Abstract
The world's nations are searching for renewable and sustainable energy and fuels due to restrictions on carbon discharges and fossil feedstock shortage.. Biomass is a renewable and sustainable resource; and its conversion is one of the research hotspot areas. This review aims to summarize the evidence gained from different methods of converting lignocellulose materials using heterogeneous catalysts. The review summarizes heterogeneous catalysts like carbon-based sulfonated acids, polymeric acids, metal oxides, and solid and magnetic nature acids, including methods to improve functionality and recyclability. The paper also discusses the approaches for enhancing the efficiency of reactions between heterogeneous catalysts and lignocellulose substrates, like ball-milling, microwave irradiation, solid acid interaction, the effect of hydrogen bonding, and CH–pi (π) bond interaction techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Lignocellulose biomass
Using nonrenewable resources and the enormous amount of CO2 released into the air is a widely accepted reason for the key contributor to international warming. The worldwide energy emergency and the greenhouse influence are the two problems that have a great chance of resolving by using biomass [1]. An extremely rapid growth in the consumption of remnant feedstocks for the creation of energy, fuels, and substances in recent years has been the main reason for helping to increase the total amount of CO2 in the atmosphere and increasing the price of crude oil nowadays [2,3,4,5]. Mika and coworkers' study indicated that the projected progressive depletion of fossil fuel supplies has received much consideration in recent years. More research is being done to find alternatives for producing energy and chemicals to fulfill the world's increasing energy needs and address environmental anxieties. There are a number of solutions to replace non-renewable energy with alternative renewable energy resources, for instance, wind, hydro, nuclear, solar, and biomass. It would be preferable to select a source that can provide sufficient fundamental components like carbon, oxygen, and hydrogen to produce chemicals. Compared to conventional energy sources like coal, oil, and natural gas, lignocellulosic biomass (which provides sufficient fundamental components) usage is the primary alternative that has been considered to solve this problem due to its wide availability, reasonable price, and renewability [1, 3, 6, 7]. The term lignocellulose denotes plant dry matter which is biomass; the so-called "lignocellulosic biomass." lignocellulosic biomass is the most abundantly accessible raw material on earth. The conversion of lignocellulose has garnered particular interest in utilizing inedible sources [8]. Hence, lignocellulosic biomass has the probability to create biofuels and biochemicals, which might cut greenhouse gas (like CO2) emissions and enhance energy security while reducing our reliance on conventional fossil fuels [9, 10].
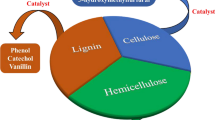
Chemical industries' reliance on fossil fuels could be reduced by using lignocellulose to fabricate chemicals and fuels. Cellulose, which is one of the most plentiful biopolymers in nature with a content of 40–50% in lignocellulose components (Fig. 1), is not a source of food for people, so using it will not take away from resources that may be used for food or other edible purposes. So, cellulose is used as a carbon–neutral feedstock since it recaptures the CO2 that chemicals produce at the end of their useful lives. Thus, using cellulose as a substitute feedstock will lower the request for remnant fuels and help mitigate the effects of climate change by CO2. Numerous catalytic systems, such as ionic liquids, aqueous media, biphasic media, and sub- and supercritical fluids, have been developed recently for the conversion of lignocellulose to more important simplest groups like cellulose degradation into glucose, 5-hydroxymethylfurfural [11], levulinic acid, 5-chloromethyl furfural, ethylene glycol, propylene glycol, and sugar alcohols [12]. The catalytic characteristics of the bimetallic catalysts were assessed employing photocatalytic oxidation of 5-hydroxymethylfurfural (5-HMF) to furandicarboxylic acid (FDCA), which was triggered by visible light. Materials with significant surface acidity and appropriate microstructure play vital roles as catalysts at diverse steps of biomass conversion to chemicals and fuels. Therefore, from the standpoint of green chemistry, the development of an effective, sustainable, and recyclable catalyst system is essential for the conversion of biomass [13]. The main focus of this review is to deliver a critical analysis of the advancements of heterogeneous solid catalysts in transforming lignocellulose into simple building block chemicals. Additionally, this review emphasizes the recyclability of the catalysts and their outlooks on strategy and manufacturing for upcoming studies.

Source and components of lignocellulose biomass [14]
1.2 Heterogeneous catalysts verses homogeneous catalysis
The dissimilarities between heterogeneous and homogeneous catalysts are that homogeneous catalysts appear identical to the reaction mixture (usually gas or liquid), but heterogeneous catalysts are regularly added to liquid or gaseous reaction mixtures as solid substances [15]. The various heterogeneous and homogeneous catalyst properties and effectivities are investigated by different researchers, as shown in Table 1 and 2.
2 Processing of lignocellulosic material by heterogeneous catalyst
2.1 Heterogeneous catalysts
Heterogeneous catalysts are more favorable for the change of lignocellulose into simple chemicals due to their simplicity of departure from the products, easy availability, and capacity to be recycled [33]. Different homogeneous catalysts, including some mineral acids like HCl and H2SO4 [34], acidic ionic liquids [35], heteropolyacids [36], and Lewis acids (e.g., Al, Zr, and Cr salts) [37] have been tried for degradation of lignocellulose. Despite homogeneous catalysts being highly efficient at catalyzing the conversion of lignocellulose, they need help with the corrosion of equipment, high separation costs, and environmental pollution [38]. Even though homogeneous catalysts are used extensively in industry, these systems have certain drawbacks that make the process more complex overall. These drawbacks include low stability, challenges in isolating and separating the final product from the reaction mixture, and recycling costly noble metals or ligands. Numerous heterogeneous catalysts, such as metal oxides, zeolites, carbonaceous acids, immobilized ionic liquids, hetero-poly acids, and acid resins have been investigated for the conversion of cellulose (an abundant component of lignocellulose) into simple platform chemicals [8].
A solid–solid (catalyst-cellulose) reaction occurs when the solid acid catalyzes the cellulose will break down. Subsequently, cellulose is water-insoluble; it is particularly challenging to hydrolyze it in micro- and mesoporous materials (such as zeolites) due to extra diffusion restrictions. The interaction of the catalyst particles with the cellulose particles greatly constrains the reaction rate. In order to ensure high reaction rates, it is desirable to build a unique catalytic system that allows for the regaining and recycling of the catalyst after the reaction [39]. Despite frequently requiring large catalyst loading and other unfavorable circumstances like microwave irradiation, some metal oxides can support Lewis and Bronsted acid sites [40]. Particularly excellent catalytic activity has been discovered for acid catalysts based on polystyrene. For instance, it has been reported that the Pan catalyst, a sulfonated chloromethyl polystyrene (CMP) polymer, can convert 93% cellulose to glucose. The idea behind this catalyst is that the sulfonic acid groups catalyze the breakage of the β- 1,4- glycosidic link while the chloride groups bind to cellulose in a custom alike to that of a cellulose enzyme [41].
