Abstract
Electrochemical biosensors fabricated based on nucleic acids have shown great potential for cancer recognition because of their low cost, fast feedback, high sensitivity, and easy operation. This review will demonstrate the impression of recent advances and applications of electrochemical biosensors that are nucleic acid-based for cancer detection. We compare electrochemical biosensors formulated on nucleic acids with those formed on antibodies and highlight some examples of electrochemical biosensors developed on nucleic acids for cancer detection, such as biosensors that use DNA or RNA aptamers to detect prostate-specific antigens, microRNA-21, or carcinoembryonic antigens. We discuss the rewards and drawbacks of these biosensors and the challenges they face, such as stability, reproducibility, interference, and standardization. We also suggest some possible directions and opportunities for future research and development, such as developing novel nucleic acid recognition elements, exploring new transducer materials and configurations, designing new signal amplification strategies, integrating electrochemical biosensors with microfluidic devices or portable instruments, and evaluating electrochemical biosensors in clinical settings with actual samples from cancer patients or healthy donors. Overall, we believe that electrochemical biosensors that are nucleic acid-based offer an auspicious alternative to conventional methods for cancer detection and have great potential to contribute to early diagnosis and effective cancer treatment.
Graphical Abstract

Article Highlights
-
Reviewing impression of recent advances in cancer detection.
-
Surveying applications of electrochemical cancer genosensors.
-
Investigating the role of nucleic acids in cancer detection.
-
Comparing electrochemical genosensors formulated on nucleic acids.
-
Showing impacts of genosensors compared to immunosensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is a leading worldwide problem that distresses millions worldwide and causes significant morbidity and mortality [1,2,3,4,5,6]. According to the information provided by (WHO), cancer is still included among the main reasons for death, known to an estimated 10 million deaths in 2020 [7,8,9,10]. Despite the advances in conventional therapies, such as surgery, chemotherapy, and radiotherapy, many cancers remain challenging to treat and cure based on their heterogeneity, resistance, and metastasis [11,12,13,14,15]. Therefore, a crucial need is to advance novel and effective cancer diagnosis and therapy strategies [16,17,18,19]. One possible approach to treat cancer is to use molecular recognition elements that can selectively attach to target molecules associated with cancer cells, such as receptors, antigens, or biomarkers [20,21,22,23,24,25,26,27].
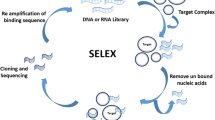
These elements can be used for various purposes, such as detecting cancer cells, delivering anticancer agents, inhibiting cancer cell growth, or modulating immune responses [28,29,30]. Among the different types of molecular recognition elements, antibodies and aptamers have attracted considerable attention due to their high precision for their targets [31,32,33,34]. Antibodies are proteins from the immune system that recognize and bind to a specific antigen. They have been widely used as diagnostic and therapeutic tools in cancer treatment, alone or in combination with other modalities. For example, antibodies can be used to detect cancer cells by immunohistochemistry or flow cytometry, to deliver anticancer agents by antibody–drug conjugates or immunotoxins [35,36,37], to inhibit cancer cell growth by blocking growth factors or oncoproteins, or to modulate immune responses by activating or suppressing immune checkpoints [38]. However, antibodies also have limitations, such as high cost, complex production, immunogenicity, toxicity, instability, and difficulty penetrating tissues and tumors. DNA or RNA molecules that can adopt exceptional three-dimensional structures and attach to a particular target molecule with high accuracy are called aptamers [39,40,41,42]. An in vitro selection method called (SELEX) is used to create them, which does not involve animals or cell cultures [43,44,45,46]. Aptamers have many advantages over antibodies: they are inexpensive, simple to make, low/no immunogenic, stable, easy to modify, and small. These characteristics make aptamers appropriate for various purposes in cancer diagnosis and therapy [47,48,49].
The detection and diagnosis of cancer pose a significant problem in contemporary medicine due to the importance of early and precise identification of cancer biomarkers, which can greatly imp1rove patient prognosis and survival [50]. Various methods for identifying cancer biomarkers have been created, such as optical, electrochemical, and magnetic biosensors [51]. Electrochemical biosensors have garnered significant attention due to their exceptional sensitivity, affordability, portability, and user-friendliness [51]. Nevertheless, there are still limitations and barriers in the process of creating and producing electrochemical biosensors [52]. These include the challenge of choosing suitable recognition elements, implementing effective signal amplification methods, and utilizing nanomaterials [53]. Hence, it is necessary to conduct a comprehensive analysis of the present achievements and trends in electrochemical biosensors used for detecting cancer biomarkers. Additionally, it is important to provide valuable perspectives and potential areas for future research and advancement [50].
Multiple compelling and significant literatures, such as Superparamagnetic nanoarchitectures for disease-specific biomarker detection, have already been published about this subject [54]. The topics covered are the cellular response to DNA damage that blocks transcription, the impact of component properties on the performance of tandem solar cells made of monolithic perovskite and organic materials, and the latest developments in electrochemical biosensors for detecting cancer biomarkers. These publications encompass a wide range of topics related to electrochemical biosensors, including the synthesis and characterization of nanomaterials, the design and optimization of signal transduction and amplification mechanisms, the integration and application of biosensors in clinical settings, and the comparison and evaluation of biosensors with other methods [55]. These literature sources, however, concentrate either on specific categories of cancer biomarkers, such as nucleic acids, proteins, or cells, or on certain types of nanomaterials, such as metal, carbon, or metal–organic frameworks [55, 56]. Therefore, there is a need for a thorough and organized examination of the existing literature on electrochemical biosensors for detecting cancer biomarkers. This review should encompass the fundamental principles, current advancements, and future prospects of these biosensors. Additionally, it should include a critical evaluation and comparison of various biosensor platforms, nanomaterials, and detection techniques [56]. This literature study would be valuable for researchers, clinicians, and engineers who have an interest in electrochemical biosensors and cancer detection. This literature review aims to provide a comprehensive and systematic overview of the recent advances in nanogenosensors based on nucleic acids for bioelectrochemical cancer detection [55]. Nanogenosensors are a novel class of biosensors that combine the advantages of nanomaterials and nucleic acids to achieve high sensitivity, selectivity, stability, and versatility for the detection of various cancer biomarkers. The review covers the basic principles, design strategies, fabrication methods, and performance evaluation of nanogenosensors, as well as the challenges and future perspectives in this field [57]. The review also compares and contrasts different types of nanomaterials and nucleic acids that can be used to construct nanogenosensors, such as metal nanoparticles, carbon nanomaterials, metal–organic frameworks, DNA, RNA, and aptamers [57]. The review highlights the potential applications of nanogenosensors for the early diagnosis, prognosis, and therapy monitoring of cancer, as well as the integration of nanogenosensors with other technologies, such as microfluidics, paper-based devices, and wearable devices [58]. The review is expected to provide valuable insights and guidance for researchers, clinicians, and engineers who are interested in the development and application of nanogenosensors for bioelectrochemical cancer detection [57].
Figure 1 depicts a schematic representation of the nanomaterials frequently used to create bioelectrochemical genosensors to detect various tumor biomarkers [28]. Genosensor design has increasingly used nanomaterials in recent years due to their distinctive properties, such as large surface area, improved reactivity, and adjustable surface chemistry [29]. The figure highlights various nanomaterials, such as gold nanoparticles, carbon nanotubes, and graphene oxide, commonly used to construct genosensors [30]. These nanomaterials can be functionalized with nucleic acid probes, antibodies, or aptamers to enable target biomolecules' specific recognition and binding. The resulting signal can be transformed into an electrochemical or optical output that can be quantified and correlated with the presence and concentration of the tumor biomarker in the sample [31].
Schematic presentation of the nanomaterials commonly exploited to establish bioelectrochemical genosensors for detecting various tumor biomarkers [59]
This review will cover the fresh developments and applications of nucleic acids-based electrochemical biosensors for cancer detection. Electrochemical biosensors are devices that can identify specific biomolecules related to diseases such as cancer by using the electrical properties of nucleic acids. Electrochemical biosensors can observe the current, voltage, or impedance changes resulting from nucleic acids connecting to their targets. We will compare electrochemical biosensors that are nucleic acid-based with those based on antibodies regarding their benefits and drawbacks. We will also show some examples of electrochemical biosensors nucleic acids-based for cancer detection, such as biosensors that use DNA or RNA aptamers to detect prostate-specific antigen (PSA), microRNA-21 (miR-21) or carcinoembryonic antigen (CEA) [41, 60].
2 Nucleic acids as recognition elements
Nucleic acids carry genetic information and can be recognition elements that bind to target molecules. Nucleic acids can be classified into two types: DNA and RNA. DNA is made of four nucleotides ATCG [61]. RNA is the combination of four nucleotides: uracil (U), cytosine (c), guanine (G), and adenine (A) [62]. DNA and RNA can form single/double-stranded structures with the complementary base coupling between A and T (or U) and C and G. Nucleic acids can be used as recognition elements that connect to target molecules by folding into exceptional three-dimensional assemblies that match the shape and charge of the target molecule. The sequence and length of the nucleic acid strand determine these structures. Nucleic acids can attach to various target molecules, like proteins, peptides, small molecules, metal ions, or other nucleic acids. Nucleic acids can be produced by an in vitro selection procedure called SELEX [63, 64]. SELEX is a method that identifies nucleic acid sequences that connect to an explicit target molecule from a large amount of accidental sequences. The basic steps of SELEX are:
-
Synthesis of a large amount of random nucleic acid sequences (usually 10^13 to 10^15 different sequences) with a fixed length (usually 20 to 80 nucleotides) and flanked by two constant regions that allow amplification (PCR) [65, 66].
-
Incubation of the amount with the target molecule under specific conditions (such as temperature, pH, salt concentration, etc.) [67].
-
Separating the bound nucleic acid sequences from the unbound ones by various methods (such as affinity chromatography, magnetic separation, filter binding, etc.) [68].
-
Elution of the bound nucleic acid sequences from the target molecule by changing the conditions (such as temperature, pH, salt concentration, etc.) [64, 69, 70].
-
Amplification of the eluted nucleic acid sequences by PCR to cause a new quantity for the following selection round [71,72,73].
-
Repetition of the selection, separation, elution, and amplification steps for several rounds (usually 5–20 rounds) until a pool of nucleic acid sequences for the target molecule is obtained [74,75,76]. The final pool of selected nucleic acid sequences can be cloned and sequenced to identify the individual sequences that bind to the target molecule. These sequences are called aptamers. DNAzyme is another type of nucleic acid that can be used as a recognition element. DNAzyme is a DNA sequence that can catalyze a specific chemical reaction, such as cleavage or ligation of another nucleic acid strand. DNAzymes can be created by SELEX or rational design. DNAzymes can attach to exact target molecules, like metal ions or other nucleic acids, and catalyze upon binding [77,78,79,80].
