Abstract
To investigate how and in what amounts biochar and methyl jasmonate can improve drought tolerance of barley. A two-year experimental study was conducted in a factorial randomized complete block design (n = 5) in the research greenhouse of Zanjan University, Iran, to investigate the possible effects of biochar and methyl jasmonate on some traits of winter barley under drought conditions. Two irrigation regimes, D0 (full irrigation in soil field capacity as control) and D1 (withholding irrigation immediately after flowering stage), three methyl jasmonate spray densities [0 (M0), 50 (M50), and 100 (M100) μM] and four levels of biochar in soil [0% (B0), 0.25% (B0.25), 0.5% (B0.5), 1% (B1) per soil weight] were used in this experiment. In this study, drought reduced two-year average leaf area (LA) by 96%, stomatal conductance (gs) by 84%, and photosynthetic water use efficiency (PWUE) by 64%. In addition, drought reduced chlorophyll-b by 1.5% and 81% and transpiration rate (Tr) by 2.5% and 78% in the first and second years, respectively. However, the application of biochar and methyl jasmonate improved all the traits studied in both D0 and D1 drought-treated plants. For most of the parameters studied, the optimal combination of biochar and methyl jasmonate that optimized water use efficiency and alleviated drought was 0.25% and 50 μM, respectively. The synergistic action of biochar and jasmonates improved the tolerance of barley to water stress.
Similar content being viewed by others
1 Introduction
Barley (Hordeum vulgare L.) is among the most strategic crop plants, providing food for loads of population of the world. However, the productivity of the barley is considerably restricted under the influence of climate change catastrophic environmental circumstances (Nasiri et al. 2023a). It is reported that the world barley production was decreased in 2021–2022 cultivation season compared with 2020–2021 mainly due to the adverse effects of drought stress (Shahbandeh 2022). It is a multidimensional kind of stress which takes place largely for the sake of restricted rainfall and a consequent dry spell. Over the past decades, the expansion in areas of dry-land agriculture has increased, and drought has caused approximately 30 billion dollars in damage to the agriculture sector (Gupta et al. 2020). Numerous studies worldwide have assessed the effects of abiotic stresses on crops and how to alleviate their effects (El-Seyed et al. 2014). Although, plants have specific adaptation mechanisms to cope with water deficits, there is an urgent need to maintain productivity at a high level under drought conditions (Dey et al. 2021; Sirhindi et al. 2020). Drought can damage plant growth and development, reducing economic yield due to adverse effects on photosynthesis, respiration, nitrogen metabolism, and absorption of other essential elements (Liu et al. 2022). Drought leads to negative effects on photosynthesis and gas exchange parameters (Bryant et al. 2021), cell wall composition Tenhaken 2014) nutrient translocation (Demidchik 2015), the transcriptional activity of genes, transposable elements (Mamnoei and Seyed Sharifi 2010), the lipid signaling (Hou et al. 2021), metabolites, proteins (Nohong and Nompo 2015), and antioxidant profile (Dey et al. 2021). Drought also results in cell dehydration by reducing relative water content, chloroplast degradation, and a severe reduction in photosynthetic pigments content, decreasing photosynthesis and final productivity (Dey et al. 2021). In addition, researchers reported that drought decreased some physiological and biochemical traits of wheat, such as photosynthetic pigments content, relative water content (RWC), net photosynthesis (An), stomatal conductance (gs), and grain yield; Furthermore, under water deficit conditions, electron transport through PSII is reduced, and the oxygen-evolving complex of photosystem II (PSII) is damaged (Gupta et al. 2020). However, there are many ways to alleviate the detrimental impacts of water deficit on soil water content, plant growth, and yield.
Several reports have documented the alleviation of water deficit stress by applying soil amendments such as biochar. Biochar has recently received attention because it improves soil fertility, carbon content, water use efficiency, and crop production (Khan et al. 2021). Biochar practically sequester carbon, enhances soil organic carbon and microbial activity and improves the whole soil quality and function which affect positively the attributes of plants in water shortage conditions (Cantrell et al. 2012). Mixing biochar with soil can diminish irrigation frequency by holding water from percolation to a deeper layer away from the root system. In addition, mixing biochar with soil increases nutrient retention (Thi Pham et al. 2021). For example, cations retention was higher in soil mixed with biochar than in other forms of organic matter (Sombroek et al. 2004). Therefore, the increase in water retention and soil fertility of soil amended with biochar mitigates the sensitivity of crops to soil water deficit (Abideen et al. 2020; Katterer et al. 2019). It is reported that, under drought circumstances, application of biochar can be effective approach in alleviating the adverse effects of it on the enzymatic activity of plant and the concentration of non-enzymatic substances. For instance, in several researches that, although the activity of plant defense system including reactive oxygen species (ROS) scavengers such as catalase, peroxidase, ascorbate peroxidase and superoxide dismutase as well as proline concentration is increased in stressful circum-stances, adding biochar, due to alleviation of drought effects, results in reduced enzymatic activity and non-enzymatic components' accumulation (Nasiri et al. 2023b). In addition, biochar application significantly enhanced plant normal functions, such as chlorophyll-a, and chlorophyll-b contents, RWC, and decreased the concentration of proline and the percentage of electrolyte leakage resulting in higher economic yield (Abd El-Mageed et al. 2021). Similarly, other researchers (Iqbal et al. 2022) reported that the biochar amendment significantly alleviated the effect of drought in broad beans (Vicia faba L.) by increasing the plants’ height, dry weight, chlorophyll content, the maximum quantum performance of photosystem II, improving the physical and biochemical properties of the soil.
Jasmonates, lipid-based plant hormones, are essential in alleviating several biotic and abiotic stresses and play a crucial role in plant response (Ali and Baek 2020). Besides, jasmonic acid (JA) regulates various plant processes, including growth, photosynthesis, and reproductive functions (Soliman et al. 2018; Munir et al. 2022; Sheteiwy et al. 2022). Furthermore, methyl jasmonate (M) relieves water deficit stress by regulating stomatal closure, ROS production, and profound root growth (Riemann et al. 2015; Iqbal et al. 2022). For example, the foliar application of methyl jasmonate can benefit plants exposed to drought by regulating enzymatic and non-enzymatic antioxidants that scavenge ROS (Fugate et al. 2018). Researchers illustrated that the foliar application of biochar can mitigate the adverse effects of drought on barley enhancing the enzymatic activity of catalase, peroxidase, ascorbate peroxidase and superoxide dismutase, improving the concentration of proline and leaf greenness index (SPAD) in leaves (Nasiri et al. 2023b). Besides, other scientists reported a substantial positive impact of spraying sugar beet subjected to drought with different levels of methyl jasmonate by positively improving RWC, fresh and dry weight, net photosynthesis rate, transpiration rate, stomatal conductance, water use efficiency (WUE), and PSII quantum efficiency (Fugate et al. 2018). Moreover, it is stated that exogenous application of methyl jasmonate relieved salinity stress by improving chlorophyll content, Hill reaction rate, transpiration, and stomatal conductance (Rezaei et al. 2018).