Due to the containing of a hydrogen-bond network among the hydroxyl groups of cellulose that is made up of β-1,4- ether linkage of D-glucose, it has an extremely organized crystalline structure. It is, therefore, challenging to break down and become insoluble in typical solvents like water. As a result, finding methods for efficiently converting cellulose into fuels and useful platform chemicals is essential for developing sustainable energy [42]. There are three basic ways to transform cellulose into useful platform chemicals and fuels. These three processes have been used to hydrolyze cellulose: homogeneous hydrolysis, heterogeneous hydrolysis, and enzymatic hydrolysis. Additionally, numerous uses of various reaction combinations like hydrolysis-oxidation have been described in the alteration of cellulose. However because heterogeneous hydrolysis is easier to separate and recycle, has a wider selection of heterogeneous catalysts available, is the most environmentally friendly method, and has high hydrothermal stability, it is more important than homogeneous hydrolysis[43,44,45]. Regarding workable methods for producing glucose from sustainable biomass, heterogeneous catalysts may be an effective alternative. With them, cellulose can be almost entirely converted, with the exception of the remaining lignin components and the creation of humin as a byproduct. Additionally, a thorough examination of the solid residues and wasted catalysts could be crucial for the development of mechanistic processes. In order to improve efficiency and selectivity, researchers have therefore started studying biomass degradation and selective transformation utilizing heterogeneous catalysts that have been designed and significantly modified on the surface with acidic functional groups [13]. Promising options are meso/nanoporous materials with a consistent arrangement of pore sizes and modified pores with acidic functional groups (-SO3H, -COOH, etc.). Large organic molecules, such as polymeric cellulose and starch found in wood-biomass feedstocks, can be processed using aluminosilicate MCM-41 (Mobil Composition of Matter), which has pore diameters ranging from 2 to 10 nm. Mesoporous materials can be used; however, since these systems need to withstand the conversion of biomass in the aqueous phase at high temperatures, efforts have been made to improve the hydrothermal stability of these catalysts through stream treatment. This is done by adding noble and/or transition metals to increase the oxygen removal capacity and/or encourage decarboxylation reactions. Research has been done on the pyrolysis of wood biomass in the presence of zeolite H-ZSM-5, wherein the carbonium ion mechanism drives the acidic zeolite sites [46]. Lewis acid sites develop at higher temperatures, although Brønsted acid sites predominate in H-ZSM-5 activated at 500 ℃. Because of its large surface area and adjustable pore size, mesoporous silica has been considered the perfect support for heterogeneous catalysts [47].
2.1.1 Hydrolysis of cellulose by sulphonated activated carbon
The cellulose conversion profitability success depends particularly on the heterogeneous catalytic hydrolysis of cellulose [12]. Wang and coworkers characterized sulfonated activated carbon as having outstanding catalytic characteristics for the hydrolysis of cellulose. These researchers found that at moderate temperatures (150 °C) the sulfonated activated carbon catalyst produced 41% monosaccharide sugar (glucose) with 95% discriminating, whereas at higher temperatures, the glucose was degraded. To compare the sulfonated activated carbon, the zeolite catalysts (H-form), and the sulfate-contained catalysts were put to the test. The sulfates (SO3H), the functional group sites of the sulfonated activated carbon catalysts and the hydrophobic parts, accounted for the consequently larger yield of glucose than H-form zeolite catalysts [48]. As Pang and coworkers' study shows, there is a 75% increased amount of glucose output with 94% cellulose change into simple sugar on sulfonated carbon catalysts. Since cellulose is a source of numerous useful compounds; glucose is the end product sought in the cellulose conversion. By hydrolyzing β-1,4-glycosidic linkages in the presence of water using a solid acid catalyst to depolymerize cellulose, glucose is produced. Cellulose is first processed to yield soluble fragments of β-1,4-glucans, which are then further hydrolyzed to produce glucose as the main product in the presence of a solid catalyst (Fig. 2) [12].
Schematic process of cellulose conversion to sugar alcohols with heterogeneous catalyst followed by hydrolytic hydrogenation (adapted from [12])
When compared to other kinds of heterogeneous acids, carbon-based solid acids ensure the maximum level of catalytic action for the hydrolysis of cellulose. These types of solid heterogeneous acids are excellent prospects for synthesizing biofuel precursors from lignocellulose due to their higher capacity for recycling and naturally accessible raw materials. The incomplete carbonization of naturally occurring polymers like cellulose or starch and the incomplete carbonization of sulfate-containing polycyclic aromatic compounds in the presence of concentrated H2SO4 was the method of preparation of carbonaceous-based sulfonated acid catalysts [49]. Fukuhara and coworkers described the process for making carbon-based catalysts with -SO3H groups by using microcrystalline cellulose at 723 K above 5 h under inert gas (N2) flow. After filtering and rinsing with hot H2O, carbonaceous acids catalysts were produced by boiling the black powder in 15 weight percent H2SO4 under N2 gas for 10 h at 353 K. The characterization of these last products revealed that the graphene sheets were uniformly functionalized with phenolic-OH, -COOH, and SO3H groups as opposed to ordinary solid acids, which only have single functional groups [50].
In comparison to the Brunauer–Emmett–Teller surface area (only 2 m2 g−1) following hydrolysis, the carbon material's effective surface area during hydrolysis was around 560 m2 g−1. The adsorption–desorption isotherm of H2O vapor revealed that more water had been absorbed into the catalyst's main body. The high catalytic activity resulted from the hydrophilic molecules' capacity to bind to cellulose chains in solution and make contact with the carbon materials –SO3H groups. Comparable studies on cellulose conversion by various solid acid catalysts have shown that carbonaceous acids perform exceptionally well. For instance, 68% of cellulose was degraded into a 4% yield of glucose and a 64% yield of β-1,4-glucan after interacting with carbon-based acids for 3 h [51].
A cellobiose adsorption experiment was performed to learn more about the enhanced hydrolytic catalysis of the amorphous carbon with -OH, -COOH, and -SO3H. The findings demonstrated that the strong contacts between the phenolic OH groups and the glycosidic bonds in β-1,4-glucan resulted in higher catalytic activity because hydrogen attaches to oxygen atoms at the glycosidic bond site. These hydrogen bonds were anticipated to attach cellulose to the catalysts' surfaces, facilitating the hydrolysis reaction. With this carbonaceous sulfonated acid, cellulose hydrolysis required lower activation energy than free H2SO4 [50]. From the reaction mixture of carbonaceous solid acids and cellulose, the amorphous carbon acid was extracted and reused a minimum of 25 times without lacking any of its activity loss. According to ion chromatography and elemental analysis, exclusively 1% of the -SO3H groups washed into the mixture through the initial reaction, and no more escaped was seen during successive reactions. The superior enactment of the carbonaceous catalyst makes it possible to use inexpensive and effective solid acids to transform cellulose into value-added compounds, which may one day be used in industrial manufacturing [49].
2.1.2 Metal oxides
Most heterogeneous catalysts with several Lewis acid positions are oxides of metals. Metal oxides are continuously produced through large holes and high definite surface areas, making it simple for reactants to arrive in the holes and come into touch with the active holes. For instance, the oxides of transition metals with a mesoporous nature have been created and used in organic chemical conversions as powerful solid acid catalysts [52]. The addition of tungsten (W) gradually raised the metal oxide acid strength, which reached its greatest reaction rate with mesoporous niobium tungsten oxide (Nb3W7 oxide). Strong acid sites and a high surface area mesoporous structure were credited with Nb3W7 oxide's outstanding catalytic performance. In order to completely convert cellulose to simple sugars, it is necessary to increase the bulk of acid sites and the superficial area of a coated oxide of transition metal. Additionally, Nanoscale metal oxide catalysts may enhance the hydrolysis reaction’s efficacy [53]. Fang and coworkers utilized Ca, Zn, and Fe Nano oxides as a catalyst in the hydrolytic conversion reaction of crystalline cellulose, and as this study showed, the glucose selectivity was 69.2%, and the cellulose conversion was 42.6%. Compared to fine particles of Ca, Zn, and Fe oxides, nano Ca, Zn, and Fe oxides performed better in terms of hydrolysis conversion rates and glucose yields [54].
