Abstract
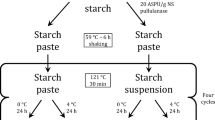
Acid phosphatase (specific activity 1.82 U/mg) from wheat germ was immobilized by entrapment in agarose gel. Blocks of 5 × 5 mm dimension were used for characterization. The optimum percent immobilization was 75.25% when agarose–enzyme mixture concentration was 1.35%. The soluble and immobilized acid phosphatase exhibited emission maxima at 335 nm, respectively, when excitation wavelength was 280 nm. The intensity of immobilized enzyme was lower than corresponding soluble enzyme. Fourier Transform Infrared spectrum exhibited bands at wave numbers 3282.2 and 1635.9 cm−1 for immobilized enzyme. The optimum pH and temperature for immobilized acid phosphatase were 5.5 and 60 °C, respectively. The Km values for soluble and immobilized acid phosphatase were 0.068 and 0.232 mM, respectively. The Vmax values for soluble and immobilized acid phosphatase were 1.92 and 2.34 µmol/min/mg protein, respectively. The immobilized enzyme retained 48% activity on 56th day when stored at 4 °C. The same enzyme blocks could be reused up to 8 cycles. The method of immobilization reported here is simple and cost effective and will be suitable for further applications.






Similar content being viewed by others
Abbreviations
- APase:
-
Acid phosphatase
- FTIR:
-
Fourier transform infrared
- p-NPP:
-
p-Nitrophenylphosphate
References
Allen SH, Nuttleman PR, Ketcham CM, Roberts RM (1989) Purification and characterization of human bone tartrate-resistant acid phosphatase. J Bone Miner Res 4:47–55
Almeida AFd, Terrasan CRF, Terrone CC, Tauk-Tornisielo SM, Carmona EC (2018) Biochemical properties of free and immobilized Candida viswanathii lipase on octyl-agarose support. Hydrolysis of triacylglycerol and soy lecithin. Process Biochem 65:71–80
Ambasht PK, Kayastha AM (1999) Plant phosphoenolpyruvate phosphatase: a review. Physiol Mol Biol Plants 5:1–6
Bagal D, Karve MS (2006) Entrapment of plant invertase within novel composite of agarose-guar gum biopolymer membrane. Anal Chim Acta 555:316–321
Belho K, Nonpiur SR, Ambasht PK (2014) Immobilization of acid phosphatase (Type I) from wheat germ on glutaraldehyde activated chitosan beads: optimization and characterization. J Proteins Proteom 4:177–183
Belho K, Nonpiur SR, Ambasht PK (2016) Purification and partial characterization of phytase from rice bean (Vigna umbellata Thunb.) germinated seeds. J Plant Biochem Biotechnol 25:327–330
Biswas TK, Cundiff C (1991) Multiple forms of acid phosphatases in germinating seeds of Vigna sinensis. Phytochemistry 41:1457–1458
Bolivar JM, Wilson L, Ferrarotti SA, Guisan JM, Fernandez-Lafuente R, Mateo C (2006) Improvement of the stability of alcohol dehydrogenase by covalent immobilization on glyoxyl-agarose. J Biotechnol 125:85–94
Bolivar JM, Lopez-Gallego F, Godoy C, Rodrigues DS, Rodrigues RC, Batalla P, Rocha-Martin J, Mateo C, Giordano RLC, Guisan JM (2009) The presence of thiolated compounds allows the immobilization of enzymes on glyoxyl-agarose at mild pH values: new strategies of stabilization by multipoint covalent attachment. Enzyme Microb Technol 45:477–483
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bull H, Murray PG, Thomas D, Fraser AM, Nelson PN (2002) Acid phosphatase. J Clin Pathol Mol Pathol 55:65–72
Chang M-Y, Juang R-S (2004) Stability and catalytic kinetics of acid phosphatase immobilized on composite beads of chitosan and activated clay. Process Biochem 39:1087–1091
Chang M-Y, Juang R-S (2007) Stability and reactivity of acid phosphatase immobilized on composite beads of chitosan and ZrO2 powders. Int J Biol Macromol 40:224–231