In light of these findings, due to the cumulative influences of climate change, drought stress is getting the most important environmental issue day by day. Furthermore, biochar amends the soil structure, enhances the microbial community and activity of the soil, resulting in the improved water availability and crop productivity under controlled environments. Methyl jasmonate also in specific and in combination with biochar can be utilized as a promising method to alleviate the drastic impacts of water shortage in a wide range of crop plants. To the best of our knowledge, no experiments have been explored to study the combined effects of biochar and methyl jasmonate on drought alleviation. According to what we stated in introduction, we aimed in this study to assess that how the individual and joint application of biochar and foliar application of methyl jasmonate influence the several physiological and biochemical traits of barley plants under drought stress and the normal conditions. We assumed that the application of biochar can ameliorate the repercussions of drought stress by improving soil properties and the external application of methyl jasmonate will also be useful due to its positive impact in elevation of the plant's enzymatic activity. The results of this study will help to determine the optimum combination of biochar amendment and methyl jasmonate for relieving the drought effect on barley.
2 Materials and Methods
2.1 Site Description
To evaluate the effects of the biochar addition to the soil and exogenous application of methyl jasmonate, an experimental glasshouse study was carried out in northwest Iran at the University of Zanjan. The study was conducted over two consecutive years (2021 and 2022) using a randomized complete block design (n = 5).
2.2 Growth Conditions
Barley (Hordeum vulgare L. var. Jolgeh) seeds were purchased from a well-known barley seed producer company in Zanjan, Iran (Zanjan Kesht Kheir Abad company) and were soaked with a density of 17 plants per pot in black plastic pots (25 cm top diameter with 30 cm height) containing 10 kg of loamy sand soil (dry weight equivalent). Seeds were sown on the 15th of October in the first year and the 22nd of October in the second year. During the experiment, the temperature and humidity of the glasshouse were set between 16–21 °C (minimum–maximum) and 60%, respectively, with natural light conditions with a day/night cycle of 14/10 h. Tables 1 and 2 show the physicochemical properties of the soil and biochar used in this experiment, which were analyzed and determined at the agricultural research center of Zanjan, Iran. The recommended doses of NPK fertilizers were based on the recommendations of the local agricultural organization for barley (100, 60, and 45 kg ha−1, respectively).
2.3 Experimental Layout
Two levels of drought stress, namely non-stress conditions (100% field capacity as a control, D0) and withholding irrigation for two weeks at the flowering stage (D1), was applied as a main factor. The field capacity (FC) is the amount of water in the soil after a rain or irrigation event when gravity has removed the remaining water from the soil.
The soils in the pots were saturated with water and left until they had constant weight. The soil was dried, and the weight difference between dry and FC soils was calculated and kept constant throughout the experiment in the D0. The treatment of D1 was started at the beginning of the flowering stage. In this treatment, the soils were kept without irrigation until the soil water potential reached under 2 mega pascal, indicating that drought stress is applied adequately. To analyze and determine the physical and chemical properties of the soil, Surface soil samples were collected from a 0–30 cm depth of the soil profile. Then the air-dried soil samples were sieved by a 2-mm sieve, homogenized, mixed, and their properties were measured according to the standard methods outlined by Lindsay and Norvell (1978) and Page et al. (1982).
Biochar was obtained from soybean stover subjected to pyrolysis temperature of 700 °C. This biochar showed these characteristics: specific surface area 419.6 m2 g, volatile matter 15.6%, ash 17.2%, cation exchange capacity 47.5 cmol kg. In terms of particle size distribution, 21.7% were between 5 and 2 mm, 24% were between 2 and 0.5 mm, 48% were between 0 and 0.5 mm. Finally, biochar showed 48% water holding capacity. It was mixed with the soil at the beginning of the experiment at four levels: 0 (B0), 0.25% (B0.25), 0.5% (B0.5), 1% (B1) w/w. To analyze the studied biochar, first the samples were homogenized, then they were divided into portions. The physicochemical properties of the biochar were determined based on method introduced by Cantrell et al. (2012) and Singh et al. (2017).
A foliar spray of methyl jasmonate (was purchased from Sigma-Aldrich company) was applied simultaneously with withholding irrigation during the flowering stage at three levels: 0 as the control (M0), 50 μM (M50), and 100 μM (M100). In both years, this method was used. The soils were changed in the second year, and biochar treatments were applied as in the first year. Samples were collected from all pots two weeks after starting D1 treatment.
Physiological and biochemical traits were determined and measured in the plant physiology laboratory at the University of Zanjan, Iran.
2.4 Leaf Area (LA)
Leaf area was measured five weeks after the flowering stage from all replications and plots, using a leaf surface meter device, model delta T, from three sampled plants from each pot selected randomly before applying drought and earmarked using colorful ribbons. Three samples from each plot were mixed and considered as one sample. The final leaf surface of the plants was calculated as the average of the three samples from each pot.
2.5 Determination of Relative Water Content of Leaf (RWC) and Water Use Efficiency (WUE)
The leaf fresh weight was measured from three randomly matured leaves from the top of each plant. Fresh leaves were weighed, placed in distilled water for 24 h, dried with tissue paper, and weighed to calculate turgid weight. The leaves were dried in an oven set (at 70 °C for 48 h) till constant weights. The RWC was calculated using the Eq. 1 (Pieczynski et al. 2013):
The water use efficiency (WUE), which is known as the ratio of plant yield to used water during the cultivation season, was calculated according to the Eq. 2 (Smart and Bingham 1974):
2.6 Photosynthetic Pigments
The fresh flagged leaves were separated and snap-frozen immediately in liquid nitrogen. A 0.5 g fresh flag leaf was used to determine the photosynthetic pigments content, according to approach introduced by Arnon (1940). To calculate chlorophyll a, b, and carotenoid contents, leaf extracts were absorbed at wavelengths of 663, 645, and 470 nm, respectively, using a UV/VIS device (PerkinElmer, Lambda 25, USA). The final concentrations of pigments were calculated based on the formulas 3, 4 and 5:
2.7 Gas Exchange Parameters and the Maximum Quantum Performance of Photosystem II
The net photosynthetic rate (NP, μM CO2 m−2 s−1), intercellular CO2 concentration (Ci, μM CO2/M air), stomatal conductance (gs, M H2O m−2 s−1) and transpiration rate (Tr, mM H2O m−2 s−1) were determined from the activity of the flagged leaves of each replicate between 8:00 and 10:00 using a portable gas analyzer device (IRGA, Model LCA4, UK). In addition, mesophyll conductance (Mc) was estimated as the ratio of An to gs (mM CO2 m−2 s−1), and leaf-intrinsic water use efficiency (μM CO2/mM H2O) was calculated as an NP to gs ratio (photosynthetic water use efficiency, PWUE). The measurements were performed at CO2 concentrations of 412 ppm, relative humidity of 60%, natural light conditions, and day/night cycle of 14/10 h in both years.