2.1.2.1 Polymer centered acids
The use of polymer-centered acids which enclosed sites of Bronsted acid as operative solid catalysts has improved numerous organic reaction processes, including lignocellulose hydrolysis reactions. One of the polymer-centered acids Amberlyst, is composed of macro-reticulated styrene–divinylbenzene resins containing sulfonic groups. They are easily accessible, reasonably priced, and stable in the majority of solvents. These acids have macroporous architectures that let tiny molecules pass through and interact with more acidic sites [55]. The solubility of the purified cellulosic substrates increases when they are treated in [BMIm]Cl (1-butyl-3-methylimidazolium chloride), which increases the difficulty of transporting the cellulose to the Polymer acid sites [53]. Cellulosic materials were hydrolyzed with Amberlyst 15DRY and then selectively transformed into sugars or cello-oligomers. The hydrolysis achievements employing the Amberlyst 15DRY revealed an initiation duration of around 1.5 h as the reaction progressed. As Cao and coworkers' study showed, no initiation time was given within the first 1.5 h when soluble p-TSA (p-toluene sulfonic acid) was utilized; it was similar to the acid sites of Amberlyst 15DRY. While the process catalyzed by p-TSA produced tiny sugars, the HPLC examination revealed that no disaccharides and monosaccharides formed within the first 1.5 h from the reducing sugars [56, 57]. The advantage of this process was that the pathway provided a cello-oligomer manufacturing approach rather than entire hydrolysis into fermentable sugars because the extremely solubilized sugars in an ionized liquid made the sugar mining and ionized liquid regaining extremely challenging. Sugars and other dehydration products were produced as the reaction time was further prolonged. The most enticing feature of the approach was its ability to selectively transform cellulose into cello-oligomers and then decompose it into simple sugars. If the hydrolysis reaction effectively stopped at different points in the series of cellulose → cello-oligomers → sugars → dehydration products, it was possible to get specific cellulose pieces or sugars for use in various biorefineries [53]. The amount of acid utilized in the reaction significantly influences the induction period. The induction time was reduced from 1.9 h to less than 5 min by aggregating the concentration of catalyst from 0.46 to 6.9 mmol of acid or hydronium ion because there was no initiation time for the formation of simple or glucose when Amberlyst 15DRY was used in larger amounts similar to those used with soluble strong acids. It may be concluded that the acid concentration significantly influenced the hydrolysis reaction. As opposed to liquid acid (H2SO4), which has an activation energy of 170 kJ mol−1, cellulose's depolymerization requires just 108 kJ mol−1 [58].
2.1.2.2 Heteropoly acid catalysts
Heteropolyacid catalysts are a kind of solid acids that are typically utilized as reusable acid catalysts in chemical reactions. They comprise clusters of initial transition metal–oxygen anions and are frequently utilized in lignocellulose biomass conversion catalytic systems. The Keggin type heteropolyacids with the general formula [XYxM(12−x)O40]n− and the symbol of heteroatom is X, and the symbol of addendum atoms are M and Y, which are the most prevalent and frequently utilized once. Because of their intriguing topologies with exceptional physicochemical characteristics, such as great proton movement, Bronsted acidity, and outstanding stability, heteropolyacids have drawn a lot of attention. They break down in polar solvents and emit H+, which has a greater acidity than common mineral acids like sulfuric acid [50]. Heteropolyacids recently demonstrated outstanding results in the hydrolytic conversion of cellulose to simple sugar glucose. They could be dried for later use after cellulose extraction with organic solvents and separated from the homogenous solution. The total yield of reducing sugars declined in the following order: according to cellulose hydrolysis comparison experiments with various acids, including mineral acids and heteropolyacids is H3PW12O40 > H4SiW12O40 > HClO4 > H2SO4 > H3PO4 [59].
The total release of reducing sugars (TRS) and glucose were produced in hydrolysis reactions utilizing H3PW12O40 in yields of 18% and 15%, respectively. Bronsted acid is very advantageous for the breaking s of cellulose's β-1,4-glycosidic linkages since cellulose conversion products closely match the hydrogen-removing enthalpy of these acids. Weak interaction between catalysts and cellulose always limits the conversion of cellulose. To obtain maximum results, the conversion reactions always call for more excellent mass ratios of catalyst to substrate, very high temperatures, and extended durations for reaction. However, they also produce many side products and have a lower selectivity for glucose [53].
2.1.2.3 Cellulase-mimic bi-functional solid acid catalysts
Cellulases contain a specific substrate-binding area for attaching to the cellulose surface and a catalyzing area for breaking the β-1,4 glycosidic cellulose linkages. This explains why they are so effective at hydrolyzing cellulose. A solid acid cellulase-mimic concept was presented to add a second cellulose-attaching part and create a bi-functional solid acid to amend the binding amid cellulose and solid acids [60, 61]. Figure 3 shows sulfonated chloromethyl polystyrene resin (bifunctional solid acid) formation, which is abbreviated as CP-SO3H, for the degradation of cellulose into simple platforms. While sulfonic acid groups on the solid acid served as the catalytic site to hydrolyze the cellulose's β-1,4 glycosidic linkages. The solid acid part chlorides served as cellulose connection groups by creating H-bonds with the hydroxyls hydrogen of cellulose as shown in Fig. 4 [62]. Due to its ability to adhere to substrates, CP-SO3H displayed significantly greater catalytic action than H2SO4 and other solid acids at equal acid packing that can convert cellulose up to 93% at extremely moderate circumstances, which are 120 °C for 10 h. Additionally, CP-SO3H has substantially lower apparent activation energy for cellulose breaking or hydrolysis than sulfuric acid. Due to the CP-ability SO3H's to hydrolyze cellulose at a lesser temperature, the unfavorable glucose degradation at high temperatures could be avoided or reduced [63].
Synthesis of cellulase-mimetic bifunctional solid acid [64]
Cellulase-mimetic bifunctional solid acid hydrolysis mechanism [64]
Numerous studies have adopted the concept of using chloride as a cellulose-attaching site in solid acids. For instance, starch and PVC were co-carbonized to produce a solid acid catalyst with a carbon basis for the hydrolysis of cellulose, with the chloride groups generated from PVC acting as cellulose adsorption area. After three rounds of this cellulose hydrolysis, it was discovered that 10–25% of the catalyst's chloride had leached. Also, the plastic waste of Polyethylene and PVC was pyrolyzed to produce a solid acid with a twofold carbon-based adsorption function that contains chloride and hydroxyl parts [65]. Recently, it was questioned if chloride components of catalysts could bind with hydrogen in cellulose. A study revealed that under the conditions of cellulose hydrolysis, by using this type of catalyst, the C–Cl bonds were easily hydrolyzed to C–OH, releasing HCl. They asserted that it is more likely that the released HCl will contribute to the bifunctional solid acid's improved ability to hydrolyze cellulose [66].
The advanced preparation method of porous polymeric solid acids by a two-step process with bi-functionality was developed by Liu and coworkers. They started with the production of pillar polymeric porous from monomers of aromatics with required cellulose-bind parts like phenylboronic acid through the polymerization method, then sulfonating the resulting polymer to familiarise -HSO3 groups with cellulose hydrolysis as shown in Fig. 5a [67, 68]. The boronic acid group, which serves as the solid acid's cellulose-attaching area, and the wide surface area provided by the solid acid's mesoporous nature were both credited with the solid acid's excellent catalytic activity towards cellulose hydrolysis. Because this can create a reversible five-membered heterocyclic ring by using two nearby cellulose –OH functional groups and linking them to catalyzing areas needed to finalize the hydrolysis step, and R−B(OH)2) acid can behave as a reliable cellulose-attaching site (Fig. 5b)[68].
a Fabrication with two-step. b Mesoporous cellulase-mimic bi-functional solid acid hydrolysis mechanism (reproduced from [68]
Following four iterations of cellulose hydrolysis recycling, the cellulase-mimic acid containing –B(OH)2 and –SO3H demonstrated a considerable decline in glucose production (94.6–74.6%) predominantly related to SO3H leaching. The same two-step methodology was used in numerous follow-up research to create bifunctional solid acid catalysts for various catalytic processes [69, 70]. A conceptual step forward in creating efficient cellulose conversion was developing a solid acid catalyst with two types of functional groups that mimic cellulase and have a specific cellulose-binding domain. The improvement of contact and binding between acids and cellulose, which is a significant shortcoming of typical unifunctional acid catalysts, was brought about by the addition of cellulose interaction functional groups to acids, which was initiated by cellulases. By choosing highly effective and reliable cellulose-connecting components, increasing the quantity of cellulose-hydrolytic connecting sites, modifying the nature of the porosity, and improving the reusability of these solid acids, the enactment of cellulase-mimetic solid acids can be improved [71].