Integrating nanostructures greatly enhances the stability, selectivity, and sensitivity of electrochemical sensors for nucleic acids [81]. Nanostructures, such as nanoparticles, nanowires, nanotubes, and nanosheets, possess high surface area, conductivity, and catalytic activity, which makes them well-suited for immobilizing and identifying nucleic acids [82]. Published works contain numerous instances of nucleic acid electrochemical sensors that incorporate nanostructures. Some examples include:
-
Iron oxide nanoparticles with a mesoporous structure are designed to mimic the behavior of peroxidase enzymes. These nanoparticles can detect DNA methylation on a global level using colorimetric and electrochemical methods [82].
-
Nanoporous superparamagnetic nanocubes containing gold are utilized for the purpose of detecting miRNA through catalytic signal amplification [81].
-
An optically stable and transparent electrode made of crystalline SiC-on-glass enables simultaneous imaging of nucleic acids and electrochemical characterization [52].
-
A biosensor made of nanostructured mesoporous gold for detecting microRNA at the atomic level [54, 83, 84].
-
A highly sensitive mesoporous gold chronocoulometric sensor, modified with carrageenan gel, is employed for the detection of microRNAs with exceptional sensitivity [52, 56].
-
Transparent crystalline cubic SiC electrodes on glass are utilized for optical microscopy and nucleic acid electrochemistry [52, 85].
-
Nanoporous films composed of a gold-silver alloy are utilized for the detection of microRNA with high sensitivity without the need for amplification [83].
These examples illustrate the flexibility and potential of integrating nanostructures into electrochemical nucleic acid sensors [57, 83]. The addition of nanostructures can improve the performance of sensors in several ways, such as expanding the electrode area, improving electron transfer, increasing the signal-to-noise ratio, and enabling multifunctional capabilities [54, 81]. Nanostructure integration enables the creation of miniaturized, portable, and cost-effective nucleic acid sensors that are ideal for point-of-care applications [52, 53]. Their discussion revolves around diverse nucleic acids and nanostructures that can be employed for constructing electrochemical sensors, a topic that is both pertinent and captivating [83, 86]. The contributions made to nanogenosensors have been significant, with the authors of these works providing valuable critique and proposing novel avenues for the field [57, 81].
In contrast, these publications tend to focus specifically on certain nanomaterials, such as metals, carbon, or metal–organic frameworks, or on specific cancer biomarkers, such as nucleic acids, proteins, or cells [51, 52]. Therefore, there is a need for a comprehensive and well-structured analysis of existing literature [55, 81]. This review should examine and differentiate between different biosensor platforms, nanomaterials, and detection methods. Additionally, it should encompass the basic principles, current status, and future potential of electrochemical biosensors for the detection of cancer biomarkers [52, 86]. This literature review would be advantageous for individuals interested in utilizing electrochemical biosensors for cancer diagnosis.
3 Electrochemical nucleic acids-based genosensing
Biosensors and microdevices have attracted much attention in biomarker detection [87,88,89,90]. Electrochemical genosensors are devices that can identify specific biomolecules associated with diseases such as cancer by using the electrical characteristics of nucleic acids [91,92,93]. Electrochemical genosensors have three main components: a recognition section, a transducer, and a signal amplifier. The recognition part is the component that attaches to the sensing biomolecule with high specificity and affinity. The recognition element can be either an antibody or a nucleic acid. This review will concentrate on electrochemical genosensors nucleic acids-based as recognition elements. The transducer is the component that transforms the connection event between the recognition element and the target into an electrical signal that can be quantified [94,95,96]. The transducer can be either an electrode or an impedance sensor. An electrode is a conductive material that can produce or measure an electric current when connected to a power source or a voltmeter. An impedance sensor is a device that can measure the resistance or capacitance of an electric circuit when an alternating current is applied. The signal amplifier is the component that enhances the electrical signal generated by the transducer to advance the sensitivity and accuracy of the sensor. The signal amplifier can be either a chemical or a physical method. A chemical method involves adding molecules or nanoparticles to increase the current or impedance change caused by the binding event. A physical method involves modifying the surface or structure of the transducer to increase its surface area or conductivity. Electrochemical genosensors, nucleic acids-based, can measure the current, voltage, or impedance changes that occur when nucleic acids interact with their targets.
Different mechanisms, such as direct electron transfer, redox reaction, charge transfer, hybridization, conformational change, catalysis, or displacement, can cause these changes [97, 98]. Electrochemical genosensors based on nucleic acids have advantages such as low cost, high sensitivity, fast response, and easy operation. However, they also face challenges such as stability, reproducibility, interference, and standardization. Therefore, further research and development are needed to improve their performance and applicability. Figure 2 presents a scheme that showcases different approaches to creating bioelectrochemical genosensors for detecting miR-21, a microRNA biomarker with clinical significance in various cancers. The figure includes a range of techniques utilized for this purpose, such as electrochemical impedance spectroscopy, surface plasmon resonance, and electrochemiluminescence, among others [99,100,101]. These methods enable the sensitive and selective detection of miR-21 in biological samples.
3.1 Examples of electrochemical genosensing nucleic acids-based for cancer detection
Electroanalytical methods have recently achieved significant progress in detecting and measuring cancer biomarkers through electrochemical DNA genosensors. These genosensors depend on the hybridization events between a DNA or PNA capture probe and the target nucleic acid sequence for detection, with the hybridization event causing a noticeable change in the electrochemical signal. These genosensors have shown high sensitivity and specificity, making them a desirable option for early cancer detection [104, 105]. In particular, several studies have developed electrochemical genosensors based on nucleic acids for biomarker detection in breast cancer. For example, Wang et al. (2018) technologically advanced a label-free electrochemical genosensors that employs DNAzyme to detect the bladder cancer biomarker miRNA-21, demonstrating high selectivity against other miRNAs [106,107,108,109]. Likewise, Liu et al. (2017) reported on an electrochemical genosensors that utilizes the hybridization chain reaction (HCR) to detect the breast cancer biomarker miRNA-21, exhibiting high specificity against other miRNAs. These studies showcase the potential of electrochemical genosensors based on nucleic acids for detecting cancer biomarkers [106,107,108, 110].
Figure 3 illustrates two related aspects of a metal–organic framework (MOF) for detecting multiple cancer markers at the same time. The first aspect (I) is a simplified illustration of the preparation process of the MOF, which involves modifying the framework with nucleic acids to increase its affinity for target biomolecules. The nucleic acid-biofunctionalized MOF can then be used as a capture agent to isolate and enrich cancer markers from complex biological samples. The MOF-based approach offers several benefits, such as high specificity, selectivity, and sensitivity, making it a promising cancer diagnosis and monitoring platform. The second aspect (II) of the figure is a MOF-assisted bioelectrochemical sensor that enables the simultaneous detection of several cancer markers in a single assay. The MOF-based sensor uses the unique properties of the framework to amplify the electrochemical signal generated by the captured cancer markers. The resulting signal can be transduced and quantified, providing quantitative information on the amount of each cancer marker in the sample. The MOF-assisted bioelectrochemical sensor offers several benefits, including high sensitivity, fast response, and low detection limits.
-
a.
Peptide-based genosensing for cancer detection
I Scheme of the nucleic acid-biofunctionalized metal–organic framework preparation process and II the MOF-assisted bioelectrochemical sensor for simultaneous detection of multiple cancer markers [111]
Peptide-based bioelectrochemical sensors are promising for identifying various disease biomarkers, especially cancer. Peptides are short chains of amino acids that can attach to precise targets. Peptides can be obtained by screening and optimizing artificial peptide libraries or by mimicking natural peptides that interact with cancer cells or enzymes [112,113,114]. Peptides can be used as recognition elements on the surface of electrodes, where they produce an electrical signal upon binding to the target. Different techniques like amperometry, voltammetry, or impedance spectroscopy can quantify the electrical signal. Peptide-based bioelectrochemical sensors have several advantages over other biosensors, such as antibodies or aptamers [115]. Peptides are more stable, cheaper, easier to synthesize and modify, and less immunogenic than antibodies. Peptides also have higher specificity, sensitivity, and versatility than aptamers, which are prone to degradation and interference by nucleases and serum proteins 2. Peptides can be designed to recognize various cancer biomarkers, such as proteins, enzymes, hormones, metabolites, or DNA mutations. Peptides can also be modified with different functional groups, such as nanoparticles, polymers, or redox mediators, to enhance the sensors' sensitivity, selectivity, and biocompatibility [113, 114, 116, 117].
Some examples of peptide-based bioelectrochemical sensors for cancer detection are: A sensor based on a peptide that mimics the binding site of (VEGF), a protein that stimulates angiogenesis in tumors. The sensor exhibited high sensitivity and selectivity for VEGF in serum samples from breast cancer patients 3. A sensor based on a peptide that attaches to matrix metalloproteinase-9 (MMP-9), an enzyme that breaks down the extracellular matrix and enables tumor invasion and metastasis [118, 119]. The sensor measured MMP-9 in serum samples from colorectal cancer patients with high accuracy. A sensor based on a peptide that recognizes the (EGFR), an overexpressed protein in many types of cancer and a target for several anticancer drugs. The sensor distinguished between wild-type and mutant EGFR in cell lysates from lung cancer cell lines. Peptide-based bioelectrochemical sensors are promising for identifying various disease biomarkers, especially cancer. They offer high sensitivity, selectivity, stability, and versatility for the diagnosis and monitoring of cancer. However, limitations still need to be addressed, such as the optimization of the peptide sequences and structures, the improvement of the electrode materials and architectures, the reduction of the nonspecific adsorption and interference, and the validation of the sensors in clinical settings. Peptide-based bioelectrochemical sensors are promising for identifying various disease biomarkers, especially cancer. Peptides are short chains of amino acids that can connect to explicit targets. Peptides can be obtained by screening and optimizing artificial peptide libraries or by mimicking natural peptides that interact with cancer cells or enzymes. Peptides can be used as recognition elements on the surface of electrodes, where they generate an electrical signal upon binding to the target. Different techniques like amperometry, voltammetry, or impedance spectroscopy can measure the electrical signal [120,121,122,123].
Peptide-based bioelectrochemical sensors have several advantages over other biosensors, such as antibodies or aptamers. Peptides are more stable, cheaper, easier to synthesize and modify, and less immunogenic than antibodies. Peptides also have higher specificity, sensitivity, and versatility than aptamers, which are prone to degradation and interference by nucleases and serum proteins 2. Peptides can be designed to recognize various cancer biomarkers, such as proteins, enzymes, hormones, metabolites, or DNA mutations. Peptides can also be modified with different functional groups, such as nanoparticles, polymers, or redox mediators, to enhance the sensors' sensitivity, selectivity, and biocompatibility [124,125,126,127].
