Abstract
Rice is the primary energy source of more than half of the global population. Challenges persist in managing phosphorus (P) in paddy soils of tropical rice-growing countries. In Sri Lanka, one specific challenge is the inconsistent yield response observed when inorganic P fertilisers are applied to paddy soils. Previous research conducted in Sri Lanka has shown that the rice yield response to added P fertilisers cannot be adequately explained by factors such as soil available P, irrigation schemes, soil texture, pH, electrical conductivity, total carbon content and available Fe and Mg concentrations. Due to the submerged conditions in which rice is grown for a significant portion of its lifespan, a unique environment controlled by redox-driven processes is developed in paddy soils. Therefore, releasing P from submerged soils is an outcome influenced by complex hydrological and biogeochemical processes, strongly influenced by inherent soil characteristics. The present review paper aimed to critically examine existing literature on soil P behaviour in submerged paddy soils of Sri Lanka, to clarify the behaviour of P under submergence, identify the factors affecting such behaviour and highlight the research gaps that need to be addressed, in order to effectively manage P in the paddy soils of Sri Lanka.
Similar content being viewed by others
1 Introduction
Rice (Oryza sativa L.) is among the top three cereal crops grown worldwide, alongside wheat and maize. It serves as a vital source of energy and nutrients for over 3.5 billion people across the globe (Gadal et al. 2019). Approximately 164 million ha of agricultural land worldwide is under rice cultivation (FAOSTAT 2022). The global production of rice was estimated as 757 million tons in 2020 (FAOSTAT 2022). The majority of rice is grown in the tropical belt (23° N–15° S), and approximately 92% of rice is produced in Asia (FAOSTAT 2022). According to the Food and Agriculture Organization’s projections, the demand for rice is expected to increase by around 1.1% per annum until 2035 (FAO 2017). However, matching the demand of the growing population by increasing the productivity of rice-growing areas is challenging. Paddy soil is specifically used for the cultivation of rice, and the proper management of nutrients in paddy soil plays a crucial role in the economic production of this staple crop.
Phosphorus is essential for rice plants in all-development stages from germination to maturity, as for any other crop. Since the plant available P concentration in many agricultural soils fails to compensate for the continuous removal of P at harvest, P fertiliser is added to soils either in the inorganic or organic form (Dougherty et al. 2004). However, being non-renewable, the natural P reserves (rock phosphate deposits) in the world used to produce inorganic P fertilisers are limited in both quantity and distribution (Cordell and White 2013; Roberts and Johnston 2015). At the same time, P enrichment in agricultural lands contributes to declining water quality in surface water bodies and raises agricultural production cost. Further, the demand for P fertilisers worldwide is expected to be increased up to 199 Mt in 2023 (Lizcano-Toledo et al. 2021). This situation has created the need for further research into the efficient use of P in agriculture.
Rice is a semi-aquatic plant. It is cultivated in submerged conditions mainly due to agronomic advantages such as suppression of weeds, storage of rainfall water during rainy seasons, reduced labour in land preparation and increased nutrient availability to plants (Datta et al. 2017). The presence of aerenchyma tissues in rice allows them to grow under submerged condition as they can transport oxygen from leaves to roots which are in water. The paddy soils are kept submerged especially during the vegetative growth period of rice. These waterlogged conditions generally last for 2–3 months depending on the age of the variety and this can significantly influence the P transformations within soil. Proper understanding of P dynamics in paddy soils is essential to minimise the loss of P to water sources and improve P utilisation to improve rice yield. Therefore, it is important to underpin the P behaviour under paddy soils in relation to applied organic or inorganic fertiliser for rice-growing.
Rice serves as the primary food for the majority of Sri Lanka’s population, which is currently estimated at 22.1 million. Sri Lankan rice consumption is 300.6 g/capita/day, ranking 9th highest in the world (Kadupitiya et al. 2022). Rice covers about 43% of the land relative to the total harvestable area of agricultural land in Sri Lanka (FAOSTAT 2022). It is grown in approximately 1.2 million ha and produces a total production of over 3.3 million tons (Central Bank Annual Report 2022).
Climate‐related challenges, production issues in paddy soils and systematic disfunctions in the industry are the major challenges in rice cultivation in Sri Lanka. Other than salinity and iron toxicity issues with paddy soils, the inconsistency of the yield response of rice to added P fertilisers remains as one of the primary challenges faced in rice cultivation in Sri Lanka. Field studies conducted over a short-term have revealed that rice yield does not respond to added P fertilisers in most of the rice-growing paddy soils of Sri Lanka (Kulasinghe et al. 2020; Sirisena et al. 2013). Unfortunately, this has led to a situation where many farmers overuse P fertilisers, despite the recommendations from the Department of Agriculture, Sri Lanka. This overuse of fertilisers poses immediate environmental issues, such as potential contamination of freshwater bodies surrounding paddy fields, and economic issues such as the high cost of importing fertilisers. It also causes long-term environmental issues such as such as cadmium and fluoride contaminations (Geretharan et al. 2018; Ubeynarayana et al. 2021) and residual P build up in soils (Menezes-Blackburn et al. 2018) which have not much been studied in Sri Lanka. The residual P can cause nutrient imbalances in soils such as changing N:P stoichiometry which regulates microbial P solubilisation and mineralisation (Dai et al. 2020) and thereby negatively affects plant growth over the long-term.
Phosphorus behaviour in soil is well studied including in submerged soils (Fageria et al. 2011; Kögel-Knabner et al. 2010; Ponnamperuma1972). Although P solubility increases with submergence, this is not visible through the yield response studies to added P fertilisers in paddy soils in Sri Lanka. Soil P management is more challenging in tropical acidic soils rich in Fe and Al oxy(hydr)oxides, compared to temperate soils. A comprehensive analysis on inherent soil characteristics that affect P behaviour in paddy soils is needed to make provisions to solve issues related to inconsistent yield response to added P fertilisers leading to efficient P management in paddy soils. Therefore, the objectives of this literature review are to analyse the previous research conducted on soil P behaviour in submerged paddy soils of Sri Lanka and to discuss the current understanding of soil P behaviour under submergence in order to identify research gaps relating to the inconsistent yield response of rice to added P fertilisers in Sri Lanka. In this review, we discuss an overview of paddy soil types, P fertiliser usage, soil available P levels and yield response studies to added P fertilisers in rice-growing paddy soils of Sri Lanka. We also examine subsequent impacts on biochemical changes and P dynamics in soil under submergence and factors affecting P dynamics under submergence, as well as suitable methods and techniques to identify P dynamics under submergence and future perspectives.
2 Topography, Climate and Rice-Growing Paddy Soils in Sri Lanka
Sri Lanka possesses two significant topographical features, namely the central highland and lowland plains. The lowland plains encompass the highland and extend to the sea. The country is divided into three regions based on elevation, namely low country (< 300 m), mid country (300–900 m) and up country (> 900 m). Sri Lanka experiences two distinct monsoonal seasons; the Southwest monsoon, locally known as the Yala season occurs from May to September, while the Northeast monsoon, referred to as the Maha season takes place from October to January. The mean annual rainfall varies significantly across the country, ranging from 900 to 5700 mm. Sri Lanka is divided in to three climatic zones based on the mean annual rainfall: dry zone (< 1750 mm), intermediate zone (1750–2500 mm) and wet zone (> 2500 mm). The average annual temperatures also differ among these zones with the dry zone experiencing temperatures of around 28 °C, the intermediate zone ranging from 24 to 26 °C and the wet zone maintaining 24 °C. The combination of rainfall and elevation has resulted in seven main agro-climatic zones in Sri Lanka. These zones are identified as low country dry zone (DL), low country intermediate zone (IL), low country wet zone (WL), mid country intermediate zone (IM), mid country wet zone (WM), up country intermediate zone (IU) and up country wet zone (WU).
Sri Lanka has highly weathered tropical soils in most part of the country (Indraratne 2020). The soils of Sri Lanka are categorised locally into 12 Great Soil Groups. Of these groups, Reddish Brown Earth (Alfisol), Low Humic Gley (Alfisol), Non-calcic Brown (Alfisol), Red/Yellow Latosol (Oxisol), Immature Brown Loam (Inceptisol), Solodized Solonetz (Alfisol), Regosol (Entisol) and Alluvial (Entisol) are present in the dry zone of Sri Lanka (Dassanayake et al. 2020a). Red Yellow Podzolic (Ultisol), Low Humic Gley (Ultisol), Reddish Brown Latosol (Ultisol) and Bog and Half Bog (Vertisol) soils can be observed in the wet zone of Sri Lanka (Dassanayake et al. 2020b).
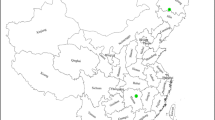
Rice covers 14% of the total land area of Sri Lanka, occupying the largest extent devoted to a single crop (Mapa 2020). Of this area, 50% is grown in the dry zone and 30% and 20% are grown in the wet and the intermediate zones, respectively. The distribution of lowland paddy lands across the agro-climatic zones of Sri Lanka is shown in Fig. 1. Reddish Brown Earth, Non-calcic Brown, Low Humic Gley and Alluvial soils are major rice-growing soils in the DL. Red Yellow Podzolic, Low Humic Gley, Non-calcic Brown, Reddish Brown Latosol, Immature Brown Loam and Alluvial are rice-growing Great Soil Groups in the IL. Red Yellow Podzolic and Reddish Brown Loam are the main rice-growing soils in the WM.
Distribution of lowland paddy lands (purple colour) in Sri Lanka across the seven agro-climatic zones. Mean annual rainfall (mm) and the elevation (m) of each zone are also provided. DL, low country dry zone; IL, low country intermediate zone; WL, low country wet zone; IM, mid country intermediate zone; WM, mid country wet zone; IU, up country intermediate zone; WU, up country wet zone
3 Phosphorus Fertiliser Usage in Rice Cultivation in Sri Lanka
In Sri Lanka, there is a tendency among farmers to excessively utilise inorganic fertilisers, despite the recommendations of the Department of Agriculture (Wimalawansa and Wimalawansa 2015). Since 1962, Sri Lanka has implemented a fertiliser subsidy programme, which has undergone various subsidy policies (Kanthilanka and Weerahewa 2018). These fertiliser subsidy programs were suspected to play a major role in encouraging farmers to overuse fertilisers as they received fertilisers at a low price (De Silva et al. 2020). Considering the consequences of P accumulation in surface water bodies, and the lack of a good yield response, it is vital to promote rational use of P fertilisers in rice cultivation.