2.1.2.4 Metal–organic frameworks
Metal–organic frameworks (MOFs) are a particular type of porous structure made of organic ligands as linkers and metal ions or clusters as joints. It is a class of crystalline materials that has drawn a lot of study interest because of their distinctive qualities. The unique properties of MOFs include their large specific surface area (> 10,000 m2 g−1), very high porosity, variable pore size (90% or more free volume), high modularity, high-order crystalline solids, and notable thermal and chemical stability [72]. Most transition metals, post-transition metals, and lanthanides have been employed as joints and coordinated with various linkers, including bi-, tri-, and N-contained cycloalkene composites. The variable joint and linker arrangements produce MOFs with different morphological and textural features [73]. Numerous novel structures are created when inorganic metals and organic linkers are combined. Some common types of MOFs are HKUST-1, MIL-53, MIL-101, UiO-66, and ZIF-8 for the Zn node and ZIF-67 for the Co node (zeolitic imidazolate frameworks) [74].
Brunauer–Emmett–Teller (BET) of large-size MOFs is a superior material for catalysis, separation, storage, and sensing applications because of its definite surface area, high pore volume, and abundance of Lewis/Bronsted acid/base sites. In these applications, MOFs can be employed alone or in combination with polymers, metal oxides, graphene, and porous carbon [73]. So, materials derived from MOF have been described as effective catalysts in the translation of lignocellulosic biomass for cellulose hydrolysis to glucose, glucose isomerization, fructose, and glucose dehydration, hydrogenation of HMF, oxidation of HMF, fructose conversion to DFF, and etherification of HMF [75].
2.1.2.5 Transformation of cellulosic biomass by pristine MOF
The transformation of cellulosic biomass by using solid acid catalysts into platform chemicals is an important method in commercial processes. The carboxymethyl-containing cellulose transformation or conversion into 5-hydroxymethylfurfural (HMF) in the aqueous state was described by using a pristine MOF like MIL-53(Al), which is a heterogeneous solid catalyst [76]. MIL-53(Al) demonstrated a 40.3% yield of HMF at 200 °C after 4 h in water media, as shown in Table 3, among numerous comparable catalysts tested for this reaction. NH3-Temperature Programmed Desorption (NH3-TPD) research discovered the existence of moderately weak Bronsted acidic locations, which are accountable for encouraging this reaction and are likely related to structural flaws, despite the absence of coordinative unsaturated locations nearby Al3+ in the best MIL-53(Al) construction. The amount of acid groups accountable for catalytic action can be determined by measuring the acidity using NH3 and additional basic molecules. Additionally, since these groups are accountable for the interaction, the distribution of acid strength should directly correlate with the catalytic action. For three cycles, MIL-53(Al) activity remained almost unchanged. Compared to the freshly solid acid catalysts, the XRD of its powder-recycled MIL-53(Al) exhibited a similar arrangement to its original crystallinity. Inductively Coupled Plasma (ICP) examination revealed the absence of Al in the hot separation experiment, demonstrating the distinguishable parts of the catalytic transformation. Bronsted acid locations, which are accountable for the hydrolysis, are present in MIL-53(Al), whereas these active sites are absent in MIL-101(Cr), explaining the greater action of MIL-53(Al) compared to MIL-101(Cr) [77].
One of the crucial procedures in the transformation of Lignocellulose biomass, in addition to the conversion of cellulosic feedstock, is the translation of saccharides into building block substances. For this purpose, the number of solids M-MOF-74 with M = Co, Ni, Zn, and Mg are produced, and their effectiveness in converting sugars to lactic acid methyl ester was examined [78]. Mg-MOF-74 produced the most methyl lactate from sucrose of all the investigated catalysts, yielding 47%, even greater than the yields produced from glucose or fructose [79]. Changing glucose to lactic acid methyl ester took three cycles using the Mg-MOF-74 catalyst. More research is still needed to determine the reasons for the enhanced production of methyl lactate from sucrose rather than glucose and to offer characterization data for the utilized catalyst. It is also essential to characterize the active spots responsible for this reaction [72].
Al-TiO2/DDA: Al-doped mesoporous dodecyl amine templated titania; Al–SiO2/RL: rubber latex bio-templated silica; Al2O3/RL: Al2O3 reinforced over rubber latex (adapted from [72]
2.1.2.6 Transformation of cellulosic biomass by functionalized MOF
Figure 6 compiles the documents that have been written about catalytically improved MOFs employing catalysts for the conversion of cellulose, identifying the MOF that was employed in the reaction, the major product, and its yield information [80]. In one important involvement, Kitagawa and colleagues described how to make MIL-101(Cr)-SO3H using a solvothermal reaction involving monosodium 2-sulfate-phthalic acid (MSBDC) and chromium (VI) oxide in the presence of water-based HCl. At 120 °C, cellulose was hydrolyzed to study the catalytic action of MIL-101(Cr)-SO3H [81]. No cellulose hydrolysis was seen without the catalyst, whereas MIL-101(Cr) revealed 0.2% glucose and xylose under comparable circumstances. Remarkably, MIL-101(Cr)-SO3H effectively stimulated the breaking of cellulose in the presence of H2O to produce 2.6% yields of glucose, 1.4%, yields of xylose, and 1.2% yields of cellobiose. This demonstrates the importance of the sulfonic acid group. The most frequent undesirable by-products of this reaction were formic acid, levulinic acid, and HMF were not present according to the 1H-NMR spectroscopy investigation. Even after thirteen uses, MIL-101(Cr)-SO3H's activity did not diminish. Comparatively, the Amberlyst-15 provided a 4.8% yield of glucose in the initial test, then after ten cycles of presentation, it declined to less than 1%. The catalytic data unequivocally demonstrates that MIL-101(Cr)-SO3H is more stable than a commercial catalyst under these circumstances [73, 82].
Functionalized MOFs can be hosts to enclose enzymes and act as active catalysts. In one of these instances, co-precipitation has resulted in the encapsulation of β-G by Cu-PABA represented as β-G@Cu-PABA (β-G: beta-glucosidase, PABA: para-aminobenzoic acid). It is anticipated that separated protein molecules would be encased in specific Cu-PABA covering, given the relationship between the diameters of β-G and Cu-PABA holes. The results of the experiments demonstrated that β-G@Cu-PABA increased movement in the breaking of carboxymethyl cellulose as well as its high resistance to heat and acids. For instance, the co-immobilized cellulase and β-G@Cu-PABA biocomposite combination produced glucose with a 98% yield; it is increased by two times in action over the co-immobilized cellulase alone. With a 70% productivity rate, the β-G@Cu-PABA biocomposite was reused eight times. The encapsulated enzyme activity can be maintained after multiple reaction cycles in which numerous undesirable products are produced. However, there are still gaps in the characterization information for used catalysts, and the causes of catalyst deactivation have not been addressed [83].