Some examples of peptide-based bioelectrochemical sensors for cancer detection are: A sensor based on a peptide that mimics the binding site of (VEGF), a protein that stimulates angiogenesis in tumors. The sensor exhibited high sensitivity and selectivity for VEGF in serum samples from breast cancer cases. A sensor based on a peptide that attaches to matrix metalloproteinase-9 (MMP-9), an enzyme that breaks down the extracellular matrix and enables tumor invasion and metastasis. The sensor measured MMP-9 in serum samples from colorectal cancer patients with high accuracy. A sensor based on a peptide that recognizes the (EGFR), an overexpressed protein in many types of cancer and a target for several anticancer drugs [118, 119, 128]. The sensor distinguished between wild-type and mutant EGFR in cell lysates from lung cancer cell lines. Peptide-based bioelectrochemical sensors are not only useful for diagnosing cancer but also for monitoring the treatment response and the prognosis of the patients. For example, a sensor based on a peptide that binds to the (PSA), a biomarker for prostate cancer, was able to quantify the changes in PSA levels after hormone therapy in prostate cancer patients [39, 63, 129,130,131]. Another sensor based on a peptide that binds to carcinoembryonic antigen (CEA), a biomarker for colorectal cancer, was able to track the CEA levels in serum samples from colorectal cancer cases before and after surgery. However, some limitations still need to be addressed for the development and application of peptide-based bioelectrochemical sensors for cancer detection [114, 121, 132, 133]. Some of these challenges are The optimization of the peptide sequences and structures to achieve high accuracy for the target and low toxicity and immunogenicity for the host [134,135,136,137]. The improvement of the electrode materials and architectures to increase the surface area, electrical conductivity, biocompatibility, and stability of the sensors. The reduction of the nonspecific adsorption and interference by other biomolecules or substances present in complex biological samples, such as serum or urine [138,139,140]. The validation of the sensors in clinical settings with large numbers of samples from different sources and conditions. The prospects of peptide-based bioelectrochemical sensors for cancer detection are bright and promising. With the advances in peptide synthesis and modification techniques, nanomaterials science, electrochemical methods, and bioinformatics tools, more novel and efficient peptide-based bioelectrochemical sensors will be designed and fabricated for various cancer biomarkers [121, 141,142,143]. These sensors will provide fast, accurate, sensitive, selective, portable, and low-cost tools for cancer patients' early diagnosis, treatment monitoring, and prognosis evaluation [144,145,146,147].
-
b.
Aptamer-based Genosensing for cancer detection
Aptamer-based bioelectrochemical sensors are another emerging technology for identifying various disease biomarkers, especially cancer. Aptamers are single-stranded DNA or RNA molecules that have the ability to fold into precise three-dimensional shapes and bind to target molecules with exceptional accuracy. Aptamers could be selected from large combinatorial libraries by (SELEX). Aptamers offer numerous advantages compared to conventional recognition agents, such as antibodies or peptides, such as greater stability, reproducibility, modifiability, and versatility compared to antibodies [39, 63, 129]. Aptamers also have higher thermal stability, lower immunogenicity, and easier regeneration than peptides [39, 63]. Figure 4 The SELEX process has undergone evolutionary changes.
-
(a)
The initial systematic evolution of ligands by exponential enrichment (SELEX) process:
the original SELEX process (a) and the significant advancements made in the last two decades (b). The methods for each part of SELEX where changes were proposed are displayed in (b). The methods focused on (b) demonstrate notable alterations to traditional SELEX in three aspects. The order is as follows: Partition (C), Elution and Amplification (D), Target Type (B), and Library Design (A) [148]
The initial SELEX (Systematic Evolution of Ligands by Exponential Enrichment) procedure is illustrated in Fig. 4a, demonstrating the fundamental stages of identifying aptamer sequences [148].
-
(b)
Technological progress in the past two decades:
Figure 4b demonstrates notable progressions in the SELEX process during the past twenty years, emphasizing alterations in critical elements [148]. Significant modifications to the conventional SELEX method:
3.1.1 Type of target
The text outlines innovations in strategies for selecting target types, highlighting adaptations that expand the range of molecular targets that can be addressed using SELEX [148].
3.1.2 Divide into separate parts
Techniques for dividing, which is a crucial stage in the SELEX procedure, have developed to enhance the segregation and extraction of aptamers bound to the desired target [148].
3.1.3 Elution and amplification
The paper showcases advancements in elution and amplification techniques, which lead to enhanced retrieval and amplification of aptamers bound to their targets [148].
These advancements collectively signify a significant change in SELEX methodology, offering greater accuracy and flexibility in aptamer discovery [148].
Aptamer-based bioelectrochemical sensors use aptamers as recognition elements on the surface of electrodes, where they generate an electrical signal upon binding to the target. Different techniques like amperometry, voltammetry, or impedance spectroscopy can measure the electrical signal. Aptamer-based bioelectrochemical sensors can achieve high sensitivity, selectivity, and rapidity for detecting various cancer biomarkers, such as proteins, nucleic acids, cells, or tissues. Aptamers can also be coupled with different signal amplification strategies, such as nanomaterials, enzymes, or catalytic DNAzymes, to enhance the performance of the sensors [129].
Some examples of aptamer-based bioelectrochemical sensors for cancer detection are: A sensor based on an aptamer that attaches to (VEGF), a protein that stimulates angiogenesis in tumors. The sensor used a gold electrode modified with gold nanoparticles and (HRP) as signal amplifiers. The sensor identified VEGF in serum samples from breast cancer patients with high sensitivity and selectivity. A sensor based on an aptamer that recognizes (HER2), a protein that is overexpressed in many types of cancer and is a target for several anticancer drugs [149,150,151]. The sensor used a glassy carbon electrode modified with graphene oxide and hemin as signal amplifiers. The sensor distinguished between HER2-positive and HER2-negative breast cancer cells with high accuracy. A sensor based on an aptamer that binds to mucin-1 glycoprotein (MUC1), a biomarker for colorectal cancer [39, 151, 152]. The sensor used a gold electrode modified with gold nanorods and glucose oxidase (GOx) as signal amplifiers. The sensor measured MUC1 in serum samples from colorectal cancer patients with high sensitivity and selectivity.
Aptamer-based bioelectrochemical sensors are not only useful for diagnosing cancer but also for monitoring the treatment response and the prognosis of the patients. For example, a sensor based on an aptamer that binds to prostate-specific antigen (PSA), a biomarker for prostate cancer, was able to quantify the changes in PSA levels after chemotherapy in prostate cancer patients [153, 154]. A different sensor, utilizing an aptamer that specifically attaches to carcinoembryonic antigen (CEA), a substance used to detect colorectal cancer, successfully monitored the levels of CEA in blood samples taken from patients with colorectal cancer both before and during surgery. However, some challenges and limitations still need to be addressed for the development and application of aptamer-based bioelectrochemical sensors for cancer detection. Some of these challenges are The optimization of the aptamer sequences and structures to achieve high accuracy for the target and low toxicity and interference by other biomolecules or substances present in complex biological samples [39]. The improvement of the electrode materials and architectures to increase the surface area, electrical conductivity, biocompatibility, and stability of the sensors. Developing novel signal amplification strategies can enhance the sensors' sensitivity and dynamic range without compromising specificity and simplicity [138, 155,156,157].
The validation of the sensors in clinical settings with large numbers of samples from different sources and conditions. The prospects of aptamer-based bioelectrochemical sensors for cancer detection are bright and promising. With the advances in aptamer selection and modification techniques, nanomaterials science, electrochemical methods, and bioinformatics tools, more novel and efficient aptamer-based bioelectrochemical sensors will be designed and fabricated for various cancer biomarkers. These sensors will provide rapid, accurate, sensitive, selective, portable, and low-cost tools for cancer patients' early diagnosis, treatment monitoring, and prognosis evaluation [63, 158,159,160].
Figure 5 describes peptide nucleic acid-based biosensors that use electrical and chemical methods to detect multiple microRNAs from cancer cells in one assay [162]. The biosensors are designed to recognize specific RNA sequences using peptide nucleic acid probes on the electrode [163]. The probes match with the target microRNAs, causing a change in the electrical signal that can be measured and related to the existence and amount of the microRNAs in the sample [164]. The biosensors also use catalytic hairpin assembly multiplication, a technique that enables the amplification of the signal produced by the matching event, thereby enhancing the sensitivity and specificity of the detection [165]. The catalytic hairpin assembly multiplication is a self-templated reaction that creates multiple copies of the target RNA sequences, greatly increasing the electrical signal. The peptide nucleic acid-based biosensors that use electrical and chemical methods with catalytic hairpin assembly multiplication offer several benefits, including high sensitivity, specificity, and selectivity, making them a potential tool for cancer diagnosis and monitoring [166].
Peptide nucleic acid-mediated bioelectrochemical genosensor for detecting multiple-microRNAs [161]
Figure 6 demonstrates the contraction of the first- and second-generation methods for identifying DNA from human papillomavirus (HPV). First-generation method uses polymerase chain reaction (PCR) to multiply the DNA and two different DNA probes, which are marked with horseradish peroxidase (HRP) and digoxigenin (DIG), respectively. The multiplied DNA and probes are then paired, and the resulting complex is identified using an anti-DIG antibody and an electrical signal generated by hydroquinone (HQ) oxidation to benzoquinone (BQ) [168]. The second-generation method replaces PCR with loop-mediated multiplication (LAMP), which multiplies the DNA at a constant temperature and requires only a single DNA probe. The probe is marked with biotin, and a streptavidin-coated magnetic bead captures the multiplied DNA and probe. The captured complex is then identified using an electrical signal generated by the oxidation of HQ to BQ. The contrast between the two methods shows that the second-generation method offers several advantages, including a simplified protocol, reduced time and cost, and improved specificity and sensitivity [169].
2. Cancer biomarker detection has also been investigated using optical genosensors, including fluorescent and colorimetric sensors. These genosensors detect changes in the optical signal when the target biomolecule binds to the probe. Fluorescent sensors are especially attractive since they can detect multiple biomarker targets with multiple fluorescent probes and have enhanced target identification sensitivity through signal multiplication methods [171].
Wei et al. [172] reviewed recent advances in electrical and optical biosensors for identifying different cancer indicators. The review emphasizes the importance of biosensors in early cancer diagnosis and treatment. The authors outline the advantages and disadvantages of each biosensor type and provide examples of their clinical applications. The review concludes that using biosensors for cancer indicator identification is promising as an early diagnosis and treatment method [172].
3. The identification of disease-related biomolecules, including cancer indicators, has been made possible through graphene-based electrochemical biosensors. Graphene has a two-dimensional structure with exceptional properties, such as high surface area, high electrical conductivity, and outstanding biocompatibility, which make it an attractive material for biosensor development. These biosensors can be functionalized with antibodies, nucleic acids, enzymes, and other biomolecules to improve their selectivity and sensitivity [173]. The constituents of an electrochemical biosensor are illustrated in Fig. 7:
-
(A)
A diagram illustrating different types of labels and label-less styles:
The schematic diagram A illustrates the two types of electrochemical biosensors: labeled and label-free. B Several species exhibit redox properties. C An electrochemical signal generator utilizing enzyme or nano-enzyme catalysis [187, 188]; D Signals obtained from a multiplex electrochemical assay [189, 190]; and E demonstrate ratiometric electrochemical biosensor [170]
Graphical representations of both labeled and label-free electrochemical biosensors, illustrating their construction and operational mechanisms [170]. In this analysis, we examine different techniques for detection, placing particular emphasis on the ways in which labels can enhance sensitivity [170].