Around 76,856 MT of Triple superphosphates (TSP; ⁓45% P2O5) has been applied to the crop sector of Sri Lanka in 2020 (AgStat 2021). About 59% of the TSP was used for rice cultivation which accounted for an average application rate of 59 kg P2O5/ha for paddy soils (AgStat 2021). Rice crops with an average grain yield of 6 Mt/ha remove about 20 kg of P per season (Rathnayake et al. 2015). However, P fertiliser requirements of paddy soils under sustainable production show a large difference among paddy soils in Sri Lanka. For example, P fertiliser requirements vary from 25 to 75 kg P2O5/ha/season in different paddy soils, even when the fields have sufficient level of soil P for optimum soil fertility (Wickramasinghe et al. 2009).
4 Rice Yield Response Studies for Added P Fertilisers in Sri Lanka
The Olsen P method is widely used in Sri Lanka to determine available P concentration in soil. The optimal threshold of Olsen P in rice-growing paddy soils varies across the agro-climatic regions in Sri Lanka. For example, in the DL and IL, the threshold ranges from 3 to 5 mg/kg, while in the WL and WM, it ranges from 10 to 35 mg/kg (Wickramasinghe et al. 2009). Approximately 44% of rice-growing soils in Sri Lanka have Olsen P concentration < 10 mg/kg (Sirisena and Suriyagoda 2018). However, these thresholds of Olsen P are not practicable in the field scale and needed to be refined as they do not comply with yield response of rice in paddy soils as explained below.
The rice yield response to added inorganic P fertilisers in paddy soils in Sri Lanka cannot be explained by Olsen P concentrations (Sirisena and Suriyagoda 2018; Suriyagoda et al. 2014). For example, Sirisena et al. (2013) conducted a field experiment in four farmer fields representing Low Humic Gley and Alluvial soils in the DL over four consecutive seasons using P fertiliser application rates of 45 kg P2O5/ha in every season and in alternative seasons, separately (Table 1). Regardless of the initial Olsen P concentration of these four fields before commencing the experiment which ranged from 1.5 to 18 mg/kg, they did not observe a significant (p > 0.05) yield increase in four seasons, whether P application was done in every season or in alternative seasons. Another study conducted at 71 experimental sites in three rice-growing districts with a range of initial Olsen P concentrations before commencing the experiment (2.7–34.2 mg/kg) showed no significant (p > 0.05) increase of rice yield to added P fertilisers (Kulasinghe et al. 2020). Although the same P fertiliser rate (59 kg P2O5/ha) had been applied to every plot in this study, the authors reported that as their general practice, most of the famers who cultivated in these experimental sites previously had applied lower or higher rates of P fertilisers than the recommendation of the Department of Agriculture, Sri Lanka. This indicates a heterogeneity of soil P management practices in the experimental sites which might have influenced the variability of initial Olsen P concentrations. An increasing trend of soil Olsen P in two paddy soils (Reddish Brown Earth and Low Humic Gley) were observed in relation to P fertiliser application in a short-term study conducted by Kendaragama et al. (2003). However, they also failed to observe a significant (p > 0.05) yield increase of rice with different P fertiliser rates (0, 25, 50, 75 and 100 kg P2O5/ha) in two consecutive seasons, despite of having high variance in soil Olsen P concentrations in the tested sites before commencing the experiment (4–49 mg/kg).
The existing literature on long-term research investigating the rice yield response to added P fertilisers in Sri Lanka is limited (Table 1). In the same manuscript, Kendaragama et al. (2003) reported results of a long-term (over 2 ½ years) research where they observed seasonal variation in soil Olsen P, with greater availability of P, and rice yield responses at high P fertiliser rates (> 75 kg P2O5/ha) during the Yala season. This research was conducted for five consecutive seasons in Low Humic Gley soil in the dry zone and recorded a significant rice yield increase (p < 0.05) only in Yala seasons with P fertiliser application. Since two different rice varieties were used in Yala (BG 276–5) and Maha (BG 450) seasons in this experiment, the significant yield increase in Yala season could be related to the performance of the variety. However, a study conducted for ten consecutive seasons in the same soil in the dry zone where the same rice variety (BG 300) was cultivated in both Yala and Maha seasons failed to report a significant (p > 0.05) yield increase despite different fertiliser rates added (25–75 kg P2O5/ha) to the experimental site where initial Olsen P concentration was 16 mg/kg (Senevirathne Banda et al. 2002). This study also reported higher yield in Yala than Maha within the ten consecutive seasons. Another study conducted in a farmer field in the dry zone having the same soil type where P fertiliser had not been applied for 10 years prior to the experiment demonstrated no significant (p > 0.05) yield response to the added P fertilisers in the subsequent three consecutive seasons including two Yala seasons and one Maha season (Kodagoda et al. 2022). These studies pinpoint the necessity for future research on residual or legacy P in paddy soils, the modelling of long-term changes in soil P in paddy soils, and the potential for developing separate P fertiliser recommendations for Yala and Maha seasons.
There are no detailed studies published on P sorption capacity of paddy soils in Sri Lanka. However, it has been reported that the P sorption capacity varies among major rice-growing soils of Sri Lanka under both dry and submerged conditions (Damayanthi 2001). This factor, along with stress conditions such as iron toxicity, nutrient deficiencies (e.g. Zn) and low pH, have been suspected as reasons that complicate the accurate assessment of yield response to added P fertilisers in most studies (Wickramasinghe et al. 2009). Kulasinghe et al. (2020) revealed that factors such as irrigation schemes, soil texture, pH, electrical conductivity, total carbon content and available Fe and Mg concentrations did not affect yield response to added P fertilisers in paddy soils tested in the three rice-growing districts. They further highlighted the importance of assessing the other factors related to rice yield response to added P fertilisers. However, no comprehensive study on the effects of these factors on inconsistent rice yield response to added P fertilisers has been undertaken to date. Further, soil P species and their transformations during submergence could play a significant role in the yield response of rice plants to added P fertilisers.
For an extended period, the factors behind the inconsistent yield response of rice to added P fertilisers in Sri Lanka have remained elusive (Sirisena and Suriyagoda 2018). In order to address this issue, it is vital to gain an understanding of P behaviour in rice-growing environments, particularly under submerged condition, and analyse the factors influencing P behaviour in such settings. Given the absence of relevant research conducted in Sri Lanka examining these factors, it becomes necessary to draw upon research conducted in similar environments internationally.
5 Biochemical Changes in Soil Under Submergence
Submerged soils are defined as “soil having free water at the surface at least during the growing season of arable crops or at least during the 2 months of the growing season of perennial crops, grassland, forest or other vegetation” (Sahrawat 2004b). Submergence causes changes in soil properties (Fig. 2) due to physical reactions and chemical and biological processes of soil–water-microbes’ interaction (Siam et al. 2019). These changes vary in different soil types based on various factors such as soil fertility levels, microbial biomass, quality and quantity of organic matter and the type of cultivar planted in soil (Fageria et al. 2011).
Major biochemical changes in soils under submergence with special reference to redox potential, soil pH and changes in nutrient availability. P, phosphorus; K, potassium; Na, sodium; Ca, calcium; Al, aluminium; Mg, magnesium; Mn, manganese; Fe, iron; Cu, copper; Mo, molybdate; S, sulphur; Zn, zinc; NH4-N, ammonium nitrogen; NO3-N, nitrate nitrogen; NO3−, nitrate; NO2−, nitrite; Mn2+, Mn3+, Mn4+, manganese cations; Fe3+, ferric ion; Fe2+, ferrous ion; SO42−, sulphate; S2−, sulphide; CO2, carbon dioxide; CH4, methane; N2, nitrogen gas; NH3, ammonia; H+, hydrogen ion; H2, hydrogen gas; O2, oxygen gas; H2O, dihydrogen monoxide; Fe2O3, ferric oxide; e−, electrode; H2CO3, carbonic acid; MnO2, manganese dioxide; HCO3−, bicarbonate
Generally, when a soil is submerged, there is a significant reduction in gas exchange between soil and the air (Ponnamperuma 1972). During this time, aerobic microbes present in the soil will utilise the limited oxygen available for their respiratory processes. As a result, the oxygen level in the soil declines rapidly. The rate of oxygen diffusion through water filled pores of a submerged soil is around 1/10000th of that of a well-drained soil with air filled pores (Howeler and Bouldin 1971). The rate of oxygen movement to soil through the overlaying water layer is slower compared to the rate of oxygen reduction inside the soil profile (Fageria et al. 2011). This creates two distinct layers in the profile: an oxidised top layer (usually 1–20 mm in thickness) and a reduced soil layer underneath (Fageria et al. 2011). The oxygen content in a submerged soil becomes depleted within a day after submergence (Kögel-knabner et al. 2010; Ponnamperuma 1972). The aerobic microbes in soil become quiescent due to a lack of oxygen under submergence and anaerobic microbes proliferate (Fageria et al. 2011). Anaerobic microbes proliferate because they use electron acceptors other than oxygen for their respiration in the following sequential order: nitrate (NO3−), Mn4+, Fe3+ and sulphate (SO42−) (Sahrawat 2012). In addition, anaerobic microbes both facultative and obligate, use dissimilation products of carbohydrates, carbon dioxide (CO2), nitrogen gas (N2) and hydrogen ions (H+) as electron acceptors (Fig. 2) in their respiration process (Fageria et al. 2011; Ponnamperuma 1972; Sahrawat 2005). Further, several electrochemical changes occur in a submerged soil, such as reduction of soil, alteration of soil pH, increasing ionic strength and specific conductance of soil solution, large shifts in mineral equilibria and availability of nutrients due to sorption/desorption reactions (Ponnamperuma 1972; Sahrawat 2005).