3 Techniques to improve solid acid contact and affinity with cellulose substrate
The performance of most currently available solid acids is not quite on par with enzymes and liquid acids despite significant attempts to increase the catalytic accomplishment of heterogeneous acids for cellulose conversion. Solid acids have an inferior affinity for cellulose, which contributes to their poor contact with and interaction with cellulose and is the primary cause of their subpar performance. In addition, cellulose's crystal structure makes it more challenging for solid acids to access and hydrolyze [67, 84, 85]. In order to develop and create solid acids with increased hydrolysis efficiency, it is essential to understand the interaction and adsorption mechanism among solid acids and both soluble intermediates and insoluble cellulose [39, 86,87,88]. Therefore, the main techniques to increase interaction and attraction among insoluble cellulose and solid acids are covered in this section.
3.1 Ball-milling
Cellulose is one of the leading constituents of lignocellulose that is highly crystalline, stiff, and difficult for solid acids to hydrolyze because an intramolecular hydrogen link causes the cellulose chain stiffness. In contrast, crystallinity is produced by an intermolecular hydrogen bond among equatorial hydroxyl clusters and axial stacking of the cellulose fragments (Fig. 7). In addition to being resistant to solid acids attack, these linkages make cellulose tough to break down, difficult to dissolve in water, and tough to dissolve in utmost organic diluters [1]. Due to the fact that the ball-milling technique effectively destroys cellulose crystal nature and minimizes the size of cellulose particle, which makes it grows in surface area. Numerous studies have found a significant rise in hydrolysis efficacy when solid acids are used as a substrate for ball-milled cellulose rather than microcrystalline cellulose [3, 89]. Kobayashi and coworkers used hard alumina balls in the dry mixing-milling process of crystalline cellulose to break down into smaller particles with an amorphous nature. Then, the amorphous cellulose and carbonaceous solid acid catalyst were mixed thoroughly [90]. This procedure increased the cellulose's physical contact with the carbon catalyst, leading to a high % cellulose conversion rate of 93% [91].
Hydrogen bonding between and in cellulose molecules and stacking between cellulose molecules [91]
3.2 Microwave irradiation
To improve the interaction between cellulose and solid acids, microwave irradiation is another technique for successfully increasing cellulose hydrolysis during solid acid-catalyzed reactions. The hydrogen bonds that hold cellulose strands together might be broken and weakened by microwave irradiation, which can make cellulose more amenable to hydrolysis [92, 93]. In order to facilitate the heterogeneous hydrolysis of cellulose, many research articles thought microwave irradiation would increase the interaction between solid acid and cellulose [90]. The microwave might improve many reactions, but the mechanisms must be better understood. However, microwave irradiation is an extremely energy-intensive procedure, making it difficult to use on a broad scale in industry [50].
3.3 Solid acids interaction
Solid acids must interact, adsorb, and come into contact with crystalline cellulose, soluble cellobiose, and oligo-glucan intermediates in sufficient amounts to hydrolyze cellulose. Otherwise, the solid acid active acidic sites cannot access glycosidic linkages to finish the hydrolysis. Improved physical interaction between cellulose and solid acids, such as through the mix-milling method mentioned above, is one way to advance the interface between cellulose and solid acids [39, 94]. In addition, adding affinity and the ability to "attach" or "bind" to cellulose to solid acids is another tactic. It was claimed that the -OH groups on the surface may hydrolyze β-1,4-glucan that had been immobilized on SiO2 and Al2O3 through ether linkages. However, the unbound cellulose could not indicate that even weak acids could hydrolyze cellulose as long as they intermingle with it closely [95, 96]. The two key mechanisms by which solid acids adsorb glucan are H-bonding and CH-π collaboration [50].
3.3.1 Effect of hydrogen bonding
Shen and coworkers reported that shapeless carbon-contained solid acids with high concentrations of COOH, SO3H, and phenolic OH groups could successfully adsorb and hydrolyze soluble long-chain β-1,4 glucan. Amberlyst-15 and Nafion NR50, the two sulfonated resins that only contain the SO3H group, were unable to hydrolyze the glucan because there was no intimate contact or adsorption on the surface between the glucan and the resins [63]. According to the adsorption research shown, phenolic -OH and COOH tried to adsorb β-1,4-glucan; the phenolic OH groups are the dominant surface coating sites; however, SO3H cannot. It was hypothesized that the formation of strong, polarised hydrogen networks between the glycosidic bonds of cellulose and the hydroxide groups of the heterogeneous catalyst induced the cellohexaose adsorption on the catalyst (Fig. 8). Because these networked hydrogens in cellulose were more powerful relative to the inter-molecular hydrogen and intra-molecular hydrogen bonds, it was desirable to couple the cellulose's β-1,4-glycosidic bonds with the catalyst's active acidic sites for conversion. Due to the effective coupling of -COOH and phenolic -OH as adsorption places to link with cellulose and strong SO3H groups as effective acidic sites to hydrolyze cellulose, the exceptional presentation of the carbonaceous solid acid was attributed [63, 97, 98]. The hydrolysis of cellulose could be efficiently catalyzed by carbon compounds containing weak acids, such as carboxylic acid, lactone groups, and phenolic –OH. In addition to hydrolyzing cellulose, functional groups were also in charge of adsorbing it by forming a hydrogen bond with its hydroxyl group. Studies using density functional theory showed that the hydrogen bonding made it more likely for the acid parts to attack the β-1,4 glycosidic link [98].
Diagrammatic representation of the hydrogen bonding-based adsorption of cellulose on a solid acid catalyst [12]
3.3.1.1 CH–pi (π) interaction
According to Fukuoka and coworkers' investigation, adsorption happened only at hydrophobic locations on the surface of carbon, but there was no involvement of hydrophilic groups. In order to eliminate the hydrophilic oxygen-containing functional groups, particularly phenolic OH and acid COOH functional groups, they heated the microporous nature of carbon. Then, hydrophilic groups were out of the way during cellobiose adsorption, and the adsorption equilibrium constant of the heat-treated carbon was quite comparable to that of the untreated carbon [99, 100]. The main mechanism for the adsorption was thought to be the hydrophobic contact between the carbon–carbon π-bonded electrons and axial CH groups, that is, when every single glucose part has 2 or 3 axial CH classes at one or other side of the glucose plane, as shown in Fig. 9 [86].
Additionally, because of the restrictions on the initial shape and mobility of water molecules, typical H-bonding linkages that are entropically unfavorable must be maintained. In addition to the CH- π interactions, the entropic release of bound water from the hydrophobic carbon surface to achieve a higher degree of freedom also encouraged the adsorption. Fan and coworkers showed the adsorption capacity of glucan oligomers on 3-dimensionally organized mesoporous carbon catalysts to be entropically and enthalpically favored, and it improved with the chain of glucans [87]. Since the longer glucan oligomers packed better on the carbon surface, there was a greater amount of longer glucan adsorbed than glucose and shorter glucan oligomers. These results suggested that the potential pouring mechanisms for the cellulose adsorption on carbon catalysts were hydrophobic rather than hydrophilic contact. It was believed that a large portion of the adsorption driving force was made up of van der Waals forces, such as the CH-π interface among the perpendicular C-H hydrophobic groups of cellulose series and polyaromatic rings π- bond electrons. The CH-π interface resembles the interaction between cellulose and cellulase (cellulose hydrolyzes enzymes); cellulase is adsorbed on cellulose by its carbohydrate-attaching groups [50, 101].
4 Recycling of heterogeneous catalysts
4.1 Challenges of recycling
The difficulty in separating homogenous acids from the hydrolyzate and recovering them is one problem of recycling. On the other hand, the solid acid/ heterogeneous catalysts might be extracted by centrifugation or filtration process from cellulose hydrolyzate and then used again in the hydrolysis of cellulose while maintaining their catalytic activity. As Zeng Pan discovered, the uncomplicated decantation allowed them to recover > 99% of the carbon-based catalysts from the products of hydrolysis, and the recuperated catalyst indicated there are no decline activities after 25 reutilizations for hydrolysis of cellulose [39, 102]. Pure cellulose hydrolysis makes it possible to reuse the solid acid catalysts and any leftover cellulose in the same system devoid of further parting. Nevertheless, lignin and unhydrolyzed cellulose coupling and even produced humins during sugar breakdown make recycling solid catalysts highly difficult when hydrolyzing actual lignocellulosic biomass [103].