-
(B)
The graphical representation displays different redox species that are essential for electrochemical biosensors [170]. This section provides a comprehensive description of the types and characteristics of redox molecules utilized in electrochemical processes that are crucial for signal transduction [170].
-
(C)
provides a detailed explanation of the process involved in generating electrochemical signals, focusing specifically on the involvement of enzymes and nano-enzymes in catalyzing the reactions [170]. Here, we will examine the mechanisms by which these catalysts enhance the accuracy and precision of biosensor signals [170].
-
(D)
Electrochemical biosensors and multiplex electrochemical assays are used for signal presentation [170]. This section highlights the proficiency and adaptability of electrochemical biosensors by illustrating their ability to detect multiple analytes simultaneously and utilize ratio-based measurements [170].
Dey et al. (2021) reviewed recent advances in graphene-based electrical biosensors for identifying various disease-related biomolecules, highlighting their advantages, including high sensitivity and selectivity, and their potential for clinical applications. However, the authors also discuss the challenges associated with these biosensors, such as reproducibility and clinical translation [174].
In cancer identification, Li et al. (2020) developed an electrical biosensor that identifies pancreatic cancer by pairing a DNA probe with cancer-associated exosomes. The biosensor exhibited high specificity against exosomes from healthy individuals. Similarly, Zhang et al. (2018) developed an electrical biosensor for identifying lung cancer by pairing a DNA probe with circulating tumor DNA. The biosensor demonstrated high selectivity against plasma DNA from healthy individuals. These studies demonstrate the potential of graphene-based electrical biosensors for cancer indicator identification [175, 176]. Figure 8 illustrates the application of electrochemical biosensors in an organ-on-a-chip system. (A) The graphic depicts a transparent sensor chip that has many electrochemical sensors incorporated into the bottom of a tissue culture flask, resulting in a device known as a detecting cell culture flask. (B) Depiction of a thorough arrangement in which the multiorgan-on-a-chip platform is situated within specifically designed microfluidics. This configuration comprises tangible sensors and a potentiostat specifically engineered for quantifying electrochemical signals. (C) Diagram illustrating the incorporation of a microfluidic sensing system with organs-on-chip, facilitating the uninterrupted measurement of a particular biomarker.
-
(A)
Flask for Cell Culture Sensing:
Electrochemical biosensors are utilized for detecting tissue-level signals in an organ-on-a-chip system.(A) Electrochemical biosensors are utilized for detecting tissue-level signals in an organ-on-a-chip system. Illustration translucent sensor chip, housing an assortment of electrochemical sensors, is integrated into the base of a tissue culture flask to produce a sensing cell culture flask device. (B) The diagram illustrates a comprehensive setup where the multiorgan-on-a-chip platform is located inside a custom-made microfluidics. The setup also includes physical sensors and a potentiostat to measure electrochemical signals [184]. (C) A diagram illustrating the integration of a microfluidic sensing system with organs-on-chip to continuously monitor a specific biomarker [184].
Transparent sensor chip is embedded at the bottom of a tissue culture flask to provide a visual representation [170]. This setup integrates various electrochemical sensors, emphasizing the integration of sensing technology in the field of cell culture [170].
-
(B)
The glass-based system for in situ monitoring comprises the following components:
This illustration showcases an in-situ system featuring amperometric oxygen sensors, potentiometric pH sensors, and an interdigital electrode. In addition, the microfluidic system incorporates amperometric biosensors for glucose and lactate, which are positioned downstream [170]. The main focus is on simultaneously monitoring multiple parameters.
-
(C)
This schematic overview provides a comprehensive description of a muscle-on-a-chip system, elucidating its structure and operational capabilities [170]. This section of the article provides detailed information on the design and application of electrochemical biosensors within the framework of an organ-on-a-chip. The focus is specifically on muscle tissue [170].
-
(D)
A schematic depiction of a comprehensive system wherein the multiorgan-on-a-chip platform is contained within a compact incubator placed on a laboratory bench [170]. The diagram depicts a comprehensive arrangement for monitoring multiple organs by integrating physical sensors and a potentiostat to measure electrochemical signals [170].
The next alternative is the Microfluidic Sensing System with Organs-on-Chip.
This diagram illustrates the incorporation of organs-on-chip into a microfluidic sensing system to enable the continuous monitoring of a specific biomarker [170]. This section aims to emphasize the capacity of electrochemical biosensors to continuously and instantly assess specific biomarkers within the organ-on-a-chip platform [170].
Cancer biomarkers, being biological molecules, can serve as indicators for the presence or progression of cancer within the body [177]. Electrochemical techniques are frequently employed for the identification of cancer biomarkers due to their rapidity, sensitivity, affordability, and user-friendly nature [177, 178]. Below are several novel and established electrochemical methods for detecting cancer biomarkers:
-
Impedance spectroscopy (EIS), or electrochemical impedance spectroscopy (EIS), is a method used to measure the impedance of an electrochemical system [177]. This includes both resistance and reactance, taking into consideration the frequency of the system [178]. EIS can detect the shift in impedance when a biomarker binds to a receptor on the electrode’s surface [177]. Electrochemiluminescence immunoassay (EIS) has been employed for the detection of various cancer biomarkers, such as prostate-specific antigen (PSA), carcinoembryonic antigen (CEA), and microRNAs [179, 180].
-
An electrochemical enzyme-linked immunosorbent assay (ELISA) variant: This method synergistically combines the precision of antibody-antigen recognition with the heightened responsiveness of electrochemical detection to attain outstanding outcomes. Two antibodies are sandwiched together around the biomarker [177]. An antibody is fixed on the surface of the electrode, while the other antibody is tagged with an electroactive enzyme [179]. The enzyme facilitates the simultaneous oxidation or reduction of a substrate, resulting in the production of an electrical signal that is directly proportional to the biomarker's concentration [179]. Electrochemical ELISA has been utilized to detect breast cancer biomarkers, including human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR).
-
Electrochemical nanobiosensors employ nanomaterials, such as nanoparticles, nanotubes, nanowires, or nanosheets, to enhance the electrochemical capabilities of the device [177]. In addition, nanomaterials have the potential to enhance the immobilization and recognition of biomarkers, while also increasing the surface area, conductivity, catalytic activity, and biocompatibility of electrodes [179]. Electrochemical nanobiosensors have been created to identify biomarkers of lung cancer [177]. EGFR, KRAS gene mutations, and microRNAs are examples of biomarkers.
Recent advancements in electrochemical biosensor technologies hold promise for detecting cancer biomarkers. Electrochemical DNA biosensors, optical biosensors, and graphene-based electrochemical biosensors have all shown potential for early cancer diagnosis and treatment. These biosensors have high sensitivity and specificity, making them attractive for clinical use [173]. However, reproducibility and clinical translation must be addressed before widespread implementation in clinical settings [181]. Further research and development in this area could lead to the creation of susceptible and specific diagnostic tools for cancer detection [182]. Electrochemical Signal Amplification Strategies are depicted in Fig. 9.
This illustration includes a variety of techniques that can be used to improve electrochemical signal detection. The letter (A) is used to denote the electrochemical detection of ELISA. (B) This illustration demonstrates electrode modification with nanoparticles for electrochemical detection of cancer biomarkers. [170, 191, 193]
The electrochemical ELISA is a schematic diagram that illustrates the electrochemical signal amplification strategy that is based on the Electrochemical Enzyme-Linked Immunosorbent Assay (ELISA) [170]. This section provides an overview of the essential components and procedures that are involved in increasing the sensitivity of electrochemical biosensors by utilizing techniques that are based on ELISA [170].
3.1.4 Signal amplification utilizing RCA circuits
This is a presentation of the schematic diagram that illustrates the strategy for signal amplification that makes use of Rolling Circle Amplification (RCA) [170]. This section provides an in-depth explanation of the process of amplification of electrochemical signals through RCA, thereby shedding light on the molecular mechanisms that are utilized for signal improvements [170].
Table 1 in this table various techniques, lists the type of biomarkers that they examined, lists their limit of detections (and have the limit of detections be normalized for easier comparison between techniques), their costs, their turnaround times, whether they are quantitative or qualitative, a summary of their strengths or weaknesses summarized [183].
4 Conclusion and future perspectives
This review has discussed the recent advances and applications of nucleic acid-based electrochemical genosensors for cancer detection. We aimed to compare the various studies and demonstrate the potential of this technology for cancer detection. We have compared nucleic acid-based electrochemical genosensors with those based on antibodies, highlighting their respective advantages and disadvantages. Additionally, we have provided examples of nucleic acid-based electrochemical genosensors for cancer detection, including those that use DNA or RNA aptamers to detect prostate-specific antigens, microRNA-21, or carcinoembryonic antigens.
Our review indicates that nucleic acid-based electrochemical genosensors offer a promising alternative to traditional cancer detection methods such as ELISA or qRT-PCR. They have several advantages: low cost, high sensitivity, fast response, and easy operation. Furthermore, they have the potential to detect various types of cancer biomarkers.
However, nucleic acid-based electrochemical genosensors face challenges like stability, reproducibility, interference, and standardization. These challenges must be addressed to optimize the performance of these genosensors for clinical use. Despite these challenges, nucleic acid-based electrochemical genosensors have shown great potential as a tool for cancer detection and could lead to the advance of more specific, sensitive, and affordable diagnostic systems. Therefore, further research and development are needed to improve their performance and applicability. We believe there are several possible directions and opportunities for future research and development in this area. One option is to develop novel nucleic acid recognition elements with higher precision for cancer biomarkers, such as aptamers with modified bases, backbones, or DNAzymes with enhanced catalytic activity. Another option is to explore new transducer materials and configurations with improved electrical properties, such as carbon nanotubes, graphene, nanowires, or nanocomposites. A third option is to design new signal amplification strategies with higher sensitivity and lower background noise, such as rolling circle amplification, exonuclease-assisted amplification, or nanoparticle-based amplification. A fourth option is integrating electrochemical genosensors with microfluidic devices or portable instruments for point-of-care testing or in vivo monitoring of cancer biomarkers. Finally, it is essential to evaluate electrochemical genosensors in clinical settings with actual samples from cancer patients or healthy donors to validate their accuracy and reliability.
In conclusion, our review has provided a comprehensive and updated overview of the state of the art and prospects of electrical genosensors based on nucleic acids for cancer identification. We believe this technology has great potential to contribute to early diagnosis and effective cancer treatment. However, further research and development are needed to improve their performance, overcome their limitations, and translate them into clinical practice. We hope our review will stimulate more interest and innovation in this field and lead to new cancer identification and therapy breakthroughs.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jahankhani K, Ahangari F, Adcock IM, Mortaz E. Possible cancer-causing capacity of COVID-19: Is SARS-CoV-2 an oncogenic agent? Biochimie. 2023;213:130–8.