Fluorescence parameters were measured in the greenhouse using a portable plant fluorescence meter (Opti Sciences, model OS-30), and the maximal quantum performance of photosystem II (MQPPSII) was calculated using Fm-F0 / Fm. In this equation, Fm is the maximum fluorescence and F0 is the minimum fluorescence. The dark adaptation period in measurements of Fm-F0 was 20 min.
2.8 Statistical Analysis
Four-way analyses of variance (ANOVA) were used in factorial based on randomized complete block design to test the effects of the main factors (i.e., year, drought, biochar, and methyl jasmonate) on the studied biochemical and physiological attributes and yield. Duncan’s test assessed the significant differences between the means of different treatments. The figures were delineated using Excel software. All statistical analyses were performed using the SAS software version 9.1.
3 Results
The ANOVA test showed significant effects for the main factors, i.e., the study year, drought treatment, methyl jasmonate) on the physiological attributes, e.g., RWC, WUE, and Chl-a, Chl-b, carotenoids contents, and MQPPSII (Table 2), leaf gas exchange parameters, e.g., NP, Tr, Ci, gs, Mc, PWUE, and drain yield of barley (P < 0.05, Table 3). However, the interactions between the different combinations of the main factors were not significant in most studied attributes.
3.1 Leaf Area (LA)
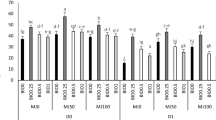
The main factors had significant effects on the leaf area (LA). Besides, the two-way (B x D, B x M) and three-way interactions (D x B x M) had significant effects on the LA (Table 2). Drought stress significantly reduced the LA, but biochar and methyl jasmonate significantly increased the LA in drought-stressed plants (Figs. 1, 2). In fact, drought decreased the two-year average of LA by 96%, while the application of biochar and methyl jasmonate increased this parameter comparable to the control. Regardless of the effect of methyl jasmonate, the interaction between biochar and methyl jasmonate was significant; the enhancement of biochar application was significantly greater in drought-non-stressed than in drought-stressed plants. Besides, the maximum increase in the LA was recorded in plants treated with B0.25 and M0, with B0.25 and M50 in drought-non-stressed plants, and in plants treated with B0.25 and M50 in drought-stressed plants. Such results indicate that the enhancing efficiency of biochar and methyl jasmonate is independent of drought stress. Furthermore, applying 1% biochar (B1) had a greater inhibitory effect on the LA, than B0.25 and B0.5, in all methyl jasmonate and drought treatments, indicating 1% biochar might be harmful to soil physicochemical or microbial properties (Fig. 2).
The effect of triple interaction between drought, biochar and methyl jasmonate on the two-year average of leaf area (LA) of barley plants. In this figure, D0 and D1 refer to normal irrigation and drought stress conditions respectively, B0, B0.25, B0.5 and B1 respectively stand for control treatment without biochar, 0.25, 0.5 and one % of biochar addition into the soil per soil weight, and M0, M50, M100 refer to control treatment without spraying methyl jasmonate, 50 and 100 μM/L of spraying
3.2 Relative Water Content and Water Use Efficiency
The relative water content (RWC) and water use efficiency (WUE) were significantly affected by the main factor (D, B and M) and the two-way interaction of biochar and methyl jasmonate (Table 2). Investigating the two-year interaction between biochar and methyl jasmonate in both irrigation systems (DS0 and DS1) illustrated that the biochar addition and methyl jasmonate spraying, individually or in combination, improved RWC and WUE (Table 4). The highest RWC (77.9%) was detected by the combined application of B0.25 and M50, and the lowest index (59.3%) was observed in the controls (i.e., B0 and M0, Table 4). Similarly, the WUE was significantly greater in the combined application of B0.25, and M50 (0.77 g/L) than in control treatments (B0 and M0, 0.29 g/L) (Table 4).
3.3 Leaf Photosynthetic Pigment Content
The concentrations of Chl-a, Chl-b, total chlorophyll, and carotenoids were significantly affected by the man factors (D, B, and M) (Table 2). The results showed significant interactions between the year and both biochar and methyl jasmonate on Chl-a, Chl-b, and total chlorophyll. The results demonstrated that drought reduced the Chl-b in the first year by 1.5% and in the second year by 81% compared with drought-non-stressed plants (Fig. 3). However, application of biochar and methyl jasmonate mitigated the adverse effects of drought in both years. In the first year, the highest accumulation of Chlorophyll-b (2.76 mg g−1 DW) was observed in plants exposed to D0-B0.25-M50 treatment, and the lowest value (0.71 mg g−1 DW) was in D1-B1-M0 treatment. In the second year, the highest concentration of Chlorophyll-b (4.5 mg g−1 DW) was detected in D0-B0.5-M100, and the lowest content (0.47 mg g−1DW) in D1-B0-M0 treatment (Fig. 3) Moreover, there was a significant interaction between biochar and methyl jasmonate on the concentration of Chl-a and total chlorophyll. The positive effect of M50 was greater in B0.25 than in the other biochar treatment. The highest accumulation of Chl-a (8.34 mg g−1 DW) and total chlorophyll (11.24 mg g−1 DW) was observed in the B0.25-M50 treatment. Still, the lowest content of Chl-a (3.93 mg g−1 DW) and total chlorophyll (5.41 mg g−1 DW) was detected in non-treated plants (B0-M0) (Table 4).
Four-way interaction between year, drought, biochar and methyl jasmonate on the concentration of chlorophyll-b (Chl-b) in barley plants. In this figure, D0 and D1 refer to normal irrigation and drought stress conditions respectively, B0, B0.25, B0.5 and B1 respectively stand for control treatment without biochar, 0.25, 0.5 and one % of biochar addition into the soil per soil weight, and M0, M50, M100 refer to control treatment without spraying methyl jasmonate, 50 and 100 μM of spraying
3.4 Maximum Quantum Performance of Photosystem II (Fv/Fm)
There were interactive effects of biochar and methyl jasmonate (B x M, Tables 2 and 4) and drought and methyl jasmonate (D × M, Tables 2 and 5) on the most important chlorophyll fluorescence parameter (MQPPSII). The investigation on the average of the two-year interaction between biochar and methyl jasmonate in both irrigation systems. The MQPPSII was significantly greater in M50 than both M0 and M100 in B0.25, B0.5, and B1, but not B0 (Table 4). In B0, there was no significant difference between M50 (0.56) and M100 (0.53), and both had significantly greater values than M0 (0.33). The highest MQPPSII (0.69) was in the B0.25-M50 treatment, but the lowest (0.33) was in B0-M0 (Table 4). Besides, drought significantly reduced MQPPSII, but methyl jasmonate's foliar application significantly increased it under drought-non-stressed and drought-stressed plants. The highest value (0.7) was observed in M50-DS0, and the lowest value (0.33) in the M0-D1 treatment (Table 5).