In order to hydrolyze eucalyptus wood, Fukuoka and colleagues developed a self-contained system that recycling and catalyst preparation achieved through a straightforward cycle as shown in Fig. 9. Eucalyptus was air oxidized to form a carbon-based solid acid (E-carbon, with 2.1 mmol/g carboxylic groups), and this acid was then used to hydrolyze eucalyptus to yield glucose and xylose. Subsequently, the catalyst was hydrolyzed and again converted using the same air oxidation, along with the insoluble components (mainly lignin) in the solid residue, to replace the catalyst. By expertly using the impurity of lignin to produce catalysts, this procedure successfully regenerated solid catalysts during the hydrolysis of real lignocellulosic biomass [104].
Schematic representation of a self-contained system as biomass substrate and catalyst source (adapted from [104])
4.2 Magnetization
Preparing solid acid catalysts with magnetic properties that can easily be isolated by using an external magnetic field from hydrolysis byproducts is a practical method of recycling solid acids. If the addition of Ni, Co, or Fe nanoparticles to solid acid catalysts does not conflict with the other solid acid functions, such as acidity and mesoporosity, magnetic solid acids may be produced. For instance, Lai and colleagues created a solid magnetic acid catalyst like Fe3O4-SBA-SO3H by inserting magnetic Fe3O4 nanoparticles into sulfonated, well-ordered mesoporous silica SBA-15 [105]. From hydrolyzing microcrystalline cellulose, the magnetic solid acid produced 26% glucose and 45% total reducing sugar from corncob. A permanent magnet could be used to separate Fe3O4-SBA-SO3H in the reaction solution easily. After three cycles of hydrolysis experiments, the solid acid's mesoporous structure and magnetic properties were still present, showing that Fe3O4-SBA-SO3H was stable under hydrothermal conditions. Similar to this, Takagaki and colleagues found that SO3H-appended magnetic silica nanoparticles could easily separate polysaccharides (starch and cellulose) and disaccharides (sucrose and cellobiose) through the hydrolysis of these substances [106].
The catalyst was created by inserting magnetic CoFe2O4 cores into a silica matrix and converting thiol clusters into powerful acidic SO3H groups. The microcrystalline cellulose hydrolysis at 150 °C for three hours only produced 7.0% glucose due to the small magnetic solid acid density (0.5 mmol/g) and the poor acid dispersion of the CoFe2O4 in water and the poorer cellulose collaboration. The magnetic nanoparticles' stability in the acidic venue throughout cellulose conversion is a common worry for magnetic solid acids catalysts. When exposed to aqueous acidic conditions, these common metal-based nanoparticles encapsulated in solid acid catalysts are prone to leaching due to their high surface energy [94].
The Zeng Pand and coworkers study also focused on the platform chemical glucose due to its use as the carbon source and the two functional groups contained, such as OH and C = O on the surface of the resultant carbon shells, which could encourage sulfonations to integrate acid sites further. For instance, when they hydrolyzed cellulose at 140 °C after 12 h, the catalyst Fe3O4@C-SO3H furnished 52.1% selectivity of glucose and 48.6% cellulose change, but not an excellent enactment as compared with previous work recording consistent sulfonated carbon catalysts in the absence of magnetization. In an effective magnetic field, the catalyst Fe3O4@C-SO3H could be recovered. The first run saw a modest decrease in the saturation magnetization of recycled Fe3O4@C-SO3H, but no further decline was seen in the second or third runs [39].
5 Conclusion and future prospects
Uses of solid acids as lignocellulose conversion catalysts and substantial advancements have been made during the past few decades. It was recognized that the efficiency of connection and interface among cellulose and the solid catalysts and the structural characteristics of cellulose and the solid catalysts all contribute to the effectiveness of catalyzing and hydrolysis. In addition to these, the heterogeneous/solid acid catalysts that have a wide face area, a great thickness of functional groups, a tough attraction, and an intimate connection with cellulose are likely to have significant catalytic action on cellulose hydrolysis (the major hydrolysis of lignocellulose). Numerous initiatives, like adding extra acid sites, developing mesoporous structures, and improving the interface and interaction between the cellulose and the solid acids by mixing and adding cellulose-binding sites to increase the efficiency of catalysts for breaking cellulose, have been successful. To improve the conversion of lignocellulose substrates into simple platform materials, it has also been possible to understand better the connections between solid acid catalysts and cellulose, including the CH-π hydrophobic nature interaction, the H-bond hydrophilic properties, and specially created cellulose-binding groups. In addition, pure and functionalized MOFs that have been employed as diverse functional catalysts to encourage bike reactions have been addressed in this review. Additionally, magnetic solid acids have been created to make separating and recycling solid acid catalysts easier. The area of tremendous interest and brimming with possibilities in future research to amend the low catalytic activity and ineffective interactions between solid acids and solid lignocellulose biomass for conversion into feedstocks is as follows: solid acids are strongly required for those with high specific catalytic activity. The technique to improve catalytic activity generally should involve adding further high-density materials and many acid sites. A successful strategy for improving a solid acid's connections with cellulose is to design cellulose-binding or cellulose-adsorbing activities specifically for that purpose. The essential and basic things are to have a deeper identification of the interactions of solid acids with catalysts and substrates. So, to improve the binding site and interaction time between substrate (like cellulose) and heterogeneous/solid acid catalysts for an effective result, scholars should consider the relative affinity and selective adsorption of substrate, intermediates, and products with solid acid catalysts.
References
Trisanti PN, et al. The degradation of cellulose in ionic mixture solutions under the high pressure of carbon dioxide. RSC Adv. 2021;11(6):3484–94.
Tong DS, et al. Catalytic hydrolysis of cellulose to reducing sugar over acid-activated montmorillonite catalysts. Appl Clay Sci. 2013;74:147–53.
Zeng M, Pan X. Insights into solid acid catalysts for efficient cellulose hydrolysis to glucose: progress, challenges, and future opportunities. Catal Revi. 2020: 1–46.
Li H, et al. Carbon-increasing catalytic strategies for upgrading biomass into energy-intensive fuels and chemicals. ACS Catal. 2018;8(1):148–87.
Kabir G, Hameed B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew Sustain Energy Rev. 2017;70:945–67.
Dhepe PL, Fukuoka A. Cellulose conversion under heterogeneous catalysis. ChemSusChem: Chem Sustain Energy Mater. 2008;1(12):969–75.
Mika LT, Cséfalvay E, Németh Á. Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem Rev. 2018;118(2):505–613.
Wang Y, et al. Temperature-responsive solid acid catalyst for cellulose hydrolysis to HMF. ChemistrySelect. 2020;5(14):4136–42.
Rai M, et al. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal Rev. 2019;61(1):1–26.
De Bhowmick G, Sarmah AK, Sen R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Biores Technol. 2018;247:1144–54.
Zhang X, et al. High-yield synthesis of 5-hydroxymethylfurfural from untreated wheat straw catalyzed by FePO4 and organic acid in a biphasic system. Energy Fuels. 2023;37(17):12953–65.
Shrotri A, Kobayashi H, Fukuoka A. Cellulose depolymerization over heterogeneous catalysts. Acc Chem Res. 2018;51(3):761–8.
Dutta S. Catalytic materials that improve selectivity of biomass conversions. RSC Adv. 2012;2(33):12575–93.
Brethauer S, Shahab RL, Studer MH. Impacts of biofilms on the conversion of cellulose. Appl Microbiol Biotechnol. 2020;104:5201–12.