Rashid K, Ahmad A, Meerasa SS, Khan AQ, Wu X, Liang L, Cui Y, Liu T. Cancer stem cell-derived exosome-induced metastatic cancer: An orchestra within the tumor microenvironment. Biochimie. 2023;212:1–11.
Caiado H, Cancela ML, Conceição N. Assessment of MGP gene expression in cancer and contribution to prognosis. Biochimie. 2023;214:49–60.
du Plessis M, Fourie C, Stone W, Engelbrecht A-M. The impact of endocrine disrupting compounds and carcinogens in wastewater: Implications for breast cancer. Biochimie. 2023;209:103–15.
Arrigoni R, Ballini A, Santacroce L, Cantore S, Inchingolo A, Inchingolo F, Di Domenico M, Quagliuolo L, Boccellino M. Another look at dietary polyphenols: challenges in cancer prevention and treatment. Curr Med Chem. 2022;29(6):1061–82.
Ozkan E, Bakar-Ates F. Ferroptosis: a trusted ally in combating drug resistance in cancer. Curr Med Chem. 2022;29(1):41–55.
Organization WH. CureAll framework: WHO global initiative for childhood cancer: increasing access, advancing quality, saving lives. , Switzerland, Geneva: World Health Organization; 2021.
Dawood KM, Raslan MA, Abbas AA, Mohamed BE, Nafie MS. Novel bis-amide-based bis-thiazoles as anti-colorectal cancer agents through Bcl-2 inhibition: synthesis, in vitro, and in vivo studies. Anti-Cancer Agents Med Chem. 2023;23(3):328–45.
Ullah A, Ullah N, Nawaz T, Aziz T. Molecular mechanisms of Sanguinarine in cancer prevention and treatment. Anti-Cancer Agents Med Chem. 2023;23(7):765–78.
Sahebnasagh A, Saghafi F, Negintaji S, Hu T, Shabani-Borujeni M, Safdari M, Ghaleno HR, Miao L, Qi Y, Wang M. Nitric oxide and immune responses in cancer: searching for new therapeutic strategies. Curr Med Chem. 2022;29(9):1561–95.
Salehi, Saba, Seyed Morteza Naghib, Hamid Reza Garshasbi, Sadegh Ghorbanzadeh, Wei Zhang. Smart stimuli-responsive injectable gels and hydrogels for drug delivery and tissue engineering applications: A review. FBB. 11 (2023): 1104126
Merighi S, Borea PA, Varani K, Vincenzi F, Jacobson KA, Gessi S. A2A adenosine receptor antagonists in neurodegenerative diseases. Curr Med Chem. 2022;29(24):4138.
Ao C, Gao L, Yu L. Research progress in predicting DNA methylation modifications and the relation with human diseases. Curr Med Chem. 2022;29(5):822–36.
Cioni P, Gabellieri E, Campanini B, Bettati S, Raboni S. Use of exogenous enzymes in human therapy: approved drugs and potential applications. Curr Med Chem. 2022;29(3):411–52.
Lucafò M, De Biasi S, Curci D, Norbedo A, Stocco G, Decorti G. Extracellular vesicles as innovative tools for assessing adverse effects of immunosuppressant drugs. Curr Med Chem. 2022;29(20):3586–600.
Crulhas BP, Basso CR, Castro GR, Pedrosa VA. Detection of prostate cancer biomarker PCA3 by using aptasensors. Curr Med Chem. 2022;29(37):5895–902.
Piasek AM, Musolf P, Sobiepanek A. Aptamer-based advances in skin cancer research. Curr Med Chem. 2023;30(8):953–73.
Yadav N, Dahiya T, Chhillar AK, Rana JS, Saini HM. Nanotechnology in cancer diagnostics and therapeutics: a review. Curr Pharm Biotechnol. 2022;23(13):1556–68.
Pusta A, Tertis M, Graur F, Cristea C, Al-Hajjar N. Aptamers and new bioreceptors for the electrochemical detection of biomarkers expressed in hepatocellular carcinoma. Curr Med Chem. 2022;29(25):4363–90.
Wu C, Chen Z, Li C, Hao Y, Tang Y, Yuan Y, Chai L, Fan T, Yu J, Ma X. CRISPR-Cas12a-empowered electrochemical biosensor for rapid and ultrasensitive detection of SARS-CoV-2 delta variant. Nano-Micro Lett. 2022;14(1):159.
Ashrafizadeh M, Aghamiri S, Tan SC, Zarrabi A, Sharifi E, Rabiee N, Kadumudi FB, Pirouz AD, Delfi M, Byrappa K, Thakur VK, Sharath Kumar KS, Girish YR, Zandsalimi F, Zare EN, Orive G, Tay F, Hushmandi K, Kumar AP, Karaman C, Karimi-Maleh H, Mostafavi E, Makvandi P, Wang Y. Nanotechnological approaches in prostate cancer therapy: integration of engineering and biology. Nano Today. 2022;45:101532.
Jiang W, Zhang Z, Ye M, Pan S, Huang G, Chen T, Zhu X. Morphology-directed radiosensitization of MoSe2 nanoplatforms for promoting cervical cancer radiotherapy. Nano Today. 2022;46:101598.
Wang S, Cheng K, Chen K, Xu C, Ma P, Dang G, Yang Y, Lei Q, Huang H, Yu Y, Fang Y, Tang Q, Jiang N, Miao H, Liu F, Zhao X, Li N. Nanoparticle-based medicines in clinical cancer therapy. Nano Today. 2022;45:101512.
Yin B, Qin Q, Li Z, Wang Y, Liu X, Liu Y, Huan S, Zhang X, Song G. Tongue cancer tailored photosensitizers for NIR-II fluorescence imaging guided precise treatment. Nano Today. 2022;45:101550.
Zheng Z, Zhu S, Lv M, Gu Z, Hu H. Harnessing nanotechnology for cardiovascular disease applications—a comprehensive review based on bibliometric analysis. Nano Today. 2022;44:101453.
Sousa DA, Carneiro M, Ferreira D, Moreira FTC, Sales MGF, Rodrigues LR. Recent advances in the selection of cancer-specific aptamers for the development of biosensors. Curr Med Chem. 2022;29(37):5850–80.
Keramat A, Kadkhoda J, Farahzadi R, Fathi E, Davaran S. The potential of graphene oxide and reduced graphene oxide in diagnosis and treatment of cancer. Curr Med Chem. 2022;29(26):4529–46.
Akim AM, Safdar N, Yasmin A, Sung YY, Muhammad TST. Cancer and disease diagnosis-biosensor as potential diagnostic tool for biomarker detection. J Adv Pharma Technol Res. 2022;13(4):243.
Kantak M, Shende P. Nucleic acid-conjugated carbohydrate nanobiosensors: a multimodal tool for disease diagnosis. Curr Pharm Des. 2022;28(30):2461–77.
Gevariya D, Priya L, Mehta S, Patel V, Bhuva D, Panjwani D, Patel S, Ahlawat P, Dharamsi A, Patel A. Bio-functional mesoporous silica nanoparticles as nano-structured carriers in cancer theranostic review on recent advancements. Curr Drug Targets. 2023;24(12):934–44.
Mehrabadi S, Velayati M, Zafari N, Hassanian SM, Mobarhan MG, Ferns G, Khazaei M, Avan A. Growth-hormone-releasing hormone as a prognostic biomarker and therapeutic target in gastrointestinal cancer. Curr Cancer Drug Targets. 2023;23(5):346–53.
Giannakodimos I. Urinary miRNAs as diagnostic biomarkers for prostatic cancer: current dilemmas and novel potential role for a digital rectal examination. Curr Med Chem. 2022;29(25):4311–3.
Scandolara TB, Barreto Pires BR, Vacario B, de Amorim ISS, Siqueira PB, Serpeloni JM, Mencalha AL, Bonvicino CR, Panis C. An overview regarding pharmacogenomics and biomarkers discovery: focus on breast cancer. Curr Top Med Chem. 2022;22(20):1654–73.
Evmorfopoulos K, Vlachostergios PJ, Sountoulides P, Tzortzis V. Emerging biomarkers in the diagnosis and treatment of testicular tumors. Curr Cancer Drug Targets. 2023;23(11):858–67.
Li M, Mei S, Yang Y, Shen Y, Chen L. Strategies to mitigate the on-and off-target toxicities of recombinant immunotoxins: an antibody engineering perspective. Antib Ther. 2022;5(3):164–76.
Wang W, Khoury JD, Miranda RN, Jorgensen JL, Xu J, Loghavi S, Li S, Pemmaraju N, Nguyen T, Medeiros LJ. Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a ten-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm. Haematologica. 2021;106(4):1047.
Gaidano V, Tenace V, Santoro N, Varvello S, Cignetti A, Prato G, Saglio G, De Rosa G, Geuna M. A clinically applicable approach to the classification of B-cell non-Hodgkin lymphomas with flow cytometry and machine learning. Cancers. 2020;12(6):1684.
Sun L, Wang X, Saredy J, Yuan Z, Yang X, Wang H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020;37:101759.
Ștefan G, Hosu O, De Wael K, Lobo-Castañón MJ, Cristea C. Aptamers in biomedicine: selection strategies and recent advances. Electrochim Acta. 2021;376:137994.
Sinitsyna VV, Vetcher AA. Nucleic acid aptamers in nanotechnology. Biomedicines. 2022;10(5):1079.
Veenuttranon K, Kaewpradub K, Jeerapan I. Screen-printable functional nanomaterials for flexible and wearable single-enzyme-based energy-harvesting and self-powered biosensing devices. Nano-Micro Lett. 2023;15(1):85.
Sheng C, Wu B, Li L, Zhao Y. Merging DNA probes with nanotechnology for RNA imaging in vivo. Curr Anal Chem. 2022;18(6):622–9.
Qi S, Duan N, Khan IM, Dong X, Zhang Y, Wu S, Wang Z. Strategies to manipulate the performance of aptamers in SELEX, post-SELEX and microenvironment. Biotechnol Adv. 2022;55:107902.
Lyu C, Khan IM, Wang Z. Capture-SELEX for aptamer selection: a short review. Talanta. 2021;229:122274.
Kohlberger M, Gadermaier G. SELEX: Critical factors and optimization strategies for successful aptamer selection. Biotechnol Appl Biochem. 2022;69(5):1771–92.
Chatterjee B, Kalyani N, Anand A, Khan E, Das S, Bansal V, Kumar A, Sharma TK. GOLD SELEX: a novel SELEX approach for the development of high-affinity aptamers against small molecules without residual activity. Microchim Acta. 2020;187:1–13.
Li L, Xu S, Yan H, Li X, Yazd HS, Li X, Huang T, Cui C, Jiang J, Tan W. Nucleic acid aptamers for molecular diagnostics and therapeutics: advances and perspectives. Angew Chem Int Ed. 2021;60(5):2221–31.