Dixon M, Webb EC, Thorne CJR, Tipton KF (1979) Enzymes, 3rd edn. Longman Group Ltd., London
dos Santos JCS, Rueda N, Torres R, Barbosa O, Goncalves LRB, Fernandez-Lafuente R (2015) Evaluation of divinylsulfone activated agarose to immobilize lipase and to tune their catalytic properties. Process Biochem 50:918–927
Duff SMG, Sarath G, Plaxton WC (1994) The role of acid phosphatases in plant phosphorus metabolism. Physiol Plant 90:791–800
Dutta S, Christena LR, Rajaram YRS (2013) Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3:1–9
Elnashar MMM (2010) Review article: immobilized molecules using biomaterials and nanobiotechnology. J Biomater Nanobiotechnol 1:61–77
Fernandez-Lopez L, Rueda N, Bartolome-Cabrero R, Rodriguez MD, Albuquerque TL, dos Santos JCS, Barbosa O, Fernandez-Lafunte R (2016) Improved immobilization and stabilization of lipase from Rhizomucor miehei on octyl-glyoxyl agarose beads by using CaCl2. Process Biochem 51:48–52
Ferreira CV, Granjeiro JM, Taga EM, Aoyama H (1998) Purification and characterization of multiple forms of soybean seed acid phosphatases. Plant Physiol Biochem 36:487–494
Gellatly KS, Moorhead GBG, Duff SMG, Lefebvre DD, Plaxton WC (1994) Purification and characterization of potato tuber acid phosphatase having significant phosphotyrosine phosphatase activity. Plant Physiol 106:223–232
Goldstein L (1976) Kinetic behavior of immobilized enzyme systems. Methods Enzymol 44:397–443
Granjeiro PA, Cavagis ADM, Leite LDC, Ferreira CV, Granjeiro JM, Aoyama H (2004) The thermal stability of castor bean acid phosphatase. Mol Cell Biochem 266:11–15
Guerrero C, Vera C, Serna N, Illanes A (2017) Immobilization of Aspergillus oryzae β-galactosidase in an agarose matrix functionalized by four different methods and application to the synthesis of lactulose. Bioresour Technol 232:53–63
Homma T, Kondo M, Kuwahara T, Shimomura M (2014) Bio- and bioelectro-catalytic properties of polyaniline/poly (acrylic acid) composite films bearing covalently-immobilized acid phosphatase. React Funct Polym 74:31–36
Huang Q, Liang W, Cai P (2005) Adsorption, desorption and activities of acid phosphatase on various colloidal particles from an Ultisol. Colloid Surf B 45:209–214
Hussain WM, Feder D, Schenk G, Guddat LW, McGeary RP (2018) Purple acid phosphatase inhibitors as leads for osteoporosis chemotherapeutics. Eur J Med Chem 157:462–479
Juang R-S, Wu F-C, Tseng R-L (2001) Solute adsorption and enzyme immobilization on chitosan beads prepared from shrimp shell wastes. Bioresour Technol 80:187–193
Ketchman CM, Baumbach GA, Bazer FW, Roberts RM (1985) The type 5 acid phosphatase from spleen of humans with hairy cell leukemia. J Biol Chem 260:5768–5776
Klabunde T, Strater N, Frohlich R, Witzel H, Krebs B (1996) Mechanism of Fe(III)–Zn (II) purple acid phosphatase based on crystal structures. J Mol Biol 259:737–748
Kurita K, Yoshino H, Nishimura S-I, Ishii S, Mori T, Nishiyama Y (1977) Mercapto-chitins: a new type of supports for effective immobilization of acid phosphatase. Carbohydr Polym 32:171–175
Pedroche J, Yust MDM, Mateo C, Fernandez-Lafunte R, Giron-Calle J, Alaiz M, Vioque J, Guisan JM, Millan F (2007) Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: correlation between enzyme-support linkages and thermal stability. Enzyme Microb Technol 40:1160–1166
Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47:185–214
Poonkuzhali K, Palvannan T (2013) Comparison of biopolymers for immobilization of laccase: eco-toxicity assessment of azo dye. Indian J Biotechnol 12:395–401
