Abstract
This study advances the understanding of nanofluid behaviour within stenosed arteries, highlighting the importance of considering multifaceted effects in the modelling process. It investigates the combined impact of pressure gradient variation, heat transfer, chemical reactions, and magnetic field effects on nano-blood flow in stenosed arteries. Unlike previous studies that made the assumption that the pulsatile pressure gradient remains constant during channel narrowing, this novel investigation introduces a variable pressure gradient. This, in turn, significantly impacts several associated parameters. The mathematical model describing nano-blood flow in a horizontally stenosed artery is solved using perturbation techniques. Analytical solutions for key variables, including velocity, temperature, concentration, wall shear stress, flow rate, and pressure gradient, are visually presented for various physical parameter values.
Article highlights
-
Velocity decreases with a higher magnetic field but increases with greater permeability, stenosis height, and nanoparticle volume fraction.
-
Temperature rises with increasing magnetic field, radiation parameter, and nanoparticle volume fraction. However, it decreases as the permeability parameter increases.
-
The pressure gradient increases as the height of constriction rises along the channel's length.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Studying blood flow through arteries under stenosed conditions is crucial due to its direct link to vascular diseases and cardiovascular dysfunction. Stenosis caused by intravascular plaques disrupts flow patterns and contributes to complications like tissue ingrowth, thrombosis, and weakened arteries. In normal physiological conditions, blood transport relies on a heart-driven pressure gradient. Mathematical modelling of blood flow through constricted channels is essential in clinical contexts, offering insights into circulatory disorders. Numerous theoretical and numerical studies have been conducted recently [1,2,3,4,5,6,7] to investigate blood flow through arteries with a single stenosis, leading to a deeper understanding of flow characteristics under various assumptions and considering diverse arterial geometries. To comprehend the impact of stenosis on arterial blood flow, multiple investigations were conducted by Hung and Tsai [8], Pedley [9], and Tu et al. [10], many of which operate under the assumption that blood behaves as a Newtonian fluid. Misra and Ghosh [11] and Ellahi et al. [12] explored blood flow in stenosed arteries, considering wall shear stress and wall resistance, while Ratchagar and Subasri [13] developed a mathematical model elucidating blood flow through arteries with composite stenosis.
The application of Magnetohydrodynamics (MHD) in physiological flow is of growing interest. MHD can be used to control blood flow by applying an appropriate magnetic field. Blood is an electrically conducting fluid, and the Lorentz force acts on the constituent particles of blood, opposing the motion of blood and reducing its velocity. Therefore, it is essential to study blood flow in the presence of a magnetic field. Many investigators have conducted research in this area [14,15,16,17,18]. The concept of electromagnetic fields in medical research was initially introduced by Kolin [19]. Subsequently, Korchevskii et al. [20] explored the potential of utilizing magnetic fields to control blood circulation within the human system. Haik et al. [21] demonstrated a 30% reduction in blood flow rate under a 10 T magnetic field, while Yadav et al. [22] observed a comparable decrease in blood flow rate under a significantly lower magnetic field of 0.002 T. The combined effect of these fields generates a Lorentz force, termed as such, which opposes liquid movement [23, 24]. In arterial studies to detect stenosis, non-invasive MRI techniques are employed, utilizing a powerful magnetic field [25]. This MRI device influences the velocity field within the specific body region it targets. Pulsatile blood flow through a porous medium affected by periodic body acceleration was investigated by Rathod and Tanveer [26]. The study incorporated a magnetic field and treated blood as an incompressible, electrically conducting fluid with couple stress properties. Sankar and Lee [27] devised a computational model to assess magnetic field effects on pulsatile blood flow through narrow arteries with mild stenosis, employing the Casson fluid model. Their investigation revealed that velocity and flow rate decrease with increasing Hartmann number. Moreover, skin friction and longitudinal impedance increase with the amplitude parameter of the artery radius. Mirza et al. [28] presented a mathematical model analysing the effect of a magnetic field on transient laminar electromagneto-hydrodynamic two-phase blood flow using a continuum approach. By solving the model analytically, they separately demonstrated that the magnetic field has effects on blood and particle velocities. They concluded that as the magnetic field's influence increases, both blood and particle velocities decrease in electromagneto-hydrodynamic two-phase blood flow. In a recent investigation conducted by Tanveer et al. [29], the study focused on entropy generation induced by a peristaltic process on a curved surface, taking into account the influence of magnetohydrodynamics (MHD), variable viscosity, and convective conditions. Their findings indicate that velocity and temperature profiles experience enhancement with higher values of the Biot number and magnetic parameter. Furthermore, Tanveer et al. [30] examined entropy generation and Joule heating effects in the context of MHD peristaltic flow over an asymmetric channel with mixed convective conditions.