Respiration of anaerobic microbes results in reduced soil conditions (Ponnamperuma 1972). During this process of respiration, organic matter is oxidised, while soil compounds are reduced. Therefore, the main requirements for soil reduction are the presence of decomposable organic matter, availability of other electron acceptors, the absence of oxygen and anaerobic microbial activity (Ponnamperuma 1972). Redox potential (Eh) of soils varies across a broad range in approximately the following manner: aerated soils (+ 700 to + 500 mV), moderately reduced soils (+ 400 to + 200 mV), reduced soils (+ 100 to − 100 mV) and highly reduced soils (− 100 to − 300 mV) (Sahrawat 2004a, 2005). The Eh of submerged soils generally ranges from + 200 to –300 mV, depending on organic matter content and reducible species (Sahrawat 2005). An incubation study conducted using paddy soils in the IL of Sri Lanka reported an Eh range of + 312 to − 368 mV during first week of submergence (Akter 2018).
Soil Eh controls the stability of several chemical compounds under submergence (Fig. 2). Under submergence, reduction of oxygen (O2) to dihydrogen oxide (H2O), NO3− to nitrogen gas (N2) and nitrous oxide gas (N2O), Mn4+ and Mn3+ to Mn2+, Fe3+ to Fe2+, SO42− to sulphide (S2−), CO2 to methane (CH4), N2 to ammonia (NH3) and H+ to hydrogen gas (H2) occur (Gao et al. 2002; Marschner 2021; Ponnamperuma 1972). Even though some overlap may occur, the sequential reduction of the above soil components generally occurs only when the level of the previous oxidant in soil is negligible. For example, NO3− reduction begins only when the oxygen concentration of soil is very low and nitrate stabilises the redox potential at 0.2–0.4 V, preventing the reduction of the oxidants that comes later (Mn4+, Fe3+, SO42− and CO2) in the sequential reduction process (Ponnamperuma 1972). Parallel to these redox reactions, gasses such as CO2, CH4, N2 and H2 will accumulate in submerged soil (Fageria et al. 2011).
Generally, the pH of submerged soils changes towards neutrality (an equilibrium in between 6.5 and 7.5) (Fageria et al. 2011). The pH of acidic soils increases, and the pH of alkaline soils decreases under submergence. Most of the redox reactions in submerged soils involve the consumption of H+ ions (Ponnamperuma 1972). The main determinant of an increase of pH in acidic soils is the reduction of Fe and Mn oxides, which consumes H+ ions (Eqs. 1 and 2) (Fageria et al. 2011).
The decomposition of organic matter causes the pH to decrease in both acidic and alkaline soils under submergence. The CO2 produced in organic matter decomposition remain in soil due to restricted diffusion through the standing water layer on the soil surface. Carbon dioxides react with H2O and form carbonic acid which dissociates into H+ and bicarbonate ions as illustrated in Eqs. 3 and 4. Thus, accumulation of CO2 will lower the pH of submerged alkaline soils (Najafi 2013; Ponnamperuma 1972). Although both consumption of H+ ions by reduction of Fe and Mn oxides and releasing of H+ ions by carbonic acid formed in organic matter decomposition occur simultaneously in acidic soils, as the net effect, the soil pH will be increased.
The redox reactions and pH changes under submergence, therefore, change the concentrations and forms of soluble nutrients in the soil (Fageria et al. 2011; Sahrawat 2012). Submergence improves the availability of several macro and micronutrients for plants and reduces the availability of some nutrients depending on other soil properties (Table 2).
6 Phosphorus Dynamics Under Submergence
The P solubility of soil increases under submerged conditions (Fageria et al. 2011; Ponnamperuma 1972; Weil and Holah 1989), and this is directly associated with the redox and pH changes which occur in submerged soils. Phosphorus is sorbed to different kinds of minerals or compounds and/or exists in the form of precipitates depending on soil properties such as pH and availability of associated ions and minerals (Fig. 3). In acidic tropical soils, P is mainly adsorbed by the Al and Fe hydrous oxides and 1:1 type clay (Sanyal and De Datta 1991). Mineralogical evidence reported the presence of Al oxy(hydr)oxides such as gibbsite and boehmite and 1:1 type clay minerals such as kaolinite in acidic wet zone soils of Sri Lanka (Indraratne 2020), thereby providing possible sources of P sorption in such soils. The water-soluble P concentration increases in acidic soils as a result of (1) hydrolysis of Fe(III) and Al phosphates, (2) release of P held by anion exchange sites on clay and hydrous oxides of Fe(III) and Al, and (3) reduction of Fe(III) to Fe(II) releasing sorbed and chemically bound P (Ponnamperuma 1972). Phosphorus release by the first two reactions is due to a pH increase in acidic soils. In neutral and alkaline soils, different forms of calcium phosphates (e.g. dicalcium or octacalcium phosphates, hydroxyl apatite and apatites) govern P concentration in the soil solution (Abolfazli et al. 2012; Sanyal and De Datta 1991). Phosphorus solubility increases in alkaline soils mainly due to release of P from Ca-P compounds when pH decreases following submergence (Fageria et al. 2011). These compounds are found in alkaline soils in the DL of Sri Lanka such as Reddish Brown Earth and Low Humic Grey, thereby providing a possible mechanism of P release under submergence in such soils. The maximum P availability to plants occurs at nearly pH 6.5 where the fixation by Fe, Al and Ca minerals is minimum as described by Penn and Camberato (2019) and Price (2006).
Comparing the behaviour of phosphorus before and after submergence of soil. Top: phosphorus sorbed to different surfaces such as colloids, amorphous materials, organic matter and other elements before submergence. Bottom: phosphorus release into the soil solution upon submergence of soil. P, phosphorus; Fe, iron; Mn, manganese; Al, aluminium; Ca, calcium; Mg, magnesium; Am, amorphous materials
The released phosphate ions by the above reactions and those released from organic matter decomposition can then become available to be uptaken by plants, re-adsorbed by hydrous oxides of Fe and Al and clay in the anaerobic zone or may be diffused into the oxidised zones and reprecipitated (Ponnamperuma 1972). Release of P under submergence depends on a collection of complex hydrological and biogeochemical processes (Dharmakeerthi et al. 2019a; Jayarathne et al. 2016). The amount and time of P release and its cumulative effect on porewater P concentration will depend on soil properties and other related factors as illustrated in Fig. 4.
Factors influencing phosphorus release during soil submergence, including soil and plant characteristics, as well as fertiliser and water management practices (This figure is based on the literature documents reviewed in the “Factors Affecting P Release in Submerged Soils” section). Eh, redox potential; MgSO4, magnesium sulphate; C, carbon; P, phosphorus; N, nitrogen; K, potassium; Fe, iron; Mn, manganese; Al, aluminium; Ca, calcium; Mg, magnesium
7 Factors Affecting P Release in Submerged Soils
Phosphorus release under submergence mainly depends on inherent soil characteristics, external factors such as soil amendments, water management, and the cultivar grown in the soil (Fig. 4). Both organic and inorganic P pools release P under submergence. Therefore, clay mineralogy, soil microorganisms and organic matter content/quality of soil can be considered as the main factors influencing P release under submergence. In addition, P sorption capacity; soil temperature; ions such as Fe, Al, Ca and Mg; and cation exchange capacity affect P release under submergence.
7.1 Clay Mineralogy
There are over 400 known phosphate minerals on earth (Pasek et al. 2017). The main mineral groups involved in P release under submergence are clay minerals, such as Fe and Mn hydroxides and oxyhydroxides, Fe phosphates (Hutchison 2003; O’Loughlin et al. 2013; Smith et al. 2021), Al hydroxides and Ca minerals (Schmieder et al. 2018). Amorphous minerals present in clay fraction (e.g. amorphous Fe(OOH)) are also capable of P sorption by complexation (Watts 2000). Several examples of the minerals involved in P sorption and release in soil are shown in Table 3.
7.2 Iron and Aluminium Ions
Tropical soils usually contain high amounts of Fe and Al oxy(hydr)oxides that sorb dissolved inorganic P (Agbenin 2003; Rakotoson et al. 2016). A study conducted using 97 paddy soil samples covering the dry and wet zones of Sri Lanka reported high total Fe content with a high variability. According to the study, total Fe content in the dry zone paddy soils varied from 4255 to 31028 mg/kg, and it varied from 2923 to 78400 mg/kg in the wet zone (Rubasinghe et al. 2021). Furthermore, Fe toxicity is largely reported in paddy soils in the WU, WM and WL of Sri Lanka (Siriwardana et al. 2019). Sorption and precipitation are the main mechanisms involved in P sorption to Fe and Al oxy(hydr)oxides. Adsorption occurs at low inorganic P concentrations, and precipitation of Fe and Al phosphates occurs at high inorganic P concentrations (Darke and Walbridge 2000).
Generally, Fe is considered the most abundant redox sensitive species in soil (Kirk 2004). However, soils with high Fe content will hinder the increases of soluble P content in submerged soils by reprecipitating with or resorbing P onto Fe(II) minerals (Amery and Smolders 2012). Reductive dissolution of Fe(III) oxy(hydr)oxides increases Fe(II) concentration of soil solution even above 1 mM under submergence (Scalenghe et al. 2002). High concentrations of Fe(II) in soil solution induce precipitations of Fe(II)/Fe(III) carbonates and hydroxides that limit P release due to sorption or co-precipitation (Amery and Smolders 2012; Chacon et al. 2006). There is also evidence suggesting that crystallinity of Fe oxy(hydr)oxides decreases with flooding, thereby increasing P sorption capacity (Darke and Walbridge 2000; Zhang et al. 2003). Unlike Fe, Al is not redox sensitive. Therefore, the solubility of Al phosphates does not change due to redox reactions under submergence. In contrast, organic matter can complex with Al and prevent precipitation of amorphous Al oxides (Darke and Walbridge 2000).
7.3 Calcium and Magnesium Ions in Alkaline Soils
Calcium and Mg are present as the dominant cations in alkaline soils. Phosphorus will precipitate as different forms of calcium phosphate (e.g. dicalcium or octacalcium phosphates, hydroxyl apatite, and less soluble apatites) in such soils (Hinsinger 2001). The pH of alkaline soils decreases with submergence. This will increase the solubility of Ca-P minerals such as octacalcium phosphate, hydroxyapatite, ß3-tricalcium phosphate and fluorapatite in calcareous soils. Furthermore, acid anions of decomposing organic matter can form complexes with Ca2+ ions increasing the solubility of Ca bound P in submerged soils (Bhattacharyya et al. 2005).