3.5 Leaf Gas Exchange Parameters
The individual effects of D, B, and M significantly affected all gas exchange parameters (Table 3). The effect of B × M interaction on NP (net photosynthesis rate) was significant; the average An of the two-year and biochar treatments was greater by 11.3% and 69.5% in B0.25 and B0.5 compared with control plants (B0), while the use of B1 decreased NP by 4.2% (Table 4). Furthermore, spraying methyl jasmonate enhanced NP by 76% and 62% with the application of M50 and M100 compared with M0. The highest photosynthesis rate (9.78 μM CO2 m−2 s−1) was obtained in B0.25-M50 treatment, and the lowest (3.65 μM CO2 m−2 s−1) was in B1-M0 (Table 4). Additionally, the application of biochar and methyl jasmonate decreased the accumulation of Ci, which was related to the increase in the net photosynthesis rate (NP) (Table 5). The highest Ci (280.9 μM CO2/M air) was detected in B0-M0, whereas the lowest (126.6 μM CO2/M air) was in B0.25-M50 treatment (Table 5). The highest mesophyll conductance (Mc) (0.1 mM CO2 m−2 s−1) was detected in B0.25-M50 and the lowest (0.02 mM CO2 0.1 mM CO2 m−2 s−1) was B0-M0 treatment (Table 4). The drought significantly elevated the Ci, but the foliar application of methyl jasmonate resulted in a significant reduction in the accumulation of CO2 (Table 5). The effect of drought and methyl jasmonate interaction on the Ci index was significant; the reduction was greater in J50 (32.1% and 17.8% in D1 and D0, respectively) than in M100 (2.3% and 17.3% in D1 and DS0, respectively), as compared to in M0 (Table 5). Similarly, the effect of drought and biochar interaction on the Ci index was significant; in the non-drought-stressed plants (D0), the Ci was significantly greater in B0.25 and B0.5 than B0 by 33.7%, and 3.05%, respectively, in D0 treatment, and by 44.5% and 15.0% in D1 treatment. However, Ci was significantly lower in B1 than B0 by 16% in D0 treatment and 20.2 in D1 treatment (Table 6). Although drought stress caused an increase in the Ci index, it caused a significant reduction in mesophyll conductance (Mc). The highest Mc value was in D0-B0.25 (0.11), and the lowest was in the D1-B1 treatment. The results indicate that the best biochar treatment in D0 and D1 was B0.25, and the worst was B1 (Tables 5 and 6).
The ANOVA result showed a significant effect for the individual effect of drought, biochar and methyl jasmonate, and year and the interaction of the four factors (i.e., year x D x B x M) on the transpiration rate (Tr) of barley plants (Table 3). The Tr was significantly reduced under drought in both year 1 (2.5%) and year 2 (78%) compared to non-drought-stressed plants (Fig. 3). However, biochar addition increased Tr in Year 2 but not in year 1. The methyl jasmonate had no clear effect on the transpiration rate in both years and drought and biochar treatments (Fig. 4).
Four-way interaction between year, drought, biochar and methyl jasmonate on transpiration rate (Tr). In this figure, D0 and D1 refer to normal irrigation and drought stress conditions respectively, B0, B0.25, B0.5 and B1 respectively stand for control treatment without biochar, 0.25, 0.5 and one % of biochar addition into the soil per soil weight, and M0, M50, M100 refer to control treatment without spraying methyl jasmonate, 50 and 100 μM of spraying
There was a significant effect of the three-way interaction of the main factors (D x B x M) on the stomatal conductance (gs) (Fig. 5) and the leaf intrinsic water use efficiency (PWUE) (Fig. 6). Drought stress significantly reduced gs by 84%; however, biochar and methyl jasmonate's individual and combined applications significantly increased it. The highest gs was in the B0.25-D0 treatment, and the lowest was B0—M0 treatment. Again, the highest dose of biochar (B1) provided the lowest gs values. Drought declined PWUE by 64% compared to non-drought-stressed plants (D0). At the same time, the individual and combined applications of biochar and methyl jasmonate significantly enhanced PWUE. The highest PWUE values were in B0.25 at all M treatments of both drought-stressed and on-drought-stressed plants (Fig. 5).
The two-year average of interaction between drought, biochar and methyl jasmonate on stomatal conductance (gs) of barley plants. In this figure, D0 and D1 refer to normal irrigation and drought stress conditions respectively, B0, B0.25, B0.5 and B1 respectively stand for control treatment without biochar, 0.25, 0.5 and one % of biochar addition into the soil per soil weight, and M0, M50, M100 refer to control treatment without spraying methyl jasmonate, 50 and 100 μM of spraying
The two-year average of interaction between drought, biochar and methyl jasmonate on photosynthetic water use efficiency (PWUE) index of barley plants. In this figure, D0 and D1 refer to normal irrigation and drought stress conditions respectively, B0, B0.25, B0.5 and B1 respectively stand for control treatment without biochar, 0.25, 0.5 and one % of biochar addition into the soil per soil weight, and M0, M50, M100 refer to control treatment without spraying methyl jasmonate, 50 and 100 μM of spraying
4 Discussion
Environmental changes affecting water availability, drought, or flooding, had major impacts on plant growth, and physiological and metabolic processes (Feng et al. 2021). Restricted supply of water can change the physiological, biochemical and photosynthetic attributes of crop plants, leading to the lower production. The present study demonstrated that drought significantly affected some parameters, resulting in substantial alterations in the grain yield of barley. However, our findings revealed that the addition of biochar into the soil and exogenous application of methyl jasmonate as a plant growth regulator, individually and in combination, alleviated the impact of drought in barley plants by maintaining cellular water levels, membrane stability, the accumulation of photosynthetic pigments, and gas exchange attributes, eventually enhancing morpho-physiological traits.