In recent times, nanofluid dynamics has emerged as a pivotal field within fluid mechanics. Nanofluids, characterized by the presence of nanoparticles at the nanometer scale, have a wide range of scientific and technological applications across various fields. They play a crucial role in medical science and therapy, including in vivo therapy, drug delivery, and the coating of medical devices for improved biocompatibility and cancer treatment. Their unique properties, such as enhanced thermal conductivities and convective heat transfer, make them valuable in heat transfer technologies, including microelectronics cooling and thermal management systems [31,32,33,34,35,36,37,38]. The study of nanofluid-blood flow is critical for treating arterial stenosis, considering changes in viscosity and the potential for innovative applications that can advance biomedical science and save lives. Choi [39] introduced the term “nanofluid” and demonstrated increased thermal conductivity in base fluids infused with nanometer-sized particles. Sandeep et al. [40] examined the impact of radiation on the pulsatile convective flow of an ethylene glycol-based nanofluid along a vertical plate. Radiation effects on the magnetohydrodynamic stagnation point flow of a nanofluid towards a stretching surface with convective boundary conditions were investigated by Akbar et al. [41]. Gireesha et al. [42] addressed the magnetohydrodynamic flow and heat transfer of a dusty fluid over a stretching surface, while Mohankrishna et al. [43] studied radiation effects on the unsteady magnetohydrodynamic natural convection flow of a nanofluid past an infinite vertical plate with a heat source. Subsequently, numerous researchers investigated diverse nano-blood flow models across varying aspects and conditions [44,45,46,47,48,49,50,51,52,53,54,55,56].
The literature review revealed that no efforts have been made to investigate the simultaneous impact of pressure gradient variation on nanofluid flow along the length of the stenosed artery. In previous research, the prevailing assumption was that the pressure gradient can be simplified into steady and unsteady components, both remaining constant with the stenosed artery’s length. Contrary to this, Chow and Abumandour et al. [57, 58] discovered a substantial variation in the pressure gradient's relationship with the restricted channel length, exerting an influence on other associated parameters. This study addresses this gap by investigating nanofluid flow in a stenosed artery, accounting for pressure gradient variation, as well as radiation, chemical reaction, and magnetic field effects. The governing equations of nanofluid flow in a horizontal stenosed artery are solved using the perturbation method. The analytical solutions of velocity, temperature, concentration, wall shear stress, flow rate, and pressure gradient are presented graphically, considering different values of the relevant physical parameters.
2 Mathematical formulation
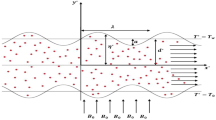
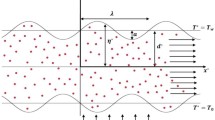
The statement describes blood as an unsteady, incompressible nanofluid flow through a porous stenosed arterial segment with heat and chemical reaction. The artery is assumed to be a three-dimensional vessel with a small radius. It can be approximated as a two-dimensional channel, and the pressure gradient is in the horizontal direction (See Fig. 1) [16, 59]. The uniform magnetic field is applied perpendicularly to the direction of the nanofluid flow. The following equations govern the flow field [16]:
Momentum equation
Energy equation
Concentration equation
Rosseland approximation for radiative heat flux, \({q}_{r}\) is defined as [60]:
where \({k}^{*}\) is the Rosseland mean absorption coefficient, \({\sigma }^{*}\) is the Stefan–Boltzmann constant. We presume that the temperature variation within the flow is sufficiently small such that \({T}^{*4}\) may be expanded in a Taylor’s series. Expanding \({T}^{*4}\) about \({T}_{\infty }\) and neglecting higher-order terms we obtain [61]:
Substituting Eqs. (4) and (5) into Eq. (2), we get:
Since the nanofluid-blood flow is driven by the pumping action of the heart, which produces a pulsatile pressure gradient, it can be approximated as a function of \({x}^{*}\) and \({t}^{*}\) [3, 62]. So, it can be taken as:
which,
\(A\) is the amplitude of the nanofluid flow and \(\omega \) is the angular frequency of the flow.