Lin Y-C, Huber GW. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ Sci. 2009;2(1):68–80.
Baskar G, et al. Recent advances in heterogeneous catalysts for biodiesel production. J Energy Environ Sustain. 2017;4(1):10–47469.
Lin L, et al. Emerging heterogeneous catalysts for biomass conversion: studies of the reaction mechanism. Chem Soc Rev. 2021;50(20):11270–92.
Dai S, Chen C. A self-supporting strategy for gas-phase and slurry-phase ethylene polymerization using late-transition-metal catalysts. Angew Chem Int Ed. 2020;59(35):14884–90.
Zaera F. Designing sites in heterogeneous catalysis: are we reaching selectivities competitive with those of homogeneous catalysts? Chem Rev. 2022;122(9):8594–757.
Sun Z, et al. Raney Ni as a versatile catalyst for biomass conversion. ACS Catal. 2021;11(16):10508–36.
Zhang B, Reek JN. Supramolecular strategies for the recycling of homogeneous catalysts. Chem Asian J. 2021;16(23):3851–63.
Romo JE, et al. Conversion of sugars and biomass to furans using heterogeneous catalysts in biphasic solvent systems. ChemCatChem. 2018;10(21):4805–16.
Kibar ME, et al. Assessment of homogeneous and heterogeneous catalysts in transesterification reaction: a mini review. ChemBioEng Rev. 2023;10(4):412–22.
Iris K, et al. Tailoring acidity and porosity of alumina catalysts via transition metal doping for glucose conversion in biorefinery. Sci Total Environ. 2020;704:135414.
Deuss PJ, Barta K, de Vries JG. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal Sci Technol. 2014;4(5):1174–96.
Farinelli G, et al. Fe-Chitosan complexes for oxidative degradation of emerging contaminants in water: Structure, activity, and reaction mechanism. J Hazard Mater. 2021;408:124662.
Mukhtar A, et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew Sustain Energy Rev. 2022;157:112012.
Chen SS, Tsang DC, Tessonnier J-P. Comparative investigation of homogeneous and heterogeneous Brønsted base catalysts for the isomerization of glucose to fructose in aqueous media. Appl Catal B. 2020;261:118126.
Wang Y, et al. Electrocatalytic oxygen reduction to hydrogen peroxide: From homogeneous to heterogeneous electrocatalysis. Adv Energy Mater. 2021;11(15):2003323.
Garcés D, et al. Aqueous-phase transformation of glucose into Hydroxymethylfurfural and Levulinic acid by combining homogeneous and heterogeneous catalysis. Chemsuschem. 2019;12(4):924–34.
Mandari V, Devarai SK. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: a critical review. BioEnergy Res. 2021:1–27.
Du Y, et al. A solid-supported arylboronic acid catalyst for direct amidation. Chem Commun. 2019;55(20):2916–9.
Védrine JC. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin J Catal. 2019;40(11):1627–36.
Jeong G-T, et al. Conversion of red-algae Gracilaria verrucosa to sugars, levulinic acid and 5-hydroxymethylfurfural. Bioprocess Biosyst Eng. 2015;38(2):207–17.
Wu C, et al. Conversion of xylose into furfural catalyzed by bifunctional acidic ionic liquid immobilized on the surface of magnetic γ-Al2O3. Catal Lett. 2017;147(4):953–63.
da Silva MJ, de Oliveira CM. Catalysis by Keggin heteropolyacid salts. Current Catalysis. 2018;7(1):26–34.
Yan L, et al. Ruthenium trichloride catalyzed conversion of cellulose into 5-hydroxymethylfurfural in biphasic system. Biores Technol. 2019;279:84–91.
Shende VS, Saptal VB, Bhanage BM. Recent advances utilized in the recycling of homogeneous catalysis. Chem Rec. 2019;19(9):2022–43.
Zeng M, Pan X. Insights into solid acid catalysts for efficient cellulose hydrolysis to glucose: progress, challenges, and future opportunities. Catal Rev. 2022;64(3):445–90.
Finzel JP. Solid acid catalysts for cellulose hydrolysis: structural investigations into the thermal stability and catalytic mechanism of chloromethyl polystyrene based solid acid catalysts. 2018.
Tyufekchiev M, et al. A new method for solid acid catalyst evaluation for cellulose hydrolysis. Sustain Chem. 2021;2(4):645–69.
Wang Y, et al. Recent developments in the catalytic conversion of cellulose. Biotechnol Biotechnol Equip. 2014;28(6):981–8.
Chen Z, et al. Acid–base synergistic catalysis of biochar sulfonic acid bearing polyamide for microwave-assisted hydrolysis of cellulose in water. Cellulose. 2019;26:751–62.
Kurnia I, et al. Hydrolysis of cellulose and woody biomass over sustainable weak-acid carbon catalysts from alkaline lignin. Fuel Process Technol. 2019;196:106175.
Binod P, et al. Hydrolysis of cellulosic and hemicellulosic biomass. In: Biofuels: alternative feedstocks and conversion processes for the production of liquid and gaseous biofuels. Elsevier; 2019. p. 447–60.
Stöcker M. Biofuels and biomass-to-liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials. Angew Chem Int Ed. 2008;47(48):9200–11.
Melero JA, Iglesias J, Morales G. Heterogeneous acid catalysts for biodiesel production: current status and future challenges. Green Chem. 2009;11(9):1285–308.
Frecha E, et al. Catalytic hydrolysis of cellulose to glucose: on the influence of graphene oxide morphology under microwave radiation. J Environ Chem Eng. 2023:109290.
Fukuhara K, et al. Structure and catalysis of cellulose-derived amorphous carbon bearing SO3H groups. Chemsuschem. 2011;4(6):778–84.
Houfani AA, et al. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenerg. 2020;134:105481.
Konwar LJ, et al. Towards carbon efficient biorefining: multifunctional mesoporous solid acids obtained from biodiesel production wastes for biomass conversion. Appl Catal B. 2015;176:20–35.
Jing S, et al. Effectively enhancing conversion of cellulose to HMF by combining in-situ carbonic acid from CO2 and metal oxides. Ind Crops Prod. 2018;126:151–7.
Huang Y-B, Fu Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013;15(5):1095–111.
Zhang F, et al. Hydrolysis of microcrystalline cellulose over Zn–Ca–Fe oxide catalyst. Petrochem Technol. 2011;40(1):43–8.
de Araujo Padilha CE, et al. Fractionation of green coconut fiber using sequential hydrothermal/alkaline pretreatments and Amberlite XAD-7HP resin. J Environ Chem Eng. 2019;7(6):103474.
Cao R, et al. Investigation on decolorization kinetics and thermodynamics of lignocellulosic xylooligosaccharides by highly selective adsorption with Amberlite XAD-16N. Food Chem. 2020;310:125934.
Nis B, Ozsel BK. Efficient direct conversion of lignocellulosic biomass into biobased platform chemicals in ionic liquid-water medium. Renew Energy. 2021;169:1051–7.
Nis B, Ozsel BK. A comprehensive experimental study on the production of key platform chemicals from raw biomass. Fuel. 2020;280:118674.
Tian J, et al. Hydrolysis of cellulose by the heteropoly acid H3PW12O40. Cellulose. 2010;17:587–94.
Agmata AB, et al. DFT simulation of HCl leaching over cellulase-mimetic solid acid catalyst for possible application in biohydrogen production. Int J Hydrogen Energy. 2021;46(27):14063–72.
Ding X, et al. High-efficiency depolymerization of microcrystalline cellulose in 1-butyl-3-methylimidazolium chloride over a magnetically recoverable cellulase-mimetic resin catalyst. J Biobased Mater Bioenergy. 2019;13(3):389–94.