The soils of the dry zone of Sri Lanka contain high amounts of Ca and Mg probably due to the presence of carbonate-bearing minerals. Further, there are evidence of high variability of Ca and Mg contents in paddy soils within the dry zone of Sri Lanka. A study conducted using 70 paddy soils samples of the dry zone revealed that Ca and Mg varied from 31 to 5600 mg/kg and 47 to 3660 mg/kg, respectively (Rubasinghe et al. 2021). This heterogeneity could be a contributing factor to inconsistent yield response of rice yield to added P fertilisers in Sri Lanka.
7.4 Manganese Content
Manganese plays a key role in P release in soils that retain P via Mn(IV) oxides (Amarawansha et al. 2015). Phosphorus is released to soil solution by the reductive dissolution of Mn(IV) oxides upon submergence (Amery and Smolders 2012; Zhang et al. 2021). A laboratory experiment conducted in the UK using two contrasting soils, a typical non-calcareous soil (sand 35%, clay 19%) and a typical brown earth soil (sand 77%, clay 11%), revealed a significant positive correlation (r = 0.622, p < 0.001) between total dissolved P and floodwater Mn concentration during 31 days of flooding (Khan et al. 2022). Manganese is present as Mn(II), MnHCO3+ and organic complexes in submerged conditions (Ponnamperuma 1972). Manganese(II) ions can be removed from soil solution due to precipitation, adsorption and formation of organic complexes.
7.5 Soil Organic Matter Content/Quality or Addition of Organic Matter
Organic matter increases soil solution P concentration by competitive sorption (Bhattacharyya et al. 2005). Organic compounds such as humic and fulvic acids and low molecular weight aliphatic acids compete with P for sorption sites (Ajmone-Marsan et al. 2006). The addition of organic matter increases the microbial biomass such as phosphate solubilising bacteria in submerged soils and thus increases phosphatase enzyme levels that mineralise organic P (Bhattacharyya et al. 2003). Organic matter releases soluble P slowly with time through mineralisation, especially in organic matter rich soils (Ajmone-Marsan et al. 2006; Maranguit et al. 2017). Organic matter, particularly dissolved organic carbon, acts as an essential carbon source for the microbes which contribute to the reductive dissolution of Fe(III) and Mn(IV) oxyhydroxides (Hanke et al. 2013; Watts 2000). Organic matter releases organic acids during anaerobic decomposition (Tsutsuki and Ponnamperuma 1987). These acids form complexes with Ca2+ ions and increase the solubility of Ca-P compounds.
7.6 Microorganisms
Anaerobic microbes use organic matter as the carbon source for their respiration. The electrons released when organic matter is oxidised are accepted by electron acceptors such as O2, NO3−, Fe(III), Mn(IV). Therefore, reductive dissolution of Fe(III) and Mn(IV) oxy(hydr)oxides, and associated P release are microbially mediated. For example, it is evident that Geobacter spp. and Anaeromyxobacter spp. are involved in the iron reduction process in paddy soils (Hori et al. 2010). Other than that, mineralisation of organic P by microbial phosphatases, and microbial decomposition of soil organic matter have positive effects on P release under submergence as described under the “Soil Organic Matter Content/Quality or Addition of Organic Matter” section.
7.7 Phosphorus Sorption Capacity
The term P sorption is used generically to explain all processes involved in removing phosphate from soil solution such as adsorption, absorption and precipitation. Phosphorus sorption capacity is affected by several soil properties such as pH, Fe and Al oxides, CaCO3, and degree of weathering (Antoniadis et al. 2016). Measures of soil P sorption have been identified as promising tools to predict the variability of rice yield response to added P fertilisers under submergence in typical P deficient paddy soils (Nishigaki et al. 2021). Above study reported a negative correlation (r = − 0.697) of P retention with increasing rice yield as a response to P fertilisers. Further, they identified air-dried soil moisture content mainly regulated by oxalate extractable Al as a parameter to predict soil P sorption.
Phosphorus sorption in soil can be explained by three possible mechanisms that involve soil pH. Gradual dissolution of Fe and Al oxides occurs at pH values between 2 and 5, and they re-precipitate as phosphates. Phosphate adsorption on clay mineral surfaces occurs at pH values between 4.5 and 7.5 and P precipitation by divalent cations occurs at pH values between 6 and 10 (Hanyabui et al. 2020). Adsorption can happen either physically or chemically. Physical adsorption occurs due to electrostatic attraction between soil colloidal surfaces and negatively charged phosphate ions. Chemical adsorption occurs with soil colloids with variable charges such as Fe and Al oxides and calcites (Zhao et al. 2021). With time, some of the adsorbed P on clay surfaces can diffuse into the colloid (absorbed) and form a specific mineral.
Two opposite effects of submergence on the P availability in paddy soils are documented. Submergence increases P availability temporarily due to reduction dissolution Fe/Mn oxy(hydr)oxides and releasing P sorbed/occluded. Based on the results of a P sorption study on two typical rice-growing soils (Ultisols and Entisols) in China, Zhang et al. (2003) reported that the submergence of paddy soils can also increase P sorption capacity and decrease P desorption with time. Under submergence, crystalline Fe and Al oxides transform to amorphous forms (Darke and Walbridge 2000). Amorphous oxides have higher reactive surface areas compared to their crystalline counterparts (Mayakaduwage et al. 2020). Therefore, P sorption by amorphous compounds of Fe and Al is high.
Degree of P saturation which is the extractable P concentration expressed as a percentage of P sorption capacity of a soil is also proven as an important parameter in predicting P release upon submergence of soil (Kumaragamage et al. 2019). A strong positive linear relationship between P release in submerged soils and degree of P saturation was reported in previous studies (Amarawansha et al. 2016; Kumaragamage et al. 2019; Sallade and Sims 1997).
7.8 Soil Amendments
Soil amendments alter P release from soil under submergence. A study conducted in Canada in clay soils revealed that application of MgSO4 at a rate of 2.5 Mg/ha reduced the P release by 21–75% among the tested soils under snowmelt flooded conditions (Vitharana et al. 2021). The authors suggest a possible reason for this reduction is an increase in Mg concentrations which enhanced P precipitation. The application of alum (Al2(SO4)3·18H2O), gypsum (CaSO4·2H2O) and manganese(IV) oxide (MnO2) was also found to decrease P release under flooding conditions (Attanayake et al. 2022; Dharmakeerthi et al. 2019b; Kumaragamage et al. 2022).
Biochar application increased the P sorption capacity in acidic soils and decreased P sorption in alkaline soils (Xu et al. 2014). However, this is not an independent effect of biochar and is likely to occur due to interactions with other organic (organic matter) and inorganic compounds (cations; Ca2+, Mg2+, Fe3+, Al3+) in soils. Biochar can act as a P source or a sink under submergence (Dharmakeerthi et al. 2019a). Previous research from the same authors showed that biochar can increase dissolved reactive P in porewater in clay soils, while it had no effect on sandy loam soils.
Organic amendments increase P availability in soil due to several mechanisms as explained in the “Soil Organic Matter Content/Quality or Addition of Organic Matter” section. Application of organic manure such as poultry and cattle manure has been reported to increase soil available P concentrations under submergence (Chen et al. 2021). Furthermore, organic fertilisers can improve release of residual P in soil (Yang and Yang 2021).
7.9 Temperature
Phosphorus release is higher in warm temperatures than the cold temperatures in anaerobic conditions (Kumaragamage et al. 2020; Sallade and Sims 1997). An incubation study conducted using five agricultural soils in Canada using warm (20 ± 2 °C) and cold (4 ± 1 °C) temperatures revealed that P release in warm temperature under anaerobic condition is significantly higher (p < 0.05) compared to that of cold temperature (Kumaragamage et al. 2020). The reason for the increasing trend of P release with increasing temperature could be an increased rate of microbially mediated redox reactions with temperature in the presence of oxidisable organic matter (Heiberg et al. 2012). Contrastingly, another experiment conducted in wetland soils in the USA revealed that cooler temperatures and anaerobic conditions decrease P sorption (Hurst et al. 2022). Similarly, an incubation study conducted by Barrow (1983) also discovered that high temperatures increase P sorption.
7.10 Water Management
Frequent alternative wetting and drying of a soil can alter pH and Eh of the water-soil system and thereby affect the P release from soil (Chacon et al. 2006). Drying events following wetting can reduce the P sorption capacity of soil by increasing the crystallinity of Fe hydroxides which can ultimately lead to increased P release from soil (Schönbrunner et al. 2012). In contrast, a soil column study conducted in China using vegetable and wheat growing soils revealed that the dissolved reactive P in floodwater after re-flooding, following a draining period of 10 days, was 3–25 times less than the dissolved reactive P recorded in floodwater during a continuous flooding of 17 days (Tian et al. 2017). Furthermore, another study conducted in England and Wales reported that the water-soluble P, especially the organic form of P increased up to 1900% with the process of drying and rapid rewetting possibly due to releasing of P from microbial biomass as they die by osmotic shock and cell rupture under rapid changes of soil moisture content (Turner and Haygarth 2001).
7.11 Presence/Absence of Plants and Cultivars
The soil rhizosphere provides different environmental conditions relative to the bulk soil. The conditions in the rhizosphere are unique to plant species and even the cultivars of the same species, thereby incurring differences in P availability within that discreet region. Root exudates accumulating in the rhizosphere include low molecular weight (e.g. organic acids, amino acids and sugars) and high molecular weight (e.g. complex carbohydrates, enzymes) organic compounds (Hinsinger et al. 2009; Pii et al. 2015). The composition of root exudates is influenced by plant cultivar and environmental factors (Aulakh et al. 2001; Pii et al. 2015). This abundance of carbon sources causes migration of microbes within a radius of 50 µm from roots (Dotaniya and Meena 2015; Pii et al. 2015). Phosphatase enzymes released by microbes mineralise organic P and release orthophosphates (Rodrı et al. 2006). In addition, rhizosphere microbes produce organic acids such as oxalic and citric acids and release protons to soil that help to decrease rhizosphere pH and compete for adsorption sites (Long et al. 2018).