The geometry of the stenosis is given as follows:
The maximum projection of the stenosis \({\delta }^{*}\) is located at a distance,\({x}^{*}={d}_{0}^{*}+\frac{{l}_{0}^{*}}{2}\), from the inlet of the artery. The stenosis length is \({l}_{0}^{*}\), and its location is\({d}_{0}^{*}\). The variable height of the channel at the stenosed portion is\({h}^{*}\left({x}^{*}\right)\).
The boundary conditions for the wall of the porous channel under the no-slip condition are as follows:
The nanofluid thermophysical properties according to Zahir et al. [63]:
We introduce the dimensionless parameters as follows:
Using the dimensionless variables stated above in Eqs. (1), (3), (6), (9), and (10) we obtain:
where, the Womersley parameter \(\alpha \), the Darcy number \(Da\), the Hartmann number\(Ha\), the radiation parameter \(Rd\), the Prandtl number \(Pr\), the Eckert number \(Ec\), the Schmidt number \(Sc\), the Soret number \(Sr\) are defined respectively by:
In dimensionless form, the geometry of the stenosis is given by:
and the dimensionless transverse coordinate is:
The corresponding new boundary conditions are:
3 Method of solution
After using dimensionless technique, the velocity\(u\), temperature \(\theta \),and concentration \(\varnothing \) can be assumed to have expansions in terms of the parameter \({\alpha }^{2}\) [62], which are of the form:
where ( \(\alpha \) < 1.0) is the Womersley frequency parameter.
The following equations are the result of substituting Eqs. (19–21) into Eqs. (13–15), and (18), then comparing the coefficients of both sides we obtain the following equations subject to the corresponding boundary conditions:
(i) Zero order:
(ii) First order:
By solving Eqs. (22–28) with the corresponding boundary conditions in Eqs. (25) and (29), we obtain:
where \({C}_{1},{C}_{2},{C}_{3},\) · · · etc. given in the appendix.
Substituting Eqs. (30–35) into Eqs. (19–21), we obtain an expression for velocity\(u\), temperature \(\theta \),and concentration \(\varnothing \) as:
After having determine \(u\), we can obtain the volumetric flow rate \(Q\), defined by:
Which on integration yields after substituting from Eq. (36) into Eq. (39) results in:
The pressure gradient \(P\left(x\right)\) can be obtained from Eq. (40):
The non-dimensional wall shear stress \({\tau }_{w}\) is a physiologically important quantity given by:
Use of Eq. (36) into Eq. (42), the wall shear stress can be written as:
4 Results and discussion
In this section, we performed numerical simulations of Eqs. (36–43) to study the effect of the biophysical parameters such as Hartmann number, Darcy number, stenosis growth parameter, radiation parameter, nanoparticle concentration, Eckert number, Prindle number, Schmidt number, and Soret number on velocity, wall shear stress, temperature, concentration, pressure gradient, and flow rate profiles which are presented graphically in Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26. Table 1 contains the default values for the biophysical parameters used in the simulation.
In addition, the thermophysical numerical parameters of blood and gold nanoparticles [51, 64] are listed in Table 2.
The velocity profiles are presented in Figs. 2, 3, 4, 5. Figure 2 demonstrates that as the Hartmann number increases, the centreline velocity decreases, leading to an increase in near-wall velocity due to conservation of mass flow rate. This behaviour results in a flattening of the velocity profile near the centreline, accompanied by a decrease in the rate of velocity change. These findings have potential implications for surgical patients as mentioned in [65]. They suggest a decrease in blood velocity during surgery. Conversely, Fig. 3 exhibits a contrasting trend related to porosity's effect on velocity. It reveals that as the Darcy number increases, the centreline velocity increases while causing a decrease in near-wall velocity. Similar to the previous case, this increase in Darcy number leads to a flatter velocity profile near the centreline with a diminishing rate of velocity change. Figure 4 delves into the influence of nanoparticle concentration on axial velocity. A notable upsurge in velocity is observed as the nanoparticle volume fraction escalates, and this can be explained by the effect of increasing nanoparticles concentration leads to a decrease in the available area for the blood to flow through the channel. Furthermore, Fig. 5 highlights a significant increase in velocity as the height of the stenosis at the throat of the channel increases. This indicates a direct correlation between flow velocity and the narrowing of the channel based on the continuity equation [53].