Sargazi S, Simge E, Mobashar A, Gelen SS, Rahdar A, Ebrahimi N, Hosseinikhah SM, Bilal M, Kyzas GZ. Aptamer-conjugated carbon-based nanomaterials for cancer and bacteria theranostics: a review. Chem Biol Interact. 2022;361:109964.
Sekhon SS, Kaur P, Kim Y-H, Sekhon SS. 2D graphene oxide–aptamer conjugate materials for cancer diagnosis. npj 2D Mater Appl. 2021;5(1):21.
García-Mendiola T, Requena-Sanz S, Martínez-Periñán E, Bravo I, Pariente F, Lorenzo E. Influence of carbon nanodots on DNA-Thionine interaction. Application to breast cancer diagnosis. Electrochimica Acta. 2020;353:136522.
Gorgannezhad L, Umer M, Kamal Masud M, Hossain MSA, Tanaka S, Yamauchi Y, Salomon C, Kline R, Nguyen NT, Shiddiky MJ. Detection of FGFR2: FAM76A fusion gene in circulating tumor RNA based on catalytic signal amplification of graphene oxide-loaded magnetic nanoparticles. Electroanalysis. 2018;30(10):2293–301.
Hasan MR, Ahommed MS, Daizy M, Bacchu MS, Ali MR, Al-Mamun MR, Aly MAS, Khan M, Hossain SI. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens Bioelectron X. 2021;8:100075.
Islam MN, Gorgannezhad L, Masud MK, Tanaka S, Hossain MSA, Yamauchi Y, Nguyen NT, Shiddiky MJ. Graphene-oxide-loaded superparamagnetic iron oxide nanoparticles for ultrasensitive electrocatalytic detection of MicroRNA. ChemElectroChem. 2018;5(17):2488–95.
Masud MK, Na J, Lin T-E, Malgras V, Preet A, Sina AAI, Wood K, Billah M, Kim J, You J. Nanostructured mesoporous gold biosensor for microRNA detection at attomolar level. Biosens Bioelectron. 2020;168:112429.
Park H, Masud MK, Na J, Lim H, Phan H-P, Kaneti YV, Alothman AA, Salomon C, Nguyen N-T, Hossain MSA. Mesoporous gold–silver alloy films towards amplification-free ultra-sensitive microRNA detection. J Mater Chem B. 2020;8(41):9512–23.
Salahuddin B, Masud MK, Aziz S, Liu C-H, Amiralian N, Ashok A, Hossain SA, Park H, Wahab MA, Amin MA. κ-Carrageenan gel modified mesoporous gold chronocoulometric sensor for ultrasensitive detection of MicroRNA. Bull Chem Soc Jpn. 2022;95(1):198–207.
Masud MK, Islam MN, Haque MH, Tanaka S, Gopalan V, Alici G, Nguyen N-T, Lam AK, Hossain MSA, Yamauchi Y. Gold-loaded nanoporous superparamagnetic nanocubes for catalytic signal amplification in detecting miRNA. Chem Commun. 2017;53(58):8231–4.
Gutiérrez-Gálvez L, García-Mendiola T, Gutiérrez-Sánchez C, Guerrero-Esteban T, García-Diego C, Buendía I, García-Bermejo ML, Pariente F, Lorenzo E. Carbon nanodot–based electrogenerated chemiluminescence biosensor for miRNA-21 detection. Microchim Acta. 2021;188:1–12.
Chiorcea-Paquim A-M. Advances in electrochemical biosensor technologies for the detection of nucleic acid breast cancer biomarkers. Sensors. 2023. https://doi.org/10.3390/s23084128.
Zhao Z, Huang C, Huang Z, Lin F, He Q, Tao D, Jaffrezic-Renault N, Guo Z. Advancements in electrochemical biosensing for respiratory virus detection: A review. TrAC, Trends Anal Chem. 2021;139:116253.
Saran R, Huang Z, Liu J. Phosphorothioate nucleic acids for probing metal binding, biosensing and nanotechnology. Coord Chem Rev. 2021;428:213624.
Stricker M, Asim MN, Dengel A, Ahmed S. CircNet: an encoder–decoder-based convolution neural network (CNN) for circular RNA identification. Neural Comput Appl. 2021. https://doi.org/10.1007/s00521-020-05673-1.
Byun J. Recent progress and opportunities for nucleic acid aptamers. Life. 2021;11(3):193.
Liu R, Zhang F, Sang Y, Katouzian I, Jafari SM, Wang X, Li W, Wang J, Mohammadi Z. Screening, identification, and application of nucleic acid aptamers applied in food safety biosensing. Trends Food Sci Technol. 2022;123:355–75.
Pearson K, Doherty C, Zhang D, Becker NA, Maher LJ III. Optimized quantitative PCR analysis of random DNA aptamer libraries. Anal Biochem. 2022;650:114712.
Qian S, Chang D, He S, Li Y. Aptamers from random sequence space: accomplishments, gaps and future considerations. Anal Chim Acta. 2022;1196:339511.
Wang L, Liu Z-J, Cao H-X, Liang G-X. Ultrasensitive colorimetric miRNA detection based on magnetic 3D DNA walker and unmodified AuNPs. Sens Actuators, B Chem. 2021;337:129813.
Ning Y, Hu J, Lu F. Aptamers used for biosensors and targeted therapy. Biomed Pharmacother. 2020;132:110902.
Wang T, Chen L, Chikkanna A, Chen S, Brusius I, Sbuh N, Veedu RN. Development of nucleic acid aptamer-based lateral flow assays: a robust platform for cost-effective point-of-care diagnosis. Theranostics. 2021;11(11):5174.
Fei Z, Cheng C, Wei R, Tan G, Xiao P. Reversible superhydrophobicity unyielding magnetic beads of flipping-triggered (SYMBOL) regulate the binding and unbinding of nucleic acids for ultra-sensitive detection. Chem Eng J. 2022;431:133953.
Li J, Zhang Z, Gu J, Stacey HD, Ang JC, Capretta A, Filipe CD, Mossman KL, Balion C, Salena BJ. Diverse high-affinity DNA aptamers for wild-type and B. 1.1. 7 SARS-CoV-2 spike proteins from a pre-structured DNA library. Nucleic Acids Res. 2021;49(13):7267–79.
Yu CY, Chan KG, Yean CY, Ang GY. Nucleic acid-based diagnostic tests for the detection SARS-CoV-2: an update. Diagnostics. 2021;11(1):53.
Fukunaga K, Yokobayashi Y. Directed evolution of orthogonal RNA–RBP pairs through library-vs-library in vitro selection. Nucleic Acids Res. 2022;50(2):601–16.
Lee J, Ryu M, Bae D, Kim H-M, Eyun S-I, Bae J, Lee K. Development of DNA aptamers specific for small therapeutic peptides using a modified SELEX method. J Microbiol. 2022;60(7):659–67.
Quinodoz SA, Bhat P, Chovanec P, Jachowicz JW, Ollikainen N, Detmar E, Soehalim E, Guttman M. SPRITE: a genome-wide method for mapping higher-order 3D interactions in the nucleus using combinatorial split-and-pool barcoding. Nat Protoc. 2022;17(1):36–75.
Liu Y, Wang N, Chan C-W, Lu A, Yu Y, Zhang G, Ren K. The application of microfluidic technologies in aptamer selection. Front Cell Dev Biol. 2021;9:730035.
Micura R, Höbartner C. Fundamental studies of functional nucleic acids: aptamers, riboswitches, ribozymes and DNAzymes. Chem Soc Rev. 2020;49(20):7331–53.
Wang Q, Wang Z, He Y, Xiong B, Li Y, Wang F. Chemical and structural modification of RNA-cleaving DNAzymes for efficient biosensing and biomedical applications. TrAC Trends Anal Chem. 2022;159:116910.
Ma X, Ding W, Wang C, Wu H, Tian X, Lyu M, Wang S. DNAzyme biosensors for the detection of pathogenic bacteria. Sens Actuators, B Chem. 2021;331:129422.
Cao X, Chen C, Zhu Q. Biosensors based on functional nucleic acids and isothermal amplification techniques. Talanta. 2022;253:123977.
Masud MK, Na J, Younus M, Hossain MSA, Bando Y, Shiddiky MJ, Yamauchi Y. Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem Soc Rev. 2019;48(24):5717–51.
Masud MK, Umer M, Hossain MSA, Yamauchi Y, Nguyen N-T, Shiddiky MJ. Nanoarchitecture frameworks for electrochemical miRNA detection. Trends Biochem Sci. 2019;44(5):433–52.
Islam MN, Masud MK, Haque MH, Hossain MSA, Yamauchi Y, Nguyen N-T, Shiddiky MJA, Biomarkers RNA. Diagnostic and prognostic potentials and recent developments of electrochemical biosensors. Small Methods. 2017;1(7):1700131.
Islam MN, Masud MK, Nguyen N-T, Gopalan V, Alamri HR, Alothman ZA, Al Hossain MS, Yamauchi Y, Lamd AK, Shiddiky MJ. Gold-loaded nanoporous ferric oxide nanocubes for electrocatalytic detection of microRNA at attomolar level. Biosens Bioelectron. 2018;101:275–81.
Phan H-P, Masud MK, Vadivelu RK, Dinh T, Nguyen T-K, Ngo K, Dao DV, Shiddiky MJ, Hossain MSA, Yamauchi Y. Transparent crystalline cubic SiC-on-glass electrodes enable simultaneous electrochemistry and optical microscopy. Chem Commun. 2019;55(55):7978–81.
Bhattacharjee R, Tanaka S, Moriam S, Masud MK, Lin J, Alshehri SM, Ahamad T, Salunkhe RR, Nguyen N-T, Yamauchi Y. Porous nanozymes: the peroxidase-mimetic activity of mesoporous iron oxide for the colorimetric and electrochemical detection of global DNA methylation. J Mater Chem B. 2018;6(29):4783–91.
Naghib SM, Zare Y, Rhee KY. A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites. Nanotechnol Rev. 2020;9(1):53–60.
Naghib SM. Two-dimensional functionalised methacrylated graphene oxide nanosheets as simple and inexpensive electrodes for biosensing applications. Micro Nano Lett. 2019;14(4):462–5.
Naghib SM, Parnian E, Keshvari H, Omidinia E, Eshghan-Malek M. Synthesis, characterization and electrochemical evaluation of polyvinylalchol/graphene oxide/silver nanocomposites for glucose biosensing application. Int J Electrochem Sci. 2018;13(1):1013–26.
Sartipzadeh O, Naghib SM, Seyfoori A, Rahmanian M, Fateminia FS. Controllable size and form of droplets in microfluidic-assisted devices: Effects of channel geometry and fluid velocity on droplet size. Mater Sci Eng, C. 2020;109:110606.