The relative water content is an index, showing the repercussions of drought on plants. Our results further demonstrated that biochar considerably improved RWC and WUE (Table 5). Such a finding also was supported by other studies (Sombroek et al. 2004; Mohi-Ud-Din et al. 2021; Alikhani et al. 2019; Xu et al. 2010). For example, biochar application under drought significantly increased the WUE in Peanut (Xu et al. 2010). Like our results, other researchers concluded that biochar amendment enhanced water use efficiency in sugar beet (Beta vulgaris L.), alleviating drought stress under extremely arid or semi-arid climates (Wang et al. 2021). Biochar increases the soil content of carbon and other nutrients, increasing relative water content and enhancing plant height, leaf number, and leaf area (Abd El-Mageed et al. 2021). Additionally, biochar increases water-holding capacity (WHC), mineral nutrients, soil microbial activity, organic carbon, available phosphorous, soil carbohydrate content, and microbial biomass of the soil, and soil aeration, leading to higher water use efficiency and wheat productivity (Cantrell et al. 2012; Wang et al. 2021). It has been reported that biochar amendment improved WUE because it enhances hydrological characteristics, the physical attributes of the soil, and soil water content, resulting in higher grain yield under natural or stress conditions (Todorova et al. 2022). On the other hand, we observed in this study that the exogenous application of methyl jasmonate improved RWC and WUE (Table 5). The same results have been presented by the other (Ahmadian et al. 2021). The methyl jasmonate, at lower densities, has positive impacts on the physiological and biochemical attributes of plants, however, higher doses of this hormone can deteriorate the responses in particular in stress conditions (Feng et al. 2021; Wei et al. 2020). Our study indicated that methyl jasmonate effect was dose-specific; the positive impact of M50 was greater than that of M100 on most studied attributes. Besides, the study also indicated that the positive effect of methyl jasmonate on different morphological, physicochemical, and grain yields of barley under drought was augmented when applied with other anti-stress elements, i.e., biochar. Water use efficiency is the ratio of plant yield to actual water used during the cultivation season and represents an efficiency indicator (Baiamonte et al. 2020). The positive effect of methyl jasmonate on WUE could be attributed to its role in protecting the photosynthetic apparatus and enhancing its function even under drought-stress conditions, leading to better grain yield in crops (Vatankhah et al. 2016).
In the present experiment, water scarcity significantly reduced the pigment contents (i.e., Chl-a, Chl-b, total chlorophyll, and CAR) in barley leaves. However, adding biochar into the soil and foliar application of methyl jasmonate protected photosynthetic pigments under drought and modulated its destructive effects. Similarly, other researchers have reported a massive reduction in chlorophyll and carotenoid contents in barley under water scarcity (Iqbal et al. 2022; Feng et al. 2021). The negative effect of drought in various crops was attributed to lowering the oxidation that impairs the biosynthesis of photosynthetic pigments, including chlorophyll and carotenoids (Rezaei et al. 2018; Bagheri et al. 2020). It is possible that drought stress negatively impacts photosynthetic enzyme activity, mainly Calvin cycle enzymes and reactive oxygen species, which negatively impacts the chloroplast and reduces carbon assimilation, decreases chlorophyll concentrations and inhibits photosynthetic activity (Shadmand and Afkari 2018). It has also been proved that the reduction in pigment concentration under drought could be due to the prevention of pigment biosynthesis and assembly of the PSI and PSII light-harvesting complexes to stop over-plus absorption and ROS production (Ahmadian et al. 2021). Moreover, drought results in a colossal disorder in absorption of nutrients, inhibiting different stages of chlorophyll production and increasing the activity of cholorophylase which lead in lower pigment production (Hashem et al. 2019). Nevertheless, similar to our results, some other researchers reported that biochar significantly increased pigment accumulation under drought conditions in two seasons (Abd El-Mageed et al. 2021). It has been reported in several studies that adding biochar to the soil increases its water-holding capacity (WHC), cation exchange capacity and physicochemical properties of the soil, as well as modifies the soil pH, improving nutrient retention and availability in the root zone, such as nitrogen, which is necessary for the production of photosynthetic pigments (Abd El-Mageed et al. 2021). It is noticeable that in this study, the application of methyl jasmonate in specific or in combination with biochar inhibited the adverse effects of drought and regulated the concentration of photosynthetic pigments. The positive impact of methyl jasmonate in reducing the detrimental influences of drought stress on photosynthetic pigments in the present study is in agreement with results observed in bean plants (Wei et al. 2020). The elevation in chlorophyll and carotenoid content in drought-stressed plants treated with methyl jasmonate was reported in bean cultivars (Bagheri et al. 2020), Rapeseed (Ahmadi et al. 2018), and wheat (Todorova et al. 2022). Some believe that first of all, methyl jasmonate restricts the stomatal conductance, resulting in lower transpiration rate, then elevates the enzymatic activity in order to detoxification of plants from Malondialdehyde and other toxic components, and eventually protects plants from destructive impacts of drought conditions such as reduction in the concentration of pigments (Nasiri et al. 2023b). Other studies also have reported that methyl jasmonate stimulates the production of antioxidants and supports plants against oxidative stress, which can be beneficial in reducing the devastating effects of drought on pigment syntheses, increasing their concentration (Fugate et al. 2018). Moreover, it has been claimed that methyl jasmonate plays an important role in up-regulating some key genes in the biosynthesis pathway of chlorophyll, which results in the improved biosynthesis of pigments (Razmi et al. 2017). It seems the combined application of biochar and methyl jasmonate, particularly at optimum doses, can be more effective in alleviating drought consequences.
Our results showed that drought in barley plants resulted in a substantial reduction in the transpiration rate (Tr), mesophyll conductance (Mc), stomatal conductance (gs), and intrinsic or photosynthetic water use efficiency (PWUE), which agree with other results in the young sugar beet (Beta vulgaris L.) (Fugate et al. 2018) and wheat (Sadaf et al. 2017). In addition, drought resulted in the buildup of intercellular carbon, a clear symptom of decreased photosynthesis rate. The negative effect of drought stress on the gas exchange attributes of plants is a common phenomenon and exacerbation of these characteristics during water scarcity is mainly caused by either stomatal restrictions (stomatal closure due to CO2 reduction) or non-stomatal limitations, which result in a reduction in the chlorophyll content, inhibition of Rubisco activase, and lower photochemical efficiency of PSII (Sadaf et al. 2017; Liu et al. 2018; Huang et al. 2017). Despite, the withholding irrigation affected some of the gas exchange parameters negatively use of biochar (0.25% soil weight) ameliorated these indicators both in full irrigation and in drought conditions, which is in accordance with findings by (Hafez et al. 2020) in barley. It seems biochar improves soil microbial, biochemical and physical properties of the soil, enhances the soil's water-holding capacity, resulting in a higher RWC, a better stomatal conductance and higher photosynthesis rate and (Demidchik 2015). All these events lead in the consumption of sub-stomatal CO2 during photosynthesis and reduce the Ci index (Abd El-Mageed et al. 2021). Similarly, spraying methyl jasmonate altered the NP and Ci significantly in peppermint plants under drought stress (Vatankhah et al. 2016). It is explained that methyl jasmonate application could increase photosynthesis by enhancing the relative water content, specific leaf area, relative growth rate, and net assimilation rate (Ahmadi et al. 2018). Our study observed that stomatal conductance was positively affected by methyl jasmonate application compared to control plants (M0). It is claimed that methyl jasmonate at higher concentrations increases ABA and ethylene concentrations in plants, which causes stomatal closure, resulting in stomatal resistance and reduced transpiration rate (Boutraa et al. 2010) Furthermore, it can lead to a series of enzymes called MAPK, which affect stomata and elevate ABA accumulation under biotic and abiotic stress conditions.