There is a potential of root exposure to high levels of harmful reduced chemical species such as hydrogen sulphide (H2S) and Fe(II) under O2 depleted conditions in submerged soils (Larsen et al. 2015). However, an oxygenated zone exists in the rhizosphere due to atmospheric O2 transfer through aerenchyma tissues (Mongon et al. 2014). This oxygen secretion by roots of rice plant enhances utilisation of P under submergence (Zhang et al. 2004).
7.12 Other Non-Phosphatic Fertilisers
Phosphorus fertilisers are applied with other non-phosphatic fertilisers such as N fertilisers (urea or ammonium sulphate) and K fertilisers (muriate of potash) in rice cultivation. Although these non-phosphatic fertilisers do not affect the P dynamics directly, they change other soil properties which affect P dynamics. For example, N fertilisers used in rice cultivation such as urea affect soil pH, electrical conductivity and soil temperature (Park et al. 2023). Two paddy field studies conducted in Inceptisols (sandy clay loam) having an acidic pH ranging from 4.8 to 5.5 revealed a linear decrease of soil pH with increasing rates of both urea and ammonium sulphate fertilisers (Fageria et al. 2010). They also reported a decrease of Ca and Mg saturation and an increase of Al saturation with increasing rates of the same N fertilisers. Potassium fertilisers increase the exchangeable K in soil (Volf et al. 2021). Large quantities of K+ in exchangeable sites can displace divalent cations such as Ca2+ and Mg2+ from exchange sites (Zaker and Emami 2019).
8 Methods and Techniques to Identify Phosphorus Dynamics in Submerged Soils
The ultimate P release in a submerged soil system is a collaborative result of complex biogeochemical reactions in soils mainly depending upon the inherent soil characteristics. It is important to study the behaviour of P under submergence at the P species level as it might explain the reasons behind poor yield response of rice under submergence of paddy soils of Sri Lanka.
There are numerous methods which can be used to study soil P speciation. The P fractionation method introduced by Chang and Jackson (1957) is considered the oldest P fractionation method (Gu and Margenot 2021). However, the Hedley sequential fractionation which was introduced by Hedley et al. (1982) is the most widely used P fractionation method. As in any analytical methods, it also has its own drawbacks despite the modifications made by Tiessen and Moir (1993). One of the major limitations of these sequential chemical extraction methods is they are limited to operationally defined fractions (Schmieder 2019). For example, Hedley fractionation separates soil P into resin-P, NaHCO3-P, NaOH-P, and HCl-P extractable fractions (Nishigaki et al. 2018). But it does not reveal P species (Gu et al. 2020; McDowell and Burkitt 2022).
When P fractionation is performed for submerged soils, it is important to collect samples under reduced conditions and handle them appropriately during P fractionation (Condron and Newman 2011) as P dynamics under submergence is controlled by the Eh of soil. Redox-driven ions such as Fe2+ and Mn2+ in submerged soils can be oxidised within a short period of time upon exposure to aerobic environment, and this will lead to an inaccurate interpretation of P levels in different soil P fractions. Moore and Coale (2000) considered these conditions and introduced a method for P fractionation in flooded soils and sediments based on the P fractionation methods by and Moore and Reddy (1994) and van Eck (1982).
Advanced technologies such as molecular analyses have evolved since the 1990s overcoming the limitations of P fractionation methods. Molecular analyses such as 31P nuclear–magnetic resonance (NMR) spectroscopy and X-ray absorption near-edge structure (XANES) spectroscopy enable an identification of P species in soils (Alam et al. 2021). However, these analyses also have several limitations (Vogel et al. 2016). Widely used methods in P dynamics studies and their associated limitations are summarised in the Table 4.
However, none of these analytical techniques can successfully identify P species in soil independently because of the complex biochemical processes of P (Liu et al. 2017). For example, for certain soils, XANES spectroscopy may not enable reliable differentiation between organic P and inorganic P adsorbed to a given soil adsorbent under certain soil conditions (Prietzel et al. 2016). Furthermore, subsequent studies have also showed that measures of organic P by P fractionation can be used to complement XANES spectroscopy (Gu and Margenot 2021). Thus, the combined use of wet chemistry and spectroscopic analytical techniques could provide detailed information on the impacts of management practices on various P species distributions and transformations (Liu et al. 2019; Negassa et al. 2020). Furthermore, the kinetics of the exchanges of P among different P pools of submerged soil can also be incorporated in the analysis to improve the overall understanding of the fate of added P fertilisers in paddy soils.
9 Conclusions and Future Perspectives
The rice yield response to added P fertilisers is inconsistent in paddy soils in Sri Lanka, even in fields with very low available P. Reference to past research conducted in paddy soils in Sri Lanka, this inconsistent yield response cannot be explained by soil test P, and it is also not correlated with soil properties such as soil texture, pH, electrical conductivity, total carbon content and available Fe and Mg concentrations of soil. The issue is also influenced by the tendency of farmers to over apply P fertilisers which can negatively impact water quality in surrounding freshwater bodies. Therefore, underpinning the scientific basis for the inconsistent rice yield responses to P is critical to increasing rice production, improving the efficiency of P fertiliser use and utilisation of legacy soil P and reducing impacts on water quality.
The following aspects should be emphasised in future research in order to improve long-term P management in paddy soils in Sri Lanka.
-
1.
The varying response of rice yield to submergence-induced P release under P fertiliser management may find its origin in the heterogeneity of inherent soil characteristics, such as Fe and Al oxy(hydr)oxides, clay mineralogy, P sorption, Ca, Mg and organic matter. Investigating the variations of these soil properties at field scale and establishing their correlation among P fertiliser application, P release and yield response in Sri Lankan paddy soils hold utmost importance. This research is crucial for advancing P management practices, including the adjustment of P fertiliser application rates and frequencies based on rice yield response across diverse paddy soils.
-
2.
There is a necessity for species level investigations on the P chemical fractions to underpin the chemical P transformations under submergence of paddy soils. Undertaking such research will provide valuable insights into the specific soil characteristics that influence the response of rice yield to the application of P fertilisers under submergence. This endeavour necessitates the development of new research methodologies that combine sequential chemical extraction techniques with advanced techniques, such as XANES spectroscopy. This integrated approach will enable a comprehensive understanding of the complex system of submerged paddy soils, potentially validating previous observations.
References
Abolfazli F, Forghani A, Norouzi M (2012) Effects of phosphorus and organic fertilizers on phosphorus fractions in submerged soil. J Soil Sci Plant Nutr 12:349–362
Agbenin JO (2003) Extractable iron and aluminum effects on phosphate sorption in a savanna Alfisol. Soil Sci Soc Am J 67:589–595. https://doi.org/10.2136/sssaj2003.5890
AgStat (2021) Agricultural statistics. Socio Economics and Planning Centre, Department of Agriculture, Sri Lanka. https://doa.gov.lk/sepc-downloads_en/#1583822518501-4cd250c2-4e98. Accessed 12 Apr 2023
Ajmone-Marsan F, Côté D, Simard RR (2006) Phosphorus transformations under reduction in long-term manured soils. Plant Soil 282:239–250. https://doi.org/10.1007/s11104-005-5929-6
Akter M (2018) Linkage between subtropical paddy soil nitrogen and iron and manganese reduction. Ph.D. Thesis, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, pp 221
Alam M, Srinivasan V, Mueller AV, Gu AZ (2021) Status and advances in technologies for phosphorus species detection and characterization in natural environment- a comprehensive review. Talanta 233:122458. https://doi.org/10.1016/j.talanta.2021.122458
Amarawansha EAGS, Kumaragamage D, Flaten D, Zvomuya F, Tenuta M (2015) Phosphorus mobilization from manure-amended and unamended alkaline soils to overlying water during simulated flooding. J Environ Qual 44:1252–1262. https://doi.org/10.2134/jeq2014.10.0457
Amarawansha G, Kumaragamage D, Flaten D, Zvomuya F, Tenuta M (2016) Predicting phosphorus release from anaerobic, alkaline, flooded Soils. J Environ Qual 45(4):1452–1459. https://doi.org/10.2134/jeq2015.05.0221
Amery F, Smolders E (2012) Unlocking fixed soil phosphorus upon waterlogging can be promoted by increasing soil cation exchange capacity. Eur J Soil Sci 63:831–838. https://doi.org/10.1111/j.1365-2389.2012.01478.x
Antoniadis V, Koliniati R, Efstratiou E, Golia E, Petropoulos S (2016) Effect of soils with varying degree of weathering and pH values on phosphorus sorption. Catena (Amst) 139:214–219. https://doi.org/10.1016/j.catena.2016.01.008