Conducting a comprehensive analysis of wall shear stress distribution along the axial distance, as illustrated in Figs. 6, 7, 8, 9, under various rheological parameters. It is a well-established fact that elevated wall shear stresses can induce damage to the vessel wall, resulting in intimal thickening. Conversely, low wall shear stresses facilitate mass transport, leading to the deposition of substances such as cholesterol, which leads to an increase in stenosis height. Figure 2 provides valuable insights, revealing that the slope of the velocity profile near the wall increases with higher Hartmann numbers. Consequently, this increase in slope translates into a corresponding rise in wall shear stress, as exemplified in Fig. 6. In sharp contrast, Fig. 7 showcases a noteworthy phenomenon. Here, a decline in wall shear stress can be observed with increasing Darcy numbers. This decrease is attributable to the diminishing slope of the velocity profile near the wall, as vividly demonstrated in Fig. 3. Furthermore, Fig. 8 sheds light on the relationship between wall shear stress and nanoparticle concentration. The data unequivocally indicate that as nanoparticle concentration increases, wall shear stress follows suit, strengthening this correlation. Figure 9 unveils yet another intriguing finding. It demonstrates that as the height of the stenosis increases, wall shear stress experiences a corresponding upsurge. This phenomenon aligns with an evident escalation in the velocity gradient near the stenotic region in Fig. 5, providing crucial insights into the interplay between stenosis height and wall shear stress.
Regarding the temperature profiles, the effect of the Hartmann number on the temperature profile is shown in Fig. 10, which shows an increase with increasing Hartmann number at the centreline. As the Hartmann number rises, the magnetic field strength becomes stronger, which in turn impedes the fluid motion as shown in Fig. 2. Reduced fluid motion leads to a lower heat transfer rate, causing the temperature to rise at the centreline. The inverse relationship between the Darcy number and temperature profile is linked to fluid permeability. In Fig. 11, as the Darcy number increases, it signifies greater fluid permeability through porous media. This increased permeability allows for enhanced heat dissipation, resulting in a lower temperature profile. As shown in Fig. 12, the increase in temperature distribution with the presence of nanoparticles can be attributed to their thermal properties. Nanoparticles exhibit efficient thermal conduction or absorption, and as the nanoparticle concentration increases, there is a notable increase in the surface area exposed to the blood flow, leading to enhanced heat transfer within the fluid. Consequently, this results in a broader temperature distribution. Figure 13 further illustrates the effect of stenosis height on the temperature profile. The positive correlation between stenosis height and temperature profile is due to the narrowing of the flow passage, which gives less time for heat transfer through the blood flow. As the stenosis height increases, the flow becomes more constrained, leading to increased flow resistance and subsequently higher temperatures along the channel. The temperature increase with an increasing Eckert number is indicative of the enhanced contribution from kinetic energy, as shown in Fig. 14. A higher Eckert number implies a greater proportion of kinetic energy in the flow, which, in turn, elevates the temperature through increased internal energy. Conversely, the decrease in temperature with an increasing Prandtl number is associated with fluid properties. A higher Prandtl number signifies a lower thermal diffusivity relative to momentum diffusivity. This results in less efficient heat conduction compared to momentum transfer, leading to a lower temperature profile, as illustrated in Fig. 15. Figure 16 shows that the temperature increases with an increasing radiation parameter, which indicates the significance of radiative heating. A higher radiation parameter signifies an intensified radiation effect within the flow, which directly contributes to elevated temperatures by adding thermal energy to the system [16].
For the concentration profiles, the influence of the Hartmann number on the concentration profile is shown in Fig. 17. The decreasing concentration profile with increasing Hartmann number is attributed to the amplified magnetic field strength, which leads to enhanced electromagnetic forces acting on the nanoparticles. This results in more efficient removal of solute from the bloodstream. Clinically, this insight can be valuable in understanding the mechanisms behind diseases like anaemia, where altered blood chemistry plays a pivotal role. It may also guide therapeutic strategies by suggesting ways to modulate magnetic fields for targeted drug delivery in blood-related disorders [66]. Figure 18 demonstrates a similar decreasing trend. The decreasing concentration profile as the Darcy number increases is linked to heightened resistance within the porous medium, reducing solute transport. This phenomenon has clinical implications for disorders related to blood clotting and thrombosis. In contrast, Fig. 19 shows that the increase in the concentration profile with rising nanoparticle concentration can be attributed to enhanced solute-NP interactions, altering the transport properties. This finding not only contributes to our understanding of blood flow dynamics but also has implications for therapeutic interventions. Manipulating nanoparticle concentration could potentially improve drug delivery to specific targets within the bloodstream, offering new avenues for disease treatment and management [67]. Figure 20 illustrates that the decrease in the concentration profile with increasing stenosis height suggests reduced diffusion and transport of solute due to the restricted area. This insight can be applied to conditions involving vascular stenosis, shedding light on the chemical reactions underlying blood flow in narrowed vessels. Clinical applications extend to disorders related to blood flow obstructions, such as atherosclerosis and thrombosis. It is observed that as mentioned by [55] the downward trend in concentration with rising Schmidt numbers in Fig. 21 can be linked to increased molecular diffusion, which disperses solute more effectively. This understanding is crucial in elucidating blood chemical reactions and their implications for diseases like diabetes, where altered blood chemistry is a hallmark. The increase in concentration with rising the Soret number in Fig. 22 can be attributed to thermal gradients driving solute transport. This phenomenon enhances our understanding of the chemical reactions taking place in pulsatile blood flow. Clinically, it may have implications for managing blood-related disorders where temperature gradients play a role, potentially offering new avenues for therapeutic interventions [68].