Arafa KK, Ibrahim A, Mergawy R, El-Sherbiny IM, Febbraio F, Hassan RY. Advances in cancer diagnosis: Bio-electrochemical and biophysical characterizations of cancer cells. Micromachines. 2022;13(9):1401.
Salahandish R, Ghaffarinejad A, Omidinia E, Zargartalebi H, Majidzadeh-A K, Naghib SM, Sanati-Nezhad A. Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens Bioelectron. 2018;120:129–36.
Kazemi F, Naghib SM, Zare Y, Rhee KY. Biosensing applications of polyaniline (PANI)-based nanocomposites: a review. Polym Rev. 2021;61(3):553–97.
Mobed A, Hasanzadeh M. Sensitive recognition of Shiga toxin using biosensor technology: an efficient platform towards bioanalysis of pathogenic bacterial. Microchem J. 2022;172:106900.
Kumar D, Prasad M, Mohan H. Biological recognition elements, electrochemical sensors. Amsterdam: Elsevier; 2022. p. 213–39.
Shen Y, Xu L, Li Y. Biosensors for rapid detection of Salmonella in food: a review. Compr Rev Food Sci Food Saf. 2021;20(1):149–97.
Bhattarai P, Hameed S. Basics of biosensors and nanobiosensors, Nanobiosensors: From Design to Applications. Basics of Biosensors and Nanobiosensors, 2020; 1–22.
Xu C, Yang Y, Gao W. Skin-interfaced sensors in digital medicine: from materials to applications. Matter. 2020;2(6):1414–45.
Deiminiat B, Rounaghi GH. A novel visible light photoelectrochemical aptasensor for determination of bisphenol A based on surface plasmon resonance of gold nanoparticles activated g-C3N4 nanosheets. J Electroanal Chem. 2021;886:115122.
Ding L, Xu S, Huang Y, Yao Y, Wang Y, Chen L, Zeng Y, Li L, Lin Z, Guo L. Surface-enhanced electrochemiluminescence imaging for multiplexed immunoassays of cancer markers in exhaled breath condensates. Anal Chem. 2022;94(21):7492–9.
Mousavi SM, Hashemi SA, Kalashgrani MY, Rahmanian V, Gholami A, Chiang W-H, Lai CW. Biomedical applications of an ultra-sensitive surface plasmon resonance biosensor based on smart MXene quantum dots (SMQDs). Biosensors. 2022;12(9):743.
Yan C, Xu J, Yang L, Yao B, Liu G, Chen W. Target-triggered substantial stacking of electroactive indicators based on digestion-to-growth regulated tandem isothermal amplification for ultrasensitive miRNA determination. Sens Actuators, B Chem. 2021;344:130280.
Zouari M, Campuzano S, Pingarrón JM, Raouafi N. Femtomolar direct voltammetric determination of circulating miRNAs in sera of cancer patients using an enzymeless biosensor. Anal Chim Acta. 2020;1104:188–98.
Lu X, Cui M, Yi Q. Detection of mutant genes with different types of biosensor methods. TrAC Trends Anal Chem. 2020;126:115860.
Hashem A, Hossain MM, Marlinda AR, Mamun MA, Sagadevan S, Shahnavaz Z, Simarani K, Johan MR. Nucleic acid-based electrochemical biosensors for rapid clinical diagnosis: advances, challenges, and opportunities. Crit Rev Clin Lab Sci. 2022;59(3):156–77.
Miranda-Castro R, Palchetti I, De-Los-Santos-Álvarez N. The translational potential of electrochemical DNA-based liquid biopsy. Front Chem. 2020;8:143.
Low SS, Ji D, Chai WS, Liu J, Khoo KS, Salmanpour S, Karimi F, Deepanraj B, Show PL. Recent progress in nanomaterials modified electrochemical biosensors for the detection of MicroRNA. Micromachines. 2021;12(11):1409.
Zhang W, Xiao G, Chen J, Wang L, Hu Q, Wu J, Zhang W, Song M, Qiao J, Xu C. Electrochemical biosensors for measurement of colorectal cancer biomarkers. Anal Bioanal Chem. 2021;413:2407–28.
Sadighbathi S, Mobed A. Genosensors, a nanomaterial-based platform for microRNA-21 detection, non-invasive methods in early detection of cancer. Clin Chim Acta. 2022;530:27–38.
El Aamri M, Yammouri G, Mohammadi H, Amine A, Korri-Youssoufi H. Electrochemical biosensors for detection of microRNA as a cancer biomarker: pros and cons. Biosensors. 2020;10(11):186.
Chang J, Wang X, Wang J, Li H, Li F. Nucleic acid-functionalized metal-organic framework-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal Chem. 2019;91(5):3604–10.
Tertis M, Hosu O, Feier B, Cernat A, Florea A, Cristea C. Electrochemical peptide-based sensors for foodborne pathogens detection. Molecules. 2021;26(11):3200.
Er S, Laraib U, Arshad R, Sargazi S, Rahdar A, Pandey S, Thakur VK, Díez-Pascual AM. Amino acids, peptides, and proteins: implications for nanotechnological applications in biosensing and drug/gene delivery. Nanomaterials. 2021;11(11):3002.
Yuan L, Liu L. Peptide-based electrochemical biosensing. Sens Actuators B Chem. 2021;344:130232.
Vidic J, Manzano M. Electrochemical biosensors for rapid pathogen detection. Curr Opin Electrochem. 2021;29:100750.
Sargazi S, Fatima I, Kiani MH, Mohammadzadeh V, Arshad R, Bilal M, Rahdar A, Díez-Pascual AM, Behzadmehr R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: a comprehensive review. Int J Biol Macromol. 2022;206:115–47.
Sheikh A, Md S, Kesharwani P. RGD engineered dendrimer nanotherapeutic as an emerging targeted approach in cancer therapy. J Control Release. 2021;340:221–42.
Mondal J, An JM, Surwase SS, Chakraborty K, Sutradhar SC, Hwang J, Lee J, Lee Y-K. Carbon nanotube and its derived nanomaterials based high performance biosensing platform. Biosensors. 2022;12(9):731.
Shakil MS, Niloy MS, Mahmud KM, Kamal MA, Islam MA. Theranostic potentials of gold nanomaterials in hematological malignancies. Cancers. 2022;14(13):3047.
Kappen J, Skorupa M, Krukiewicz K. Conducting polymers as versatile tools for the electrochemical detection of cancer biomarkers. Biosensors. 2022;13(1):31.
Vanova V, Mitrevska K, Milosavljevic V, Hynek D, Richtera L, Adam V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens Bioelectron. 2021;180:113087.
Farshchi F, Hasanzadeh M. Microfluidic biosensing of circulating tumor cells (CTCs): recent progress and challenges in efficient diagnosis of cancer. Biomed Pharmacother. 2021;134:111153.
Bhakta S, Mishra P. Molecularly imprinted polymer-based sensors for cancer biomarker detection. Sens Actuators Rep. 2021;3:100061.
Wasilewski T, Gębicki J, Kamysz W. Bio-inspired approaches for explosives detection. TrAC, Trends Anal Chem. 2021;142:116330.
Awang MS, Bustami Y, Hamzah HH, Zambry NS, Najib MA, Khalid MF, Aziah I, Abd Manaf A. Advancement in salmonella detection methods: from conventional to electrochemical-based sensing detection. Biosensors. 2021;11(9):346.
Dahiya T, Yadav S, Yadav N, Mann A, Sharma M, Rana J. Monitoring of BNP cardiac biomarker with major emphasis on biosensing methods: a review. Sens Int. 2021;2:100103.
Sinha K, Das Mukhopadhyay C. Quantitative detection of neurotransmitter using aptamer: from diagnosis to therapeutics. J Biosci. 2020;45:1–12.
Song W, Song Y, Li Q, Fan C, Lan X, Jiang D. Advances in aptamer-based nuclear imaging. Eur J Nucl Med Mol Imaging. 2022;49(8):2544–59.
Guan B, Zhang X. Aptamers as versatile ligands for biomedical and pharmaceutical applications. Int J Nanomed. 2020;15:1059–71.
Liu S, Xu Y, Jiang X, Tan H, Ying B. Translation of aptamers toward clinical diagnosis and commercialization. Biosens Bioelectron. 2022;208:114168.
Zheng XT, Tan YN. Recent development of nucleic acid nanosensors to detect sequence-specific binding interactions: from metal ions, small molecules to proteins and pathogens. Sens Int. 2020;1:100034.
Ucar A, González-Fernández E, Staderini M, Avlonitis N, Murray AF, Bradley M, Mount AR. Miniaturisation of a peptide-based electrochemical protease activity sensor using platinum microelectrodes. Analyst. 2020;145(3):975–82.
Roy S, Dastidar MG, Sharma V, Kim BS, Rajak RC. Application of geno-sensors and nanoparticles in gene therapy: a new avenue for gene delivery, nanomaterials in clinical therapeutics: synthesis and applications. USA, New Jersey, Hoboken: Wiley; 2022. p. 177–204.
Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, Zheng Q, Li G, Teng Y, Wu M. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther. 2022;7(1):166.
Philippe GJ, Craik DJ, Henriques ST. Converting peptides into drugs targeting intracellular protein–protein interactions. Drug Discov Today. 2021;26(6):1521–31.
Ilangala AB, Lechanteur A, Fillet M, Piel G. Therapeutic peptides for chemotherapy: trends and challenges for advanced delivery systems. Eur J Pharm Biopharm. 2021;167:140–58.
Aiman S, Alhamhoom Y, Ali F, Rahman N, Rastrelli L, Khan A, Ahmed A, Khan A, Li C. Multi-epitope chimeric vaccine design against emerging Monkeypox virus via reverse vaccinology techniques-a bioinformatics and immunoinformatics approach. Front Immunol. 2022;13:985450.
Beaver K, Dantanarayana A, Minteer SD. Materials approaches for improving electrochemical sensor performance. J Phys Chem B. 2021;125(43):11820–34.
Liu S, Lai C, Liu X, Li B, Zhang C, Qin L, Huang D, Yi H, Zhang M, Li L. Metal-organic frameworks and their derivatives as signal amplification elements for electrochemical sensing. Coord Chem Rev. 2020;424:213520.
Herrmann A, Haag R, Schedler U. Hydrogels and their role in biosensing applications. Adv Healthcare Mater. 2021;10(11):2100062.
Kazemi Y, Dehghani S, Nosrati R, Taghdisi SM, Abnous K, Alibolandi M, Ramezani M. Recent progress in the early detection of cancer based on CD44 biomarker; nano-biosensing approaches. Life Sci. 2022;300:120593.
Pal K, Asthana N, Aljabali AA, Bhardwaj SK, Kralj S, Penkova A, Thomas S, Zaheer T, Gomes de Souza F. A critical review on multifunctional smart materials ‘nanographene’emerging avenue: nano-imaging and biosensor applications. Crit Rev Solid State Mater Sci. 2022;47(5):691–707.