Attanayake CP, Kumaragamage D, Amarawansha G, Hettiarachchi GM, Indraratne SP, Goltz DM (2022) Phosphorus release and speciation in manganese(IV) oxide and zeolite-amended flooded soils. Environ Sci Technol 56:8082–8093. https://doi.org/10.1021/acs.est.2c01185
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148. https://doi.org/10.1055/s-2001-15205
Barrow NJ (1983) A mechanistic model for describing the sorption and desorption of phosphate by soil. J Soil Sci 34:733–750. https://doi.org/10.1111/j.1365-2389.1983.tb01068.x
Beauchemin S, Hesterberg D, Chou J, Beauchemin M, Simard RR, Sayers DE (2003) Speciation of phosphorus in phosphorus-enriched agricultural soils using x-ray absorption near-edge structure spectroscopy and chemical fractionation. J Environ Qual 32:1809–1819. https://doi.org/10.2134/jeq2003.1809
Bhattacharyya P, Chakrabarti K, Chakraborty A (2003) Effect of MSW compost on microbiological and biochemical soil quality indicators. Compost Sci Util 11:220–227. https://doi.org/10.1080/1065657X.2003.10702130
Bhattacharyya P, Chakrabarti K, Chakraborty A, Nayak DC (2005) Effect of municipal solid waste compost on phosphorous content of rice straw and grain under submerged condition. Arch Agron Soil Sci 51:363–370. https://doi.org/10.1080/03650340500201634
Central Bank Annual Report (2022) Production of tea, rubber, coconut and other export agriculture crops. In: National output, expenditure, income and employment. https://www.cbsl.gov.lk/sites/default/files/cbslweb_documents/publications/annual_report/2022/en/15_Appendix.pdf. Accessed 01 Jun 2023
Chacon N, Flores S, Gonzalez A (2006) Implications of iron solubilization on soil phosphorus release in seasonally flooded forests of the lower Orinoco River, Venezuela. Soil Biol Biochem 38:1494–1499. https://doi.org/10.1016/j.soilbio.2005.10.018
Chang SC, Jackson ML (1957) Fractionation of soil phosphorus. Soil Sci 84:133–144. https://doi.org/10.1097/00010694-195708000-00005
Chen GL, Xiao L, Xia QL, Wang Y, Yuan JH, Chen H, Wang SQ, Zhu YY (2021) Characterization of different phosphorus forms in flooded and upland paddy soils incubated with various manures. ACS Omega 6(4):3259–3266. https://doi.org/10.1021/acsomega.0c05748
Condron LM, Newman S (2011) Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J Soils Sediments 11(5):830–840. https://doi.org/10.1007/s11368-011-0363-2
Cordell D, White S (2013) Sustainable phosphorus measures: strategies and technologies for achieving phosphorus security. Agron 3:86–116. https://doi.org/10.3390/agronomy3010086
Dai Z, Liu G, Chen H, Chen C, Wang J, Ai S, Wei D, Li D, Ma B, Tang C, Brookes PC, Xu J (2020) Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J 14:757–770. https://doi.org/10.1038/s41396-019-0567-9
Damayanthi MRC (2001) Phosphorus retention ability of five Sri Lankan soils under flooded and dry conditions. University of Peradeniya, Sri Lanka
Darke AK, Walbridge MR (2000) Al and Fe biogeochemistry in a floodplain forest: implications for P retention. Biogeochemistry 51:1–32. https://doi.org/10.1023/A:1006302600347
Dassanayake AR, De Silva GGR, Mapa RB (2020) Major soils of the dry zone and their classification. In: Mapa RB (ed) The soils of Sri Lanka World Soils Book Series, 1st edn. Springer, Switzerland, pp 49–68
Dassanayake AR, Senarath A, Hettiarachchi LSK, Mapa RB (2020) Major soils of the wet zone and their classification. In: Mapa RB (ed) The soils of Sri Lanka. World Soils Book Series, 1st edn. Springer, Switzerland, pp 83–94
Datta A, Ullah H, Ferdous Z (2017) Water management in rice. In: Chauhan B, Jabran K, Mahajan G (eds) Rice Production Worldwide. Springer, Cham, pp 255–277. https://doi.org/10.1007/978-3-319-47516-5_11
De Silva LHN, Edirisinghe JC, Udyanga NWBAL, Gimhani DR (2020) Farmer perception, environmental awareness, and overuse of fertilizer in Kalpitiya: a preliminary investigation using bayesian econometrics. Appl Econ Bus 4:20–32. https://doi.org/10.4038/aeb.v4i1.42
Dharmakeerthi RS, Kumaragamage D, Goltz D, Indraratne SP (2019a) Phosphorus release from unamended and gypsum- or biochar-amended soils under simulated snowmelt and summer flooding conditions. J Environ Qual 48:822–830. https://doi.org/10.2134/jeq2019.02.0091
Dharmakeerthi RS, Kumaragamage D, Indraratne SP, Goltz D (2019b) Gypsum amendment reduces redox-induced phosphorous release from freshly manured, flooded soils to floodwater. J Environ Qual 48:127–135. https://doi.org/10.2134/jeq2018.08.0308
Dotaniya ML, Meena VD (2015) Rhizosphere effect on nutrient availability in soil and its uptake by plants: a review. Proc Nat Acad Sci, India Sect B: Biol Sci 85:1–12. https://doi.org/10.1007/s40011-013-0297-0
Dougherty WJ, Fleming NK, Cox JW, Chittleborough DJ (2004) Phosphorus transfer in surface runoff from intensive pasture systems at various scales. J Environ Qual 33:1973–1988. https://doi.org/10.2134/jeq2004.1973
Fageria NK, Dos Santos AB, Moraes MF (2010) Influence of urea and ammonium sulfate on soil acidity indices in lowland rice production. Commun Soil Sci Plant Anal 41(13):1565–1575. https://doi.org/10.1080/00103624.2010.485237
Fageria NK, Carvalho GD, Santos AB, Ferreira EPB, Knupp AM (2011) Chemistry of lowland rice soils and nutrient availability. Commun Soil Sci Plant Anal 42:1913–1933. https://doi.org/10.1080/00103624.2011.591467
FAO (2017) The future of food and agriculture and challenges-trends and challenges. https://www.fao.org/3/i6583e/i6583e.pdf. Accessed 06 May 2023
FAOSTAT (2022) Data. https://www.fao.org/faostat/en/#data. Accessed 19 Apr 2022
Gadal N, Shrestha J, Poudel MN, Pokharel B (2019) A review on production status and growing environments of rice in Nepal and in the world. Arch Agric Environ Sci 4:83–87. https://doi.org/10.26832/24566632.2019.0401013
Gao S, Tanji KK, Scardaci SC, Chow AT (2002) Comparison of redox indicators in a paddy soil during rice-growing season. Soil Sci Soc Am J 66:805–817. https://doi.org/10.2136/sssaj2002.8050
Geretharan T, Jeyakumar P, Bretherton M, Anderson CWN (2018) Defining a standard method to measure the total and bioavailable concentration of fluorine in New Zealand soils. Microchem J 142:94–101. https://doi.org/10.1016/j.microc.2018.06.018
Gu C, Margenot AJ (2021) Navigating limitations and opportunities of soil phosphorus fractionation. Plant Soil 459:13–17. https://doi.org/10.1007/s11104-020-04552-x
Gu C, Dam T, Hart SC, Turner BL, Chadwick OA, Berhe AA, Hu Y, Zhu M (2020) Quantifying uncertainties in sequential chemical extraction of soil phosphorus using XANES spectroscopy. Environ Sci Technol 54:2257–2267. https://doi.org/10.1021/acs.est.9b05278
Hanke A, Cerli C, Muhr J, Borken W, Kalbitz K (2013) Redox control on carbon mineralization and dissolved organic matter along a chronosequence of paddy soils. Eur J Soil Sci 64:476–487. https://doi.org/10.1111/ejss.12042
Hanyabui E, Apori SO, Frimpong KA, Atiah K, Abindaw T, Ali M, Yeboah AJ, Byalebeka J (2020) Phosphorus sorption in tropical soils. AIMS Agric Food 5:599–616. https://doi.org/10.3934/agrfood.2020.4.599
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Heiberg L, Koch CB, Kjaergaard C, Jensen HS, Hansen HCB (2012) Vivianite precipitation and phosphate sorption following iron reduction in anoxic soils. J Environ Qual 41:938–949. https://doi.org/10.2134/jeq2011.0067
Helfenstein J, Tamburini F, von Sperber C, Massey MS, Pistocchi C, Chadwick OA, Vitousek PM, Kretzschmar R, Frossard E (2018) Combining spectroscopic and isotopic techniques gives a dynamic view of phosphorus cycling in soil. Nat Commun 9(1):1–9. https://doi.org/10.1038/s41467-018-05731-2
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195. https://doi.org/10.1023/A:1013351617532
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. https://doi.org/10.1007/s11104-008-9885-9
Hori T, Mu A, Igarashi Y, Conrad R, Friedrich MW (2010) Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J 4:267–278. https://doi.org/10.1038/ismej.2009.100
Howeler RH, Bouldin DR (1971) Diffusion and consumption of oxygen in submerged soils. Soil Sci Soc Amer Proc 35:202–208. https://doi.org/10.2136/sssaj1971.03615995003500020014x
Hurst NR, VanZomeren CM, Berkowitz JF (2022) Temperature, redox, and amendments alter wetland soil inorganic phosphorus retention dynamics in a Laurentian Great Lakes priority watershed. J Great Lakes Res 48:935–943. https://doi.org/10.1016/j.jglr.2022.05.010
Hutchison KN (2003) Dissolution of phosphate in a phosphorus-enriched Ultisol as affected by microbial reduction. M.Sc. Thesis, North Carolina State University
Indraratne SP (2020) Soil mineralogy. In: Mapa RB (ed) The soils of Sri Lanka. World Soils Book Series, 1st edn. Springer, Switzerland, pp 35–47
Jayarathne PDKD, Kumaragamage D, Indraratne S, Flaten D, Goltz D (2016) Phosphorus release to floodwater from calcareous surface soils and their corresponding subsurface soils under anaerobic conditions. J Environ Qual 45:1375–1384. https://doi.org/10.2134/jeq2015.11.0547
Kadupitiya HK, Madushan RND, Gunawardhane D, Sirisena D, Rathnayake U, Dissanayaka D, Ariyaratne M, Marambe B, Suriyagoda L (2022) Mapping productivity-related spatial characteristics in rice-based cropping systems in Sri Lanka. J Geovisualization Spat 6. https://doi.org/10.1007/s41651-022-00122-0
Kanthilanka H, Weerahewa J (2018) Do price subsidies lead to over application of fertilizers? An analysis of Kethata-Aruna program of Sri Lanka. Trop Agric Res 30(4):133–142. https://doi.org/10.4038/tar.v30i4.8335
Kendaragama KM, Senevirathne Banda KM, Bandara PT (2003) Influence of rice crop on soil phosphorus availability in relation to phosphate fertilizer application. Ann Sri Lanka Dep Agric 5:129–139. https://drive.google.com/file/d/1JKUtp9HvHTG6LoMCe9CczFzCCEpWPKU2/view
