mahlooji M, Seyed Sharifi R, Razmjoo J, Sabzalian MR, Sedghi M (2018) Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 56:549–556. https://doi.org/10.1007/s11099-017-0699-y
Massa D, Melito S (2019) Signaling molecules in ecophysiological response mechanisms of salt-stressed plants. In: Iqbal et al (ed) Plant Signaling Molecules, 1st edn. Woodhead Publishing, Sawston, pp 1–18. https://doi.org/10.1016/j.scienta.2016.03.033
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1104/pp.87.3.547
Pandey M, Penna S (2017) Time course of physiological, biochemical, and gene expression changes under short-term salt stress in Brassica juncea L. Crop J 5:219–230. https://doi.org/10.1016/j.cj.2016.08.002
Parada RB, Marisol V, Marguet ER (2019) Antioxidant and antiglycation properties of extracts of Diplotaxis tenuifolia [Propiedades antioxidante y antiglicosilante de extractos de Diplotaxis tenuifolia]. Bionatura 4:852–855
Petretto GL, Urgeghe PP, Massa D, Melito S (2019) Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol Biochem 141:30–39. https://doi.org/10.1016/j.plaphy.2019.05.012
Purty RS, Kumar G, Singla-Pareek SL, Pareek A (2008) Towards salinity tolerance in Brassica: an overview. Physiol Mol Biol Plants 14:39–49. https://doi.org/10.1007/s12298-008-0004-4
Qiu R, Yang Z, Jing Y, Liu C, Luo X (2018) Effects of irrigation water salinity on the growth, gas exchange parameters, and ion concentration of hot pepper plants modified by leaching fractions. Hortscience 53:1050–1055. https://doi.org/10.21273/HORTSCI13078-18
Rahnama A, James RA, Poustini K, Munns R (2010) Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct Plant Biol 37:255–263. https://doi.org/10.1071/FP09148
Sagervanshi A, Naeem A, Geilfus CM, Kaiser H, Mühling KH (2021) One-time abscisic acid priming induces long-term salinity resistance in Vicia faba: Changes in key transcripts, metabolites, and ionic relations. Physiol Plant 172:146–161. https://doi.org/10.1111/ppl.13315
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hortic 103:93–99. https://doi.org/10.1016/j.scienta.2004.04.009
Seemann JR, Critchley C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164:151–162. https://doi.org/10.1007/BF00396077
Shadish WR, Cook TD, Campbell DT (2002) Experimental and quasi-experimental designs for generalized causal inference. Houghton Mifflin Company, New York
Shariatinia F, Azari A, Rahimi A, Panahi B, Madahhosseini S (2021) Germination, growth, and yield of rocket populations show strong ecotypic variation under NaCl stress. Sci Hortic 278:109841. https://doi.org/10.1016/j.scienta.2020.109841
Urlić B, Dumičić G, Romić M, Ban SG (2017) The effect of N and NaCl on growth, yield, and nitrate content of salad rocket (Eruca sativa Mill.). J Plant Nutr 40:2611–2618. https://doi.org/10.1080/01904167.2017.1381122
Vile D, Garnier E, Shipley B (2005) Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann Bot 96:1129–1136. https://doi.org/10.1093/aob/mci264
Zhao C, Zhang H, Song C, Zhu J-K, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. The Innovation 1:100017. https://doi.org/10.1016/j.xinn.2020.100017
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement. This study was in part funded to Department of Agricultural Science, University of Sassari, by Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022).
Author information
Authors and Affiliations
Contributions
Sonia Cacini: Investigation, Data curation, Writing—Original Draft. Paola Antonia Deligios: Data curation, Formal analysis, Writing—Original draft. Daniele Massa: Conceptualization, Methodology, Supervision, Resources. Pasquale Tripodi: Investigation, Formal analysis, Validation. Federica Alchera: Investigation, Data curation. Luigi Ledda: Investigation, Formal analysis. Marco Ginepro: Investigation, Data curation, Writing—Original Draft. Sara Melito: Conceptualization, Methodology, Supervision, Validation, Resources, Writing—Original Draft. All the authors equally contributed to Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cacini, S., Deligios, P.A., Massa, D. et al. Salinity Tolerance of Diplotaxis tenuifolia Varieties Growing in Spring–Summer Season Under Mediterranean Greenhouse and Optimal Growing Conditions. J Soil Sci Plant Nutr 24, 5931–5945 (2024). https://doi.org/10.1007/s42729-024-01950-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01950-3