Plants' tolerance against stress function can be due to harming the PII center or lack of water during the plant growth season (Mamnoei and Seyed Sharifi 2010). So the chlorophyll fluorescence mirrors the initial process of photosynthesis, consist of absorbing light energy and transmitting excitation and photochemical energy responses and the degree of damage due to drought stress or other stressful conditions is reverberate measuring this index (Zhang et al. 2022) In our research, drought significantly reduced the maximum quantum performance of photosystem II (MQPPSII). Nonetheless, applying biochar and methyl jasmonate showed a significant positive change in MQPPSII. The reduction in this index under water limitation has been reported in several studies (Iqbal et al. 2022; Fallahi et al. 2013; Pandey et al. 2012; Abbaspour et al. 2011). Chlorophyll fluorescence parameters are in a close relation with the different processes of the photosynthesis (Xia et al. 2019). It is reported that biochar can be beneficial in reduction of the shutdown of active reaction centers which in turn increases the performance of PSII (Zhang et al. 2018). Moreover, it seems that, biochar affects this parameter positively, increasing the accumulation of photosynthetic pigment which eventually results in the higher rates of the photosynthesis (Yang et al. 2021). Furthermore, it has been reported that reducing the amount of chlorophyll-a, a determining indicator of PSII efficiency under stress conditions, is the main reason for the reduction in the MQPPSII (Boutraa et al. 2010). The same as our results, others observed a considerable increase in the function of PSII under drought stress due to using biochar. Besides, it suggested that, biochar, as an organic source of nitrogen, phosphorus, and potassium, significantly improves the efficiency of photosystem II (Iqbal et al. 2022). It has been reported that a high nitrogen concentration increases MQPPSII, an important index of the photosynthetic capability of plants (Klughammer and Schreiber 2008; Kościelniak et al. 2006). Therefore, the improvement in photosynthesis and water use efficiencies could be attributed to the tremendous advantages of biochar, such as improving soil organic carbon, physical soil properties, and soil water holding capacity that inhibits nutrient leaching and increases the availability of essential nutrients (Sombroek et al. 2004) Similarly, methyl jasmonate can protect the photosynthetic system against several stresses by maintaining chloroplast membrane stability and pigment concentration under stress conditions, which leads to decreased Fv, Fm, F0, and increased Fv/Fm (Liu et al. 2018). What is more, Fatma et al. (2021) reported a considerable influence of methyl jasmonate, in protection of the PSII system, maintaining the stability of a series of chloroplast protein named D1 and the acceleration of enzymatic activity under stress-induced conditions. Additionally, it has been reported that applying methyl jasmonate reduces the photo-inhibition in stressed plants compared to control plants, which results in the higher photosynthetic efficiency of PSII (Sirhindi et al. 2020).
5 Conclusions
It can be conclude that, in the present work, drought resulted in destructive repercussions on many parameters such as the net photosynthesis rate, transpiration rate, stomatal conductance, and photosynthetic pigments. However, use of biochar and methyl jasmonate acclimatized barley plants to water shortage, which is associated with enhanced morpho-physiological and biochemical traits. Soil content of biochar at 0.25% w/w and methyl jasmonate leaf applied at 50 μM protected plants against drought stress. Importantly, higher doses of biochar and methyl jasmonate had negative effects, highlighting the need to understand better the optimal doses and mechanisms of action of these amendments. Overuse of biochar and methyl jasmonate can be futile as the biochar increases the soil porosity which causes negative impact on the water availability. Additionally overuse of methyl jasmonate can cause negative effect on the stomatal conductance and molecular attributes of the plant under drought conditions. Last but not least, more experimental studies are needed to figure out more about the influence of different doses of biochar along with different doses of methyl jasmonate on several crop plant to identify the best doses of them for relieving the adverse effects of water shortage.
Data Availability
All data are available by connecting the first author through e-mail address. Furthermore, the extra data and materials used in this study will be made available by the authors upon request as much as possible.
References
Abbaspour H, Saeidi Sar S, Afshari H (2011) Improving drought tolerance of Pistacia vera L. seedlings by arbuscular mycorrhiza under greenhouse conditions. J Med Plant Res 5:7065–7072. https://doi.org/10.5897/JMPR11.1448
Abd El-Mageed T, Belal E, Rady M, Abd El-Mageed S, Mansour E, Awad M, Semida W (2021) Acidified biochar as a soil amendment to drought stressed (Vicia faba L.) plants: Influences on growth and productivity, nutrient status, and water use efficiency. Agronomy 11:1–18. https://doi.org/10.3390/agronomy11071290
Abideen Z, Koyro HW, Huchzermeyer B, Ansari R, Zulfiqar F, Gul B (2020) Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol 22:259–266. https://doi.org/10.1111/plb.13054
Ahmadi F, Karimi K, Struik PC (2018) Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S Afr J Bot 115:5–11. https://doi.org/10.1016/j.sajb.2017.11.018
Ahmadian K, Jalilian J, Pirzad A (2021) Nano-fertilizers improved drought tolerance in wheat under deficit irrigation. Agric Water Manag 244:1–13. https://doi.org/10.1016/j.agwat.2020.106544
Ali MS, Baek KH (2020) Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci 21:1–19. https://doi.org/10.3390/ijms21020621
Alikhani O, Abbaspour H, Safipour afshar A, Motevalizadeh Kakhaki AR (2019) Effects of methyl jasmonate on cadmium accumulation, antioxidant capacity and some physiological traits of wheat seedlings (Triticum aestivum L.). IJPB 32:886–897. (In Persian with English summary). https://dorl.net/dor/20.1001.1.23832592.1398.32.4.2.1
Arnon DI (1940) Copper enzymes in isolated chloroplast polyphenol oxidase. J Plant Physiol 45:100–114. https://doi.org/10.1104/pp.24.1.1