Xu Q, et al. Enhanced enzymatic hydrolysis of corncob by synthesized enzyme-mimetic magnetic solid acid pretreatment in an aqueous phase. ACS Omega. 2019;4(18):17864–73.
Shen S, et al. Preparation of a novel carbon-based solid acid from cocarbonized starch and polyvinyl chloride for cellulose hydrolysis. Appl Catal A. 2014;473:70–4.
Zhang Y, et al. An ultra-sensitive electrochemical aptasensor based on Co-MOF/ZIF-8 nano-thin-film by the in-situ electrochemical synthesis for simultaneous detection of multiple biomarkers of breast cancer. Microchem J. 2023;187:108316.
Yuan S, et al. Double-adsorption functional carbon based solid acids derived from copyrolysis of PVC and PE for cellulose hydrolysis. Fuel. 2019;237:895–902.
Tyufekchiev M, et al. Cellulase-inspired solid acids for cellulose hydrolysis: structural explanations for high catalytic activity. ACS Catal. 2018;8(2):1464–8.
Yang Q, Pan X. Bifunctional porous polymers bearing boronic and sulfonic acids for hydrolysis of cellulose. ACS Sustain Chem Eng. 2016;4(9):4824–30.
Li G, et al. Chemocatalytic conversion of cellulose into key platform chemicals. Int J Polym Sci. 2018.
Dong K, et al. Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chem Eng J. 2018;334:1055–64.
Woodward RT, et al. Hypercrosslinked microporous polymer sorbents for the efficient recycling of a soluble acid catalyst in cellulose hydrolysis. Green Chem. 2018;20(10):2374–81.
Li J, et al. Hypercrosslinked organic polymer based carbonaceous catalytic materials: sulfonic acid functionality and nano-confinement effect. Appl Catal B. 2015;176:718–30.
Fang R, et al. Metal organic frameworks for biomass conversion. Chem Soc Rev. 2020;49(11):3638–87.
Liao Y-T, Matsagar BM, Wu KC-W. Metal–organic framework (MOF)-derived effective solid catalysts for valorization of lignocellulosic biomass. ACS Sustain Chem Eng. 2018;6(11):13628–43.
Abdelhamid HN, Mathew AP. Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted Applications: A review. Coord Chem Rev. 2022;451:214263.
Fang R, Luque R, Li Y. Efficient one-pot fructose to DFF conversion using sulfonated magnetically separable MOF-derived Fe3O4 (111) catalysts. Green Chem. 2017;19(3):647–55.
Zi G, et al. Catalytic hydrothermal conversion of carboxymethyl cellulose to value-added chemicals over metal–organic framework MIL-53 (Al). Carbohyd Polym. 2015;115:146–51.
Sudarsanam P, et al. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem Soc Rev. 2018;47(22):8349–402.
Lu X, Wang L, Lu X. Catalytic conversion of sugars to methyl lactate over Mg-MOF-74 in near-critical methanol solutions. Catal Commun. 2018;110:23–7.
Wang Q, Astruc D. State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis. Chem Rev. 2019;120(2):1438–511.
Qin Y, Guo J, Zhao M. Metal–organic framework-based solid acid materials for biomass upgrade. Trans Tianjin Univ. 2021;27(6):434–49.
Akiyama G, et al. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv Mater. 2011;23(29):3294–7.
Li J, et al. Metal–organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev. 2018;47(7):2322–56.
Dhakshinamoorthy A, et al. Engineering UiO-66 metal organic framework for heterogeneous catalysis. ChemCatChem. 2019;11(3):899–923.
Yabushita M, et al. Quantitative evaluation of ball-milling effects on the hydrolysis of cellulose catalysed by activated carbon. Catal Sci Technol. 2014;4(8):2312–7.
Yang Q, Pan X. Synthesis and application of bifunctional porous polymers bearing chloride and sulfonic acid as cellulase-mimetic solid acids for cellulose hydrolysis. BioEnergy Res. 2016;9:578–86.
Yabushita M, et al. Entropically favored adsorption of cellulosic molecules onto carbon materials through hydrophobic functionalities. Chemsuschem. 2014;7(5):1443–50.
Hashemi D, et al. Design principles for the energy level tuning in donor/acceptor conjugated polymers. Phys Chem Chem Phys. 2019;21(2):789–99.
Chung P-W, et al. Long-chain glucan adsorption and depolymerization in zeolite-templated carbon catalysts. ACS Catal. 2015;5(11):6422–5.
Li X, et al. Effective low-temperature hydrolysis of cellulose catalyzed by concentrated H3PW 12O40 under microwave irradiation. RSC Adv. 2012;2(17):6921–5.
Kobayashi H, Fukuoka A. Development of solid catalyst–solid substrate reactions for efficient utilization of biomass. Bull Chem Soc Jpn. 2018;91(1):29–43.
Gan L, Zhu J, Lv L. Cellulose hydrolysis catalyzed by highly acidic lignin-derived carbonaceous catalyst synthesized via hydrothermal carbonization. Cellulose. 2017;24:5327–39.
Zhai C, et al. Revealing the importance of non-thermal effect to strengthen hydrolysis of cellulose by synchronous cooling assisted microwave driving. Carbohyd Polym. 2018;197:414–21.
Jiang Y, et al. Effective saccharification of lignocellulosic biomass over hydrolysis residue derived solid acid under microwave irradiation. Green Chem. 2012;14(8):2162–7.
Hu L, et al. Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl Catal B. 2015;174:225–43.
Gazit OM, Katz A. Understanding the role of defect sites in glucan hydrolysis on surfaces. J Am Chem Soc. 2013;135(11):4398–402.
Atun S, et al. Antibacterial activity of nanoparticles produced by ethanol extract of boesenbergia rotunda rhizome loaded with chitosan and alginic acid against Streptococcus mutans by in vitro.
To AT, Chung PW, Katz A. Weak-acid sites catalyze the hydrolysis of crystalline cellulose to glucose in water: importance of post-synthetic functionalization of the carbon surface. Angew Chem Int Ed. 2015;54(38):11050–3.
Kobayashi H, et al. Synergy of vicinal oxygenated groups of catalysts for hydrolysis of cellulosic molecules. J Phys Chem C. 2015;119(36):20993–9.
Foo GS, Sievers C. Synergistic effect between defect sites and functional groups on the hydrolysis of cellulose over activated carbon. Chemsuschem. 2015;8(3):534–43.
Chung P-W, et al. Glucan adsorption on mesoporous carbon nanoparticles: effect of chain length and internal surface. Langmuir. 2012;28(43):15222–32.
Payne CM, et al. Multiple functions of aromatic-carbohydrate interactions in a processive cellulase examined with molecular simulation. J Biol Chem. 2011;286(47):41028–35.
Tagusagawa C, et al. Highly active mesoporous Nb–W oxide solid-acid catalyst. Angew Chem Int Ed. 2010;49(6):1128–32.
Suganuma S, et al. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc. 2008;130(38):12787–93.
Kobayashi H, et al. Hydrolysis of woody biomass by a biomass-derived reusable heterogeneous catalyst. Chem Sci. 2016;7(1):692–6.
Lai D-M, et al. Hydrolysis of biomass by magnetic solid acid. Energy Environ Sci. 2011;4(9):3552–7.
Takagaki A, et al. Hydrolysis of sugars using magnetic silica nanoparticles with sulfonic acid groups. Chem Lett. 2011;40(10):1195–7.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
ATW: writing, original draft preparation, Editing; INA and RKB: conceptualization, manuscript editing; MG and AS: provided strategic guidance, supervision;
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wasie, A.T., Tadesse, M.G., Wotango, A.S. et al. Heterogeneous catalytic conversion of lignocellulose: towards green and renewable chemicals. Discov Appl Sci 6, 37 (2024). https://doi.org/10.1007/s42452-024-05680-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05680-0