Jiang Z, Feng B, Xu J, Qing T, Zhang P, Qing Z. Graphene biosensors for bacterial and viral pathogens. Biosens Bioelectron. 2020;166:112471.
Sohrabi H, Bolandi N, Hemmati A, Eyvazi S, Ghasemzadeh S, Baradaran B, Oroojalian F, Majidi MR, de la Guardia M, Mokhtarzadeh A. State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: a critical review. Microchem J. 2022;177:107248.
Mohammadi R, Naderi-Manesh H, Farzin L, Vaezi Z, Ayarri N, Samandari L, Shamsipur M. Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: a review. J Pharm Biomed Anal. 2022;212:114628.
Yang S-M, Lv S, Zhang W, Cui Y. Microfluidic point-of-care (POC) devices in early diagnosis: a review of opportunities and challenges. Sensors. 2022;22(4):1620.
Haleem A, Javaid M, Singh RP, Suman R, Rab S. Biosensors applications in medical field: a brief review. Sens Int. 2021;2:100100.
Aquino-Jarquin G, Toscano-Garibay JD. RNA aptamer evolution: two decades of SELEction. Int J Mol Sci. 2011;12(12):9155–71.
Varty K, O’Brien C, Ignaszak A. Breast cancer aptamers: current sensing targets, available aptamers, and their evaluation for clinical use in diagnostics. Cancers. 2021;13(16):3984.
Han L. Current trends in electrochemical aptasensor for tumor-associated growth factor receptor detection in serum. Int J Electrochem Sci. 2022;17(6):220673.
Pandey N, Biswas D, Dutta N, Hansda A, Dutta G, Mukherjee G. Sensing soluble immune checkpoint molecules and disease-relevant cytokines in cancer: a novel paradigm in disease diagnosis and monitoring. Front Sens. 2022;3:789771.
Lakhera P, Chaudhary V, Jha A, Singh R, Kush P, Kumar P. Recent developments and fabrication of the different electrochemical biosensors based on modified screen printed and glassy carbon electrodes for the early diagnosis of diverse breast cancer biomarkers. Mater Today Chem. 2022;26:101129.
Dejous C, Krishnan UM. Sensors for diagnosis of prostate cancer: looking beyond the prostate specific antigen. Biosens Bioelectron. 2021;173:112790.
Aidoo-Brown J, Moschou D, Estrela P. Multiplexed prostate cancer companion diagnostic devices. Sensors. 2021;21(15):5023.
Ates HC, Nguyen PQ, Gonzalez-Macia L, Morales-Narváez E, Güder F, Collins JJ, Dincer C. End-to-end design of wearable sensors. Nat Rev Mater. 2022;7(11):887–907.
Daniel M, Mathew G, Anpo M, Neppolian B. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: an overview. Coord Chem Rev. 2022;468:214627.
Numan A, Gill AA, Rafique S, Guduri M, Zhan Y, Maddiboyina B, Li L, Singh S, Dang NN. Rationally engineered nanosensors: a novel strategy for the detection of heavy metal ions in the environment. J Hazard Mater. 2021;409:124493.
Kordasht HK, Hasanzadeh M. Aptamer based recognition of cancer cells: recent progress and challenges in bioanalysis. Talanta. 2020;220:121436.
Shahbazi N, Zare-Dorabei R, Naghib SM. Multifunctional nanoparticles as optical biosensing probe for breast cancer detection: a review. Mater Sci Eng C. 2021;127:112249.
Anand U, Chandel AKS, Oleksak P, Mishra A, Krejcar O, Raval IH, Dey A, Kuca K. Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors. Appl Microbiol Biotechnol. 2022;106(8):2827–53.
Fu P, Xing S, Xu M, Zhao Y, Zhao C. Peptide nucleic acid-based electrochemical biosensor for simultaneous detection of multiple microRNAs from cancer cells with catalytic hairpin assembly amplification. Sens Actuators, B Chem. 2020;305:127545.
Hanoglu SB, Man E, Harmanci D, Tozan Ruzgar S, Sanli S, Keles NA, Ayden A, Tuna BG, Duzgun O, Ozkan OF. Magnetic nanoparticle-based electrochemical sensing platform using ferrocene-labelled peptide nucleic acid for the early diagnosis of colorectal cancer. Biosensors. 2022;12(9):736.
Moccia M, Antonacci A, Saviano M, Caratelli V, Arduini F, Scognamiglio V. Emerging technologies in the design of peptide nucleic acids (PNAs) based biosensors. TrAC, Trends Anal Chem. 2020;132:116062.
Song C-W, Bae SH, Bong KW, Han C-S. Label-free multiplex detection of miRNA-assayed hydrogel barcode using a low-aspect-ratio micropore sensor. Sens Actuators, B Chem. 2023;380:133376.
Fu S, Zhang T, Jiang H, Xu Y, Chen J, Zhang L, Su X. DNA nanotechnology enhanced single-molecule biosensing and imaging. TrAC, Trends Anal Chem. 2021;140:116267.
Kumar RR, Kumar A, Chuang C-H, Shaikh MO. Recent advances and emerging trends in cancer biomarker detection technologies. Ind Eng Chem Res. 2023;62(14):5691–713.
Bartosik M, Jirakova L. Electrochemical analysis of nucleic acids as potential cancer biomarkers. Curr Opin Electrochem. 2019;14:96–103.
Li M, Yin F, Song L, Mao X, Li F, Fan C, Zuo X, Xia Q. Nucleic acid tests for clinical translation. Chem Rev. 2021;121(17):10469–558.
Abolmaali SS, Alimardani V, Farahavar G, Najafi H, Shafiee M, Tanideh N, Tamaddon AM, Ahadian S. Nanotechnology-based approaches against COVID-19, emerging nanomaterials and nano-based drug delivery approaches to combat antimicrobial resistance. Amsterdam: Elsevier; 2022. p. 305–64.
Cui F, Zhou Z, Zhou HS. Measurement and analysis of cancer biomarkers based on electrochemical biosensors. J Electrochem Soc. 2019;167(3):037525.
Kumar S, Singh R. Recent optical sensing technologies for the detection of various biomolecules. Opt Laser Technol. 2021;134:106620.
Stanciu LA, Wei Q, Barui AK, Mohammad N. Recent advances in aptamer-based biosensors for global health applications. Annu Rev Biomed Eng. 2021;23:433–59.
Arshad F, Nabi F, Iqbal S, Khan RH. Applications of graphene-based electrochemical and optical biosensors in early detection of cancer biomarkers. Colloids Surf, B. 2022;212:112356.
Akgönüllü S, Denizli A. Recent advances in optical biosensing approaches for biomarkers detection. Biosens Bioelectron. 2022;12:100269.
Li S, Ma Q. Electrochemical nano-sensing interface for exosomes analysis and cancer diagnosis. Biosens Bioelectron. 2022;214:114554.
Makler A, Asghar W. Exosomal biomarkers for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn. 2020;20(4):387–400.
Alharthi SD, Bijukumar D, Prasad S, Khan AM, Mathew MT. Evolution in biosensors for cancers biomarkers detection: a review. J Bio Tribo Corros. 2021;7(2):42.
Dhanapala L, Krause CE, Jones AL, Rusling JF. Printed electrodes in microfluidic arrays for cancer biomarker protein detection. Biosensors. 2020;10(9):115.
Son MH, Park SW, Sagong HY, Jung YK. Recent advances in electrochemical and optical biosensors for cancer biomarker detection. BioChip J. 2023;17(1):44–67.
Gajdosova V, Lorencova L, Kasak P, Tkac J. Electrochemical nanobiosensors for detection of breast cancer biomarkers. Sensors. 2020;20(14):4022.
Keenan KE, Delfino JG, Jordanova KV, Poorman ME, Chirra P, Chaudhari AS, Baessler B, Winfield J, Viswanath SE, deSouza NM. Challenges in ensuring the generalizability of image quantitation methods for MRI. Med Phys. 2022;49(4):2820–35.
Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction—evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18(3):135–51.
Xiang Y, Hu C, Wu G, Xu S, Li Y. Nanomaterial-based microfluidic systems for cancer biomarker detection: recent applications and future perspectives. TrAC Trends Anal Chem. 2023;158:116835.
(2016) Aptamer-Based Microfluidic Electrochemical Biosensor for Monitoring Cell-Secreted Trace Cardiac Biomarkers Analytical Chemistry 88(20) 10019-10027 https://doi.org/10.1021/acs.analchem.6b02028.
(2016) Electrochemical chip-based genomagnetic assay for detection of high-risk human papillomavirus DNA Biosensors and Bioelectronics 83300-305 https://doi.org/10.1016/j.bios.2016.04.035.
(2018) Genomagnetic LAMP-based electrochemical test for determination of high-risk HPV16 and HPV18 in clinical samples Analytica Chimica Acta 104237-43 https://doi.org/10.1016/j.aca.2018.08.020.
Xiaoli, Zhu Jinghua, Yang Min, Liu Yao, Wu Zhongming, Shen Genxi, Li (2013) Sensitive detection of human breast cancer cells based on aptamer–cell–aptamer sandwich architecture Analytica Chimica Acta 76459-63 https://doi.org/10.1016/j.aca.2012.12.024.
(2009) Rapid Identification and Quantification of Tumor Cells Using an Electrocatalytic Method Based on Gold Nanoparticles Analytical Chemistry 81(24) 10268-10274 https://doi.org/10.1021/ac902087k.
Liyuan, Wang Feng, Feng Zhanfang, Ma (2015) Novel electrochemical redox-active species: one-step synthesis of polyaniline derivative-Au/Pd and its application for multiplexed immunoassay Abstract Scientific Reports 5(1) https://doi.org/10.1038/srep16855.
(2019) A new ratiometric electrochemical immunoassay for reliable detection of nuclear matrix protein 22 Analytica Chimica Acta 1086103-109 https://doi.org/10.1016/j.aca.2019.08.017.
(2017) A label-free ratiometric electrochemical DNA sensor for monitoring intracellular redox homeostasis Chemical Communications 53(46) 6215-6218 https://doi.org/10.1039/C7CC03239K.
Shin, S.R., Zhang, Y.S., Kim, D.J., Manbohi, A., Avci, H., Silvestri, A., Aleman, J., Hu, N., Kilic, T., Keung, W. and Righi, M., 2016. Aptamer-based microfluidic electrochemical biosensor for monitoring cell-secreted trace cardiac biomarkers. Analytical chemistry, 88(20), pp.10019-10027.
John, A., Benny, L., Cherian, A.R. et al. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: a review. J Nanostruct Chem 11, 1–31 (2021). https://doi.org/10.1007/s40097-020-00372-8.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
BMK collected the papers and wrote the draft. SMN edited and revised the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
None.
Consent for publication
None.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kangarshahi, B.M., Naghib, S.M. Nanogenosensors based on aptamers and peptides for bioelectrochemical cancer detection: an overview of recent advances in emerging materials and technologies. Discov Appl Sci 6, 47 (2024). https://doi.org/10.1007/s42452-024-05681-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05681-z