Bagheri S, Hassandokht MR, Mirsoleiman A, Mousavi A (2020) Effects of palm leaf biochar on the availability of soil nutrients, leaf nutrient concentration, and physiological characteristics of melon plants (Cucumis melo L.) under drought stress. Acta Agrobot 73:7311. https://doi.org/10.5586/aa.7311
Baiamonte G, Minacapilli M, Crescimanno G (2020) Effects of biochar on irrigation management and water use efficiency for three di_erent crops in a desert sandy soil. Sustainability 12:1–19. https://doi.org/10.3390/su12187678
Boutraa T, Akhkha A, Al-Shoaibi A, Alhejeli A (2010) Effect of water stress on growth and Water Use Efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J Taibah Univ Sci 3:39–48. https://doi.org/10.1016/S1658-3655(12)60019-3
Bryant C, Fuenzalida TI, Brothers N, Mencuccini M, Sack L, Binks O, Ball MC (2021) Shifting access to pools of shoot water sustains gas exchange and increases stem hydraulic safety during seasonal atmospheric drought. Plant Cell Environ 44:2898–2911. https://doi.org/10.1111/pce.14080
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428. https://doi.org/10.1016/j.biortech.2011.11.084
Demidchik V (2015) Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ Exp Bot 109:212–228. https://doi.org/10.1016/j.envexpbot.2014.06.021
Dey P, Datta D, Pattnaik D, Dash D, Saha D, Panda D, Bhatta BB, Parida S, Mishra UN, Chauhan J, Pandey H, Singhal RK (2021) Physiological, biochemical, and molecular adaptation mechanisms of photosynthesis and respiration under challenging environments. In: Plant perspectives to global climate changes, 1st edn. Elsevier Academic Press, pp 79–100
Fallahi G, Hatami A, Naseri R (2013) Growth analysis of six corn hybrids under drought conditions in Kermanshah province. Iran JCEP 7:181–196 ((In Persian with English summary))
Fatma M, Iqbal N, Sehar Z, Alyemeni MN, Kaushik P, Khan NA, Ahmad P (2021) Methyl jasmonate protects the PSII System by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 10:1216. https://doi.org/10.3390/antiox10081216
Feng WY, Yang F, Cen R, Liu J, Qu ZY, Miao QF (2021) Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J Environ Manage 277:111331. https://doi.org/10.1016/j.jenvman.2020.111331
Fugate KK, Lafta AM, Eide DJ, Li G, Lulai EC, Olson LL, Deckard EL, Khan MFR, Finger LF (2018) Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris L.) plants. J Agron Crop Sci 204:1–11. https://doi.org/10.1111/jac.12286
Gupta A, Sinha R, Fernandes JL, Abdelrahman M, Burritt DJ, Tran LP (2020) Phytohormones regulate convergent and divergent responses between individual and combined drought and pathogen infection. Crit Rev Biotechnol 40:320–340. https://doi.org/10.1080/07388551.2019.1710459
Hafez EM, Kheir AMS, Badawy S, Rashwan E, Farig M, Osman H (2020) Differences in physiological and biochemical attributes of wheat in response to single and combined salicylic acid and biochar subjected to limited water irrigation in saline sodic soil. Plants 9(1346):1–22. https://doi.org/10.3390/plants9101346
Hashem A, Kumar A, Al-Dbass AM, Alqarawi AA, Al-Arjani A, Singh G, Farooq M, Abd-Allah EF (2019) Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J Biol Sci 26:614–624. https://doi.org/10.1016/j.sjbs.2018.11.005
Hou WH, Zhang YX, Wang HJ, Zhang QX, Hou ML, Cong BM (2021) Effects of nitrogen application level on leaf photosynthetic characteristics and chlorophyll fluorescence characteristics of Leymus chinensis. Acta Agrestia Sin 29:532–536. https://doi.org/10.11733/j.issn.1007-0435.2021.03.014
Huang H, Liu B, Liu L, Song S (2017) Jasmonate action in plant growth and development. J Exp Bot 68:1349–1359. https://doi.org/10.1093/jxb/erw495
Iqbal S, Wang X, Mubeen I, Kamran M, Kanwal I, Díaz GA, Abbas A, Parveen A, Nauman Atiq M, Alshaya H, Zin El-Abedin T, Fahad S (2022) Phytohormones Trigger Drought Tolerance in Crop Plants: Outlook and Future perspectives. Front Plant Sci 12:799318. https://doi.org/10.3389/fpls.2021.799318
Katterer T, Roobroeck D, Andren O, Kimutai G, Karltun E, Kirchmann H, Nyberg G, Vanlauwe B, Roing de Nowina K (2019) Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. F Crop Res 235:18–26. https://doi.org/10.1016/j.fcr.2019.02.015
Khan Z, Khan MN, Zhang K, Lua T, Zhu K, Hu L (2021) The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of rapeseed through regulation of soil status and nutrients availability. Ind Crops Prod 171:1–11. https://doi.org/10.1016/j.indcrop.2021.113878
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAN 1:201–247
Kościelniak J, Filek W, Biesaga-Kościelniak J (2006) The effect of drought stress on chlorophyll fluorescence in Lolium Festuca hybrids. Acta Physiol Plant 28(2):149–158. https://doi.org/10.1007/s11738-006-0041-y
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Liu H, Bao G, Dou Z, Liu H, Bai J, Chen Y, Yuan Y, Zhang X, Xi J (2022) Response characteristics of highland barley under freeze-thaw, drought and artemisinin stresses. BMC Plant Biol 22(126):1–12
Liu X, Pan Y, Zhu X, Yang T, Bai J, Sun Z (2018) Drought evolution and its impact on the crop yield in the North China plain. J. Hydrol. 564:984–996. https://doi.org/10.1186/s12870-022-03520-0
Mamnoei E, Seyed Sharifi R (2010) Study the effects of water deficit on chlorophyll fluorescence indices and the amount of proline in six barley genotypes and its relation with canopy temperature and yield. J Plant Biol 2(5):51–62. https://dorl.net/dor/20.1001.1.20088264.1389.2.5.6.1
Mohi-Ud-Din M, Talukder D, Rohman M, Ahmed J, Jagadish SVK, Islam T, Hasanuzzaman M (2021) Exogenous application of methyl jasmonate and salicylic acid mitigates drought-induced oxidative damages in French bean (Phaseolus vulgaris L.). Plants 10:1–17. https://doi.org/10.3390/plants10102066
Munir N, Hanif M, Abideen Z, Sohail M, El-Keblawy A, Radicetti E, Haider G (2022) Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 12:2069. https://doi.org/10.3390/agronomy12092069
Nasiri S, Andalibi B, Tavakoli Zaniani A, Delavar MA (2023a) Investigation the impacts of mineral biochar and methyl jasmonate application on biochemical characteristics and yield of barley under drought stress. ESCS 16:561–574. https://doi.org/10.22077/escs.2023.4823.2076 (In Persian with English summary).