Khan SU, Hooda PS, Blackwell MSA, Busquets R (2022) Effects of drying and simulated flooding on soil phosphorus dynamics from two contrasting UK grassland soils. Eur J Soil Sci 73(1):e13196. https://doi.org/10.1111/ejss.13196
Kirk G (2004) Biological processes in the soil and floodwater. In: The biogeochemistry of submerged soils. John Wiley & Sons, Ltd, pp 135-163. https://doi.org/10.1111/j.1365-2389.2004.0694a.x
Kodagoda MM, Thrikawala S, Abeysiriwardena DS de Z (2022) Yield response of rice to added phosphorous and potassium fertilizer in the dry zone of Sri Lanka. J Agric Sci - Sri Lanka 17:270–279. https://doi.org/10.4038/jas.v17i2.9742
Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14. https://doi.org/10.1016/j.geoderma.2010.03.009
Kruse J, Abraham M, Amelung W et al (2015) Innovative methods in soil phosphorus research: a review. J Plant Nutr Soil Sc 178:43–88. https://doi.org/10.1002/jpln.201400327
Kulasinghe HPGTN, Vitharana UWA, Dharmakeerthi RS, Sirisena DN, Rathnayake WMUK (2020) Exploring the yield response of paddy (Oryza sativa L.) under varying levels of soil nitrogen, phosphorus and potassium. Trop Agric Res 31(4):1–12. https://doi.org/10.4038/tar.v31i4.8416
Kumaragamage D, Amarawansha GS, Indraratne SP, Jayarathne K, Flaten DN, Zvomuya F, Akinremi OO (2019) Degree of phosphorus saturation as a predictor of redox-induced phosphorus release from flooded soils to floodwater. J Environ Qual 48(6):1817–1825. https://doi.org/10.2134/jeq2019.04.0154
Kumaragamage D, Concepcion A, Gregory C, Goltz D, Indraratne S, Amarawansha G (2020) Temperature and freezing effects on phosphorus release from soils to overlying floodwater under flooded-anaerobic conditions. J Environ Qual 49:700–711. https://doi.org/10.1002/jeq2.20062
Kumaragamage D, Weerasekara CS, Perry M, Akinremi OO, Goltz D (2022) Alum and gypsum amendments decrease phosphorus losses from soil monoliths to overlying floodwater under simulated snowmelt flooding. Water 14(4):559. https://doi.org/10.3390/w14040559
Larsen M, Santner J, Oburger E, Wenzel WW, Glud RN (2015) O2 dynamics in the rhizosphere of young rice plants (Oryza sativa L.) as studied by planar optodes. Plant Soil 390:279–292. https://doi.org/10.1007/s11104-015-2382-z
Liu J, Yang J, Cade-Menun BJ, Hu Y, Li J, Peng C (2017) Molecular speciation and transformation of soil legacy phosphorus with and without long- term phosphorus fertilization: Insights from bulk and microprobe spectroscopy. Sci Rep 7:15354. https://doi.org/10.1038/s41598-017-13498-7
Liu J, Sui P, Cade-Menun BJ, Hu Y, Yang J, Huang S, Ma Y (2019) Molecular-level understanding of phosphorus transformation with long-term phosphorus addition and depletion in an alkaline soil. Geoderma 353:116–124. https://doi.org/10.1016/j.geoderma.2019.06.024
Lizcano-Toledo R, Reyes-Martín MP, Celi L, Fernández-Ondoño E (2021) Phosphorus dynamics in the soil–plant–environment relationship in cropping systems: a review. Appl Sci 11(23):11133. https://doi.org/10.3390/app112311133
Lombi E, Susini J (2009) Synchrotron-based techniques for plant and soil science: opportunities, challenges and future perspectives. Plant Soil 320:1–35. https://doi.org/10.1007/s11104-008-9876-x
Long X, Yao H, Huang Y, Wei W, Zhu Y (2018) Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol Biochem 118:103–114. https://doi.org/10.1016/j.soilbio.2017.12.014
Mapa RB (2020) Land use. In: Mapa RB (ed) The soils of Sri Lanka. World Soils Book Series, 1st edn. Springer, Switzerland, pp 95–102
Maranguit D, Guillaume T, Kuzyakov Y (2017) Effects of flooding on phosphorus and iron mobilization in highly weathered soils under different land-use types: short-term effects and mechanisms. Catena (Amst) 158:161–170. https://doi.org/10.1016/j.catena.2017.06.023
Marschner P (2021) Processes in submerged soils – linking redox potential, soil organic matter turnover and plants to nutrient cycling. Plant Soil 464:1–12. https://doi.org/10.1007/s11104-021-05040-6
Mayakaduwage S, Mosley LM, Marschner P (2020) Threshold for labile phosphate in a sandy acid sulfate soil. Geoderma 371:114359. https://doi.org/10.1016/j.geoderma.2020.114359
McDowell RW, Burkitt LL (2022) In recognition of Mike Hedley: fate of fertiliser in soil and mobilisation of recalcitrant nutrients. Nutr Cycl Agroecosyst 124:131–134. https://doi.org/10.1007/s10705-022-10243-z
Menezes-Blackburn D, Giles C, Darch T et al (2018) Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: a review. Plant Soil 427:5–16. https://doi.org/10.1007/s11104-017-3362-2
Mongon J, Konnerup D, Colmer TD, Rerkasem B (2014) Responses of rice to Fe2+ in aerated and stagnant conditions: growth, root porosity and radial oxygen loss barrier. Funct Plant Biol 41:922–929. https://doi.org/10.1071/FP13359
Moore P, Coale F (2000) Phosphorus fractionation in flooded soils and sediments. In: Pierzynski GM (ed) Methods of phosphorus analysis for soils, sediments, residuals, and waters. Southern Cooperative Series Bulletin No#336, North Carolina State University, pp 60–64. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=c3713db4ecdc1f92ca2ea685ef940117bcfd27f2. Accessed 15 Jan 2023
Moore PA, Reddy KR (1994) Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida. J Environ Qual 23:955–964. https://doi.org/10.2134/jeq1994.00472425002300050016x
Najafi N (2013) Changes in pH, EC and concentration of phosphorus in soil solution during submergence and rice growth period in some paddy soils of North of Iran. Int J Agric: Res Rev 3(2):271–280
Negassa W, Michalik D, Klysubun W, Leinweber P (2020) Phosphorus speciation in long-term drained and rewetted peatlands of Northern Germany. Soil Syst 4:1–20. https://doi.org/10.3390/soilsystems4010011
Nishigaki T, Sugihara S, Kobayashi K, Hashimoto Y, Kilasara M, Tanaka H, Watanabe T, Funakawa S (2018) Fractionation of phosphorus in soils with different geological and soil physicochemical properties in southern Tanzania. Soil Sci Plant Nutr 64:291–299. https://doi.org/10.1080/00380768.2018.1436406
Nishigaki T, Tsujimoto Y, Rakotoson T, Rabenarivo M, Andriamananjara A, Asai H, Andrianary HB, Rakotonindrina H, Razafimbelo T (2021) Soil phosphorus retention can predict responses of phosphorus uptake and yield of rice plants to P fertilizer application in flooded weathered soils in the central highlands of Madagascar. Geoderma 402:115326. https://doi.org/10.1016/j.geoderma.2021.115326
O’Loughlin EJ, Boyanov MI, Flynn TM, Gorski CA, Hofmann SM, McCormick ML, Scherer MM, Kemner KM (2013) Effects of bound phosphate on the bioreduction of lepidocrocite (γ - FeOOH) and maghemite (γ -Fe2O3) and formation of secondary minerals. Environ Sci Technol 47:9157–9166. https://doi.org/10.1021/es400627j
Park JR, Jang YH, Kim EG, Lee GS, Kim KM (2023) Nitrogen fertilization causes changes in agricultural characteristics and gas emissions in rice field. Sustainability 15(4):3336. https://doi.org/10.3390/su15043336
Pasek MA, Gull M, Herschy B (2017) Phosphorylation on the early earth. Chem Geol 475:149–170. https://doi.org/10.1016/j.chemgeo.2017.11.008
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9(6):120. https://doi.org/10.3390/agriculture9060120
Pfahler V, Adu-Gyamfi J, O’Connell D, Tamburini F (2022) The use of the δ18OP to study P cycling in the environment. In: Adu-Gyamfi J, Verena Pfahler V (eds) Oxygen isotopes of inorganic phosphate in environmental samples. Springer International Publishing, pp 1–16. https://doi.org/10.1007/978-3-030-97497-8
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A Review Biol Fertil Soils 51:403–415. https://doi.org/10.1007/s00374-015-0996-1
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96. https://doi.org/10.1016/S0065-2113(08)60633-1
Price G (2006) Australian Soil Fertility Manual. Fertilizer Industry Federation of Australia, Inc. & CSIRO, Collingwood
Prietzel J, Dümig A, Wu Y, Zhou J, Klysubun W (2013) Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim Cosmochim Acta 108:154–171. https://doi.org/10.1016/j.gca.2013.01.029
Prietzel J, Klysubun W, Werner F (2016) Speciation of phosphorus in temperate zone forest soils as assessed by combined wet-chemical fractionation and XANES spectroscopy. J Plant Nutr Soil Sc 179:168–185. https://doi.org/10.1002/jpln.201500472
Rakotoson T, Rabeharisoa L, Smolders E (2016) Effects of soil flooding and organic matter addition on plant accessible phosphorus in a tropical paddy soil: an isotope dilution study. J Plant Nutr Soil Sc 179:765–774. https://doi.org/10.1002/jpln.201500383
Rathnayake WMUK, De Silva RP, Dayawansa NDK (2015) Variability of some important soil chemical properties of rainfed low land paddy fields and its effect on land suitability for rice- cultivation. Trop Agric Res 26:506–516. https://doi.org/10.4038/tar.v26i3.8113
Rivard C, Lanson B, Cotte M (2016) Phosphorus speciation and micro-scale spatial distribution in North-American temperate agricultural soils from micro X-ray fluorescence and X-ray absorption near-edge spectroscopy. Plant Soil 401:7–22. https://doi.org/10.1007/s11104-015-2494-5
Roberts TL, Johnston AE (2015) Phosphorus use efficiency and management in agriculture. Resour Conserv Recycl 105:275–281. https://doi.org/10.1016/j.resconrec.2015.09.013
Rodrı H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21. https://doi.org/10.1007/s11104-006-9056-9
Rubasinghe RT, Gunatilake SK, Chandrajith R (2021) Climatic control of major and trace elements in paddy soils from wet and dry regions of Sri Lanka. Environmental Challenges 5:100361. https://doi.org/10.1016/j.envc.2021.100361