Prakash O, Jaiswal N (2011) Immobilization of thermostable α-amylase on the agarose and agar matrices and its application in starch stain removal. World Appl Sci J 13:572–577
Reddy KRC, Kayastha AM (2006) Improved stability of urease upon coupling to alkylamine and arylamine glass and its analytical use. J Mol Catal B Enzym 38:104–112
Schenk G, Ge Y, Carrington LE, Wynne CJ, Searle IR, Caroll BJ, Hamilton S, de Jersey J (1999) Binuclear metal centres in plant purple acid phosphatases: Fe-Mn in sweet potato and Fe-Zn in soybean. Arch Biochem Biophys 370:183–189
Schenk G, Mitic N, Hanson GR, Combo P (2013) Purple acid phosphatase: a journey into the function and mechanism of a colourful enzyme. Coord Chem Rev 257:473–482
Sheffield DJ, Harry TR, Smith AJ, Rogers LJ (1995) Corallina officinalis bromoperoxidase immobilized on agarose. Phytochemistry 38:1103–1107
Siar E-H, Zaak H, Kornecki JF, Zidoune MN, Barbosa O, Fernandez-Lafunte R (2017) Stabilization of ficin extract by immobilization on glyoxyl-agarose. Preliminary characterization of the biocatalyst performance in hydrolysis of proteins. Process Biochem 58:98–104
Srivastava PK, Anand A (2014) Immobilization of acid phosphatase from Vigna aconitifolia seeds on chitosan beads and its characterization. Int J Biol Macromol 64:150–154
Tardioli PW, Vieira MF, Vieira AMS, Zanin SM, Betancor L, Mateo C, Fernandez-Lorente G, Guisan JM (2011) Immobilization–stabilization of glucoamylase: chemical modification of the enzyme surface followed by covalent attachment on highly activated glyoxyl-agarose supports. Process Biochem 46:409–412
Vincent JB, Crowder MW, Averill BA (1992) Hydrolysis of phosphate monoesters: a biological problem with multiple chemical solutions. Trends Biochem Sci 17:105–110
Wang R, Cai Q, Tong D, Nie L, Yao S (1998) Enzymatic analysis of acid phosphatase and microanalysis of Cu++ and Ag+ with a SAW-impedence sensor. Enzyme Microb Technol 22:36–43
Yamato S, Kawakami N, Shimada K, Ono M, Idei N, Itoh Y (2000) Preparation and characterization of immobilized acid phosphatase used for an enzyme reactor: evaluation in flow-injection analysis and pre column liquid chromatography. Anal Chem Acta 406:191–199
Zaak H, Siar E-H, Kornecki JF, Fernandez-Lopez L, Pedrero SG, Virgen-Ortiz JJ, Fernandez-Lafuente R (2017) Effect of immobilization rate and enzyme crowding on enzyme stability under different conditions. The case of lipase from Thermomyces lanuginosus immobilized on octyl agarose beads. Process Biochem 56:117–123
Zhu J, Huang Q, Pigna M, Violante A (2010) Immobilization of acid phosphatase on uncalcined and calcined Mg/Al-CO3 layered double hydroxides. Colloid Surf B 77:166–173
Acknowledgements
The research facilities provided in the Department through UGC DRS III and DBT NER is acknowledged. Ms. Tutu Kalita is grateful to UGC for research fellowship in the form of JRF and SRF. The authors are grateful to Head, Department of Chemistry, NEHU, for providing FTIR facility and Prof. Ghanshyam Bez, Department of Chemistry, NEHU for useful discussions.
Author information
Authors and Affiliations
Contributions
TK conducted the experiments and was involved in writing the manuscript. PKA was the mentor, conceived the ideas, wrote discussion and shaped the overall manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalita, T., Ambasht, P.K. Immobilization and characterization of acid phosphatase from wheat germ (Type I) in agarose gel. J Proteins Proteom 10, 291–297 (2019). https://doi.org/10.1007/s42485-019-00023-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42485-019-00023-9