Nasiri S, Andalibi B, Tavakoli Zaniani A, Delavar E-K, Zwieten LV, Mastinu, (2023b) Using biochar and foliar application of methyl jasmonate mitigates destructive effects of drought stress against some biochemical characteristics and yield of barley (Hordeum vulgare L.). Gesunde Pflanzen 75:1689–1703. https://doi.org/10.1007/s10343-023-00853-0
Nohong B, Nompo S (2015) Effect of water stress on growth, yield, proline and soluble sugars contents of Signal grass and Napier grass species. Am -Eurasian J Sustain Agric 9:14–21
Page AL, Miller HR, Keeney RD (1982) Methods of soil analysis, Part 2. Chemical and microbiological properties, 2nd ed. American Society of Agronomy (ASA), Madison. https://doi.org/10.2134/agronmonogr9.2.2ed
Pandey HC, Baig MJ, Bhatt RK (2012) Effect of moisture stress on chlorophyll accumulation and nitrate reductase activity at vegetative and flowering stage in Avena species. Agric Sci Res J 2:111–118
Pieczynski M, Marczewski W, Hennig J, Dolata J, Bielewicz D, Piontek P, Wyrzykowska A, Krusiewicz D, Strzelczyk-Zyta D, Konopka-Postupolska D (2013) Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol J 11:459–469. https://doi.org/10.1111/pbi.12032
Razmi N, Ebadi A, Daneshian J, Jahanbakhsh S (2017) Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J Plant Interact 12:457–464. https://doi.org/10.1080/17429145.2017.1392623
Rezaei H, Saeidi-Sar Ebadi S, Abbaspour H (2018) The effect of spraying of methyl jasmonate and Epi-brassinolide on photosynthesis, chlorophyll fluorescence and leaf stomatal traits in black mustard (Brassica nigra L.) under salinity. JISPP 7:53–62. http://jispp.iut.ac.ir/article-1-929-en.html
Riemann M, Dhakarey R, Hazman M, Miro B, Kohli A, Nick P (2015) Exploring jasmonates in the hormonal network of drought and salinity responses. Front Plant Sci 6:1077. https://doi.org/10.3389/fpls.2015.01077
Sadaf J, Shah GA, Shahzad K, Ali N, Shahid M, Ali S (2017) Improvements in wheat productivity and soil quality can accomplish by coapplication of biochars and chemical fertilizers. Sci. Total Environ 607–608:715–724. https://doi.org/10.1016/j.scitotenv.2017.06.178
Shadmand H, Afkari A (2018) The effect of superabsorbent polymer application on some biochemical traits and relative water content of bean cultivars under drought tension. Crop Physiol J 10:61–77. (In Persian with English summary). http://dorl.net/dor/20.1001.1.2008403.1397.10.39.4.8
Shahbandeh M (2022) World barley production from 2008/2009 to 2021/2022. Available via DIALOG. http://www.statista.com/statistics/271973/world-barley-production-since-2008/
Sheteiwy M, Ulhassan Z, Qi W, Lu H, Abd-El-gawad H, Minkina T, Dawood MF (2022) Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front Plant Sci 13:2022. https://doi.org/10.3389/fpls.2022.886862
Singh B, Camps-Arbestain M, Lehmann J (2017) Biochar: a guide to analytical methods. CSIRO Publishing, Clayton, Australia
Sirhindi G, Mushtaq R, Singh Gill S, Sharma P, Abd Allah E, Ahmad P (2020) Jasmonic acid and methyl jasmonatemodulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci Rep 10:9322. https://doi.org/10.1038/s41598-020-65309-1
Smart R, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260. https://doi.org/10.1104/pp.53.2.258
Soliman S, El-Keblawy A, Mosa KA, Helmy M, Wani SH (2018) Understanding the phytohormones biosynthetic pathways for developing engineered environmental stress-tolerant crops. J Crop Improv, Transgenic Approaches 2:417–450. https://doi.org/10.1007/978-3-319-90650-8_15
Sombroek WIM, Ruivo MDL, Fearnside PM, Glaser B, Lehmann J (2004) Amazonian dark earths. Springer, Dordrecht
Tenhaken R (2014) Cell wall remodeling under abiotic stress. Front Plant Sci 5:1–9. https://doi.org/10.3389/fpls.2014.00771
Thi Pham DP, Hang NNT, Viet OT, Van LN, Viet AN, Lan PDT, Van NV (2021) Sandy soil reclamation using biochar and clay-rich soil. J Ecol Eng 22:26–35. https://doi.org/10.12911/22998993/137445
Todorova D, Aleksandrov V, Anev S, Sergiev I (2022) Photosynthesis alterations in wheat plants induced by herbicide, soil drought or flooding. Agronomy 12:1–13. https://doi.org/10.3390/agronomy12020390
Vatankhah E, Kalantari B, Andalibi B (2016) Effect of methyl jasmonate on some physiological and biochemical responses of peppermint (Mentha Piperita L.) under salt stress. JISPP 5:157–171. 20.1001.1.23222727.1395.5.17.18.5 (In Persian with English summary)
Wang S, Zheng J, Wang Y, Yang Q, Chen T, Chen Y, Chi D, Xia G, Siddique K, Wang T (2021) Photosynthesis, chlorophyll fluorescence, and yield of peanut in response to biochar application. Ind Crops Prod 12:650432. https://doi.org/10.3389/fpls.2021.650432
Wei W, Yang H, Fan M, Chen H, Guo D, Cao J, Kuzyakov Y (2020) Biochar effects on crop yields and nitrogen loss depending on fertilization. Sci Total Environ 702:134423. https://doi.org/10.1016/j.scitotenv.2019.134423
Xia L, Zhao R, Wang YQ, Jin HY, Wu XD, Ge JZ, Zang FY, Li ZF, Wang JL (2019) Effects of drought stress on photosynthesis and chlorophyll fluorescence characteristics of summer maize Effects of drought stress on photosynthesis and chlorophyll fluorescence characteristics of summer maize. J North China Agric 34:106–114. https://doi.org/10.7668/hbnxb.201751385
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and re-watering. Plant Signal Behav 5:649–654. https://doi.org/10.4161/psb.5.6.11398
Yang C, Du SM, Zhang DQ, Li XD, Shi YH, Shao YH (2021) A method for estimating the relative chlorophyll content of wheat leaves based on chlorophyll fluorescence parameters for estimating the relative chlorophyll content of wheat leaves. J Appl Ecol 32:175–181. https://doi.org/10.13287/j.1001-9332.202101.009
Zhang JH, Wu BWR, Wang GL, Jia CL, Zhang QP (2018) Effect of biochar application on PS II photochemical characteristics of alfalfa leaves. Shandong Agric Sci 50:66–71. https://doi.org/10.14083/j.issn.1001-4942.2018.09.015
Zhang X, Liu W, Lv Y (2022) Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of naked oat (Avena nuda L.). Sci Rep 12:11199. https://doi.org/10.1038/s41598-022-15322-3
Acknowledgements
The authors would like to thank the principals from the University of Zanjan, Zanjan, Iran, who contributed by preparing technical and physical facilities during years 2021-2022.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. This study was not financially supported by any organization.
Author information
Authors and Affiliations
Contributions
S.N. contributed to the investigation, literature research, and writing of the manuscript; project administration and methodology were conducted by B.A.; discussion was conducted by A.M. and M.A.D., data analysis was supported by A.T. and A.E.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nasiri, S., Andalibi, B., Tavakoli, A. et al. Jasmonates Improve Drought Tolerance of Hordeum vulgare L. After Biochar Treatment. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01692-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01692-2