Sahrawat KL (2004a) Iron toxicity in wetland rice and the role of other nutrients. J Plant Nutr 27:1471–1504. https://doi.org/10.1081/PLN-200025869
Sahrawat KL (2004b) Organic matter accumulation in submerged soils. Adv Agron 81:169–201
Sahrawat KL (2005) Fertility and organic matter in submerged rice soils. Curr Sci 88(5):735–739. http://www.jstor.org/stable/24111259
Sahrawat KL (2012) Soil fertility in flooded and non-flooded irrigated rice systems. Arch Agron Soil Sci 58:423–436. https://doi.org/10.1080/03650340.2010.522993
Sallade YE, Sims JT (1997) Phosphorus transformations in the sediments of Delaware’s agricultural drainageways: II. Effect of reducing conditions on phosphorus release. J Environ Qual 26:1579–1588. https://doi.org/10.2134/jeq1997.00472425002600060018x
Sanyal SK, De Datta SK (1991) Chemistry of phosphorus transformations in soil. In: Stewart BA (ed) Advances in Soil Science. Springer, New York, pp 1–120. https://doi.org/10.1007/978-1-4612-3144-8_1
Scalenghe R, Edwards AC, Marsan FA, Barberis E (2002) The effect of reducing conditions on the solubility of phosphorus in a diverse range of European agricultural soils. Eur J Soil Sci 53:439–447. https://doi.org/10.1046/j.1365-2389.2002.00462.x
Schmieder F, Bergström L, Riddle M, Gustafsson JP, Klysubun W, Zehetner F, Condron L, Kirchmann H (2018) Phosphorus speciation in a long-term manure-amended soil profile – evidence from wet chemical extraction, 31P-NMR and P K-edge XANES spectroscopy. Geoderma 322:19–27. https://doi.org/10.1016/j.geoderma.2018.01.026
Schmieder F (2019) Phosphorus speciation in Swedish arable soils with high leaching potential. Ph.D. Thesis, Swedish University of Agricultural Sciences
Schönbrunner IM, Preiner S, Hein T (2012) Impact of drying and reflooding of sediment on phosphorus dynamics of river-floodplain systems. Sci Total Environ 432:329–337. https://doi.org/10.1016/j.scitotenv.2012.06.025
Senevirathne Banda KM, Kendaragama KMA, Ethakada DMG (2002) Response of rice to added phosphorus on Low Humic Gley soil under major irrigation in the Polonnaruwa district. Ann Sri Lanka Dep Agric 4:95–108. https://drive.google.com/file/d/1F9fbu_gB9mo7-lPKfLFdvACSUL3lOp9l/view
Siam HS, Mahmoud SA, Taalab AS, Ageeb GW (2019) A review of electrochemical changes in submerged soils. Plant Arch 19:1965–1973
Sirisena DN, Herath HMAB, Wanninayake WMN (2013) Response of rice to application of phosphorus fertilizer in Polonnaruwa district of the low country dry zone. Ann Sri Lanka Dep Agric 15:237–243. https://drive.google.com/file/d/10Ccv0-utJ2W--RfdEVwPS5aU896V8QJO/view
Sirisena D, Suriyagoda DB (2018) Toward sustainable phosphorus management in Sri Lankan rice and vegetable-based cropping systems: a review. Agric Nat Resour 52:9–15. https://doi.org/10.1016/j.anres.2018.03.004
Siriwardana KGDI, Weerasinghe WDP, Priyantha GDA, Chandrasekara KKD, Rupasinghe MDN, Wickramasinghe WRKDWKV, Dissanayake I, Wijesinghe MR (2019) Screening of selected rice varieties and advanced breeding lines against iron toxicity under field conditions in the low country wet zone of Sri Lanka. Trop Agric Res 30:33. https://doi.org/10.4038/tar.v30i2.8307
Smith GJ, McDowell RW, Condron LM, Daly K, Ó hUallacháin D, Fenton O, (2021) Reductive dissolution of phosphorus associated with iron-oxides during saturation in agricultural soil profiles. J Environ Qual 50:1207–1219. https://doi.org/10.1002/jeq2.20256
Suriyagoda L, De Costa WAJM, Lambers H (2014) Growth and phosphorus nutrition of rice when inorganic fertiliser application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil 384:53–68. https://doi.org/10.1007/s11104-014-2049-1
Tamburini F, Pfahler V, Bünemann EK, Guelland K, Bernasconi SM, Frossard E (2012) Oxygen isotopes unravel the role of microorganisms in phosphate cycling in soils. Environ Sci Technol 46(11):5956–5962. https://doi.org/10.1021/es300311h
Tian J, Dong G, Karthikeyan R, Li L, Harmel RD (2017) Phosphorus dynamics in long-term flooded, drained, and reflooded soils. Water 9(7):531. https://doi.org/10.3390/w9070531
Tiessen H, Moir J (1993) Characterization of available P by sequential extraction. In: Carter RB (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, pp 75–86
Tsutsuki K, Ponnamperuma FN (1987) Behavior of anaerobic decomposition products in submerged soils effects of organic material amendment, soil properties, and temperature. Soil Sci Plant Nutr 33:13–33. https://doi.org/10.1080/00380768.1987.10557549
Turner BL, Haygarth PM (2001) Phosphorus solubilization in rewetted soils. Nat Brief Commun 411:258
Ubeynarayana N, Jeyakumar P, Bishop P, Pereira RC, Anderson CWN (2021) Effect of soil cadmium on root organic acid secretion by forage crops. Environ Pollut 268:115839. https://doi.org/10.1016/j.envpol.2020.115839
van Eck GTM (1982) Forms of phosphorus in particulate matter from the Hollands Diep/Haringvliet, The Netherlands. Hydrobiologia 91:665–681. https://doi.org/10.1007/BF00000066
Vitharana UWA, Kumaragamage D, Balasooriya BLWK, Indraratne SP, Goltz D (2021) Phosphorus mobilization in unamended and magnesium sulfate-amended soil monoliths under simulated snowmelt flooding. Environ Pollut 287:117619. https://doi.org/10.1016/j.envpol.2021.117619
Vogel C, Rivard C, Tanabe I, Adam C (2016) Microspectroscopy–promising techniques to characterize phosphorus in soil. Commun Soil Sci Plant Anal 47:2088–2102. https://doi.org/10.1080/00103624.2016.1228942
Volf MR, Crusciol CAC, de Azevedo AC, Thompson ML, Kovar JL, Rosolem CA (2021) Potassium bioavailability in a tropical kaolinitic soil. Agron 11(10). https://doi.org/10.3390/agronomy11102016
Watts CJ (2000) The effect of organic matter on sedimentary phosphorus release in an Australian reservoir. Hydrobiologia 431:13–25. https://doi.org/10.1023/A:1004046103679
Weil RR, Holah SS (1989) Effect of submergence on availability of certain plant nutrients in three Ultisol catenas. Plant Soil 114:147–157. https://doi.org/10.1007/BF02220793
Weyers E, Strawn DG, Peak D, Moore AD, Baker LL, Cade-Menun B (2016) Phosphorus speciation in calcareous soils following annual dairy manure amendments. Soil Sci Soc Am J 80:1531–1542. https://doi.org/10.2136/sssaj2016.09.0280
Wickramasinghe WMADB, Sirisena DN, Bandara WMJ, Wijewardena JDH (2009) Response of rice to application of phosphorus in Sri Lankan soils. In:Kumaragamage D, Satyanarayana T, Harmandeep-Singh, Majumdar K (Eds). Use of phosphorus and potassium fertilizers in Sri Lankan agriculture. 197–200. International Plant Nutrition Institute (IPNI) Gurgaon Haryana India.
Wimalawansa SA, Wimalawansa SJ (2015) Protection of watersheds, and control and responsible use of fertiliser to prevent phosphate eutrophication of reservoirs. Int J Res Environ Sci 1(2):1–18
Wu YH, Prietzel J, Zhou J, Bing HJ, Luo J, Yu D, Sun SQ, Liang JH, Sun HY (2014) Soil phosphorus bioavailability assessed by XANES and Hedley sequential fractionation technique in a glacier foreland chronosequence in Gongga Mountain, Southwestern China. Sci China Earth Sci 57:1860–1868. https://doi.org/10.1007/s11430-013-4741-z
Xu G, Sun J, Shao H, Chang SX (2014) Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol Eng 62:54–60. https://doi.org/10.1016/j.ecoleng.2013.10.027
Yang X, Post WM (2011) Phosphorus transformations as a function of pedogenesis: a synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 8:2907–2916. https://doi.org/10.5194/bg-8-2907-2011
Yang M, Yang H (2021) Utilization of soil residual phosphorus and internal reuse of phosphorus by crops. PeerJ 9:e11704. https://doi.org/10.7717/peerj.11704
Zaker M, Emami H (2019) Effect of potassium to bivalent cations ratio in irrigation water on some physical and hydraulic properties of sandy loam soil. Soil Environ 38(1):66–74. https://doi.org/10.25252/SE/19/71752
Zhang Y, Lin X, Werner W (2003) The effect of soil flooding on the transformation of Fe oxides and the adsorption/desorption behavior of phosphate. J Plant Nutr Soil Sc 166:68–75. https://doi.org/10.1002/jpln.200390014
Zhang Y, Lin X, Werner W (2004) Effects of aerobic conditions in the rhizosphere of rice on the dynamics and availability of phosphorus in a flooded soil - a model experiment. J Plant Nutr 167:66–71. https://doi.org/10.1002/jpln.200320349
Zhang S, Yang X, Hsu L, Liu Y, Wang S, White JR, Shaheen SM, Chen Q, Rinklebe J (2021) Soil acidification enhances the mobilization of phosphorus under anoxic conditions in an agricultural soil: investigating the potential for loss of phosphorus to water and the associated environmental risk. Sci Total Environ 793:148531. https://doi.org/10.1016/j.scitotenv.2021.148531
Zhao Y, Li Y, Yang F (2021) Critical review on soil phosphorus migration and transformation under freezing-thawing cycles and typical regulatory measurements. Sci Total Environ 751:141614. https://doi.org/10.1016/j.scitotenv.2020.141614
Funding
The authors are thankful for financial support from the World Bank funded AHEAD Scholarship (AHEAD/PhD/R3/Agri/336) of Sri Lanka and the Kathleen Spragg Agricultural Research Award, New Zealand, for the PhD study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palihakkara, J., Burkitt, L., Jeyakumar, P. et al. Exploring Phosphorus Dynamics in Submerged Soils and Its Implications on the Inconsistent Rice Yield Response to Added Inorganic Phosphorus Fertilisers in Paddy Soils in Sri Lanka. J Soil Sci Plant Nutr 24, 1–20 (2024). https://doi.org/10.1007/s42729-023-01553-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01553-4