The effect of stenosis height on the pressure gradient can be seen in Fig. 23, where the pressure gradient increases with the height of the stenosis as it increases the flow resistance along the length of the stenosed arterial segment and this observation agrees qualitatively well with [58]. This rise in resistance is a consequence of the narrowing of the artery's cross-sectional area due to the stenosis. According to fundamental principles of fluid dynamics, an increase in flow resistance necessitates a higher pressure gradient to sustain the flow rate. In practical terms, this means that as stenosis height increases, the heart must generate greater force to maintain adequate blood flow through the narrowed region, which subsequently results in an elevated pressure gradient. These observations hold significance in the assessment of hemodynamic consequences associated with arterial stenosis, offering valuable insights into conditions such as atherosclerosis.
Finally, Fig. 24 illustrates the volumetric flow rate's periodic oscillations over time, reflecting the pulsatile nature of blood flow driven by the heart's rhythmic contractions. Notably, an increase in stenosis height leads to a marked decline in the volumetric flow rate. This reduction stems from stenosis-induced narrowing of the artery's cross-sectional area, leading to heightened flow resistance, as demonstrated in Fig. 23. Consequently, the heart's ability to pump the same amount of blood diminishes. Beyond these mathematical trends, the clinical repercussions of heightened stenosis and increased flow resistance are profound. Elevated resistance restricts blood flow to the specific vascular bed supplied by the affected artery, potentially causing tissue ischemia. Furthermore, slowed blood circulation in the presence of stenosis triggers platelet adhesion to the vessel wall, fostering platelet aggregation and thrombus formation [62]. This thrombus may detach or fibrillate, leading to abrupt physiological changes. Most critically, clotting within a stenosed coronary artery can impair myocardial contractility, reduce ventricular function, and, in severe cases, precipitate sudden cardiac death, often attributed to ventricular fibrillation. These findings emphasize the intricate connection between stenosis, blood flow dynamics, and the predictive value of flow rate measurement in assessing narrowing issues' pivotal role in cardiovascular health and pathology.
5 Conclusion
The innovative use of a variable steady pressure gradient represents a notable departure from prior studies. Conventional research primarily concentrated on fixed steady pressure gradients for studying pulsatile blood flow in stenotic arteries and associated phenomena. This research represents a substantial step forward in comprehending nanofluid behaviour in constricted arteries, emphasizing the importance of multifactorial considerations in modelling. The investigation explores the confluence of diverse factors, including pressure gradients, heat transfer, chemical reactions, and magnetic fields, influencing the flow of nanoscale blood particles within stenosed arteries. We have obtained exact solutions and thoroughly examined the effects of crucial parameters. The results and observations align well with findings reported in references [16, 53, 55, 58]. The following is a summary of the main conclusions from the graphical representations:
-
The velocity profile decreases with rising in the magnetic field at the centreline, while it increases with increasing the permeability parameter. Additionally, velocity rises with greater stenosis height and nanoparticle volume fraction.
-
The artery wall shear stress in the stenosis segment decreases with higher permeability parameter but increases with stenosis height, magnetic field, and nanoparticle volume fraction.
-
The temperature profile increases with rising magnetic field, nanoparticle volume fraction, stenosis height, Eckert number, and radiation parameter, while decreasing with increasing the permeability parameter and Prandtl number.
-
The concentration profile decreases with increasing magnetic field, the permeability parameter, stenosis height, and Schmidt number, but increases with rising nanoparticle volume fraction and Soret number.
-
The pressure gradient rises as the constriction height increases along the channel's length.
-
The volumetric flow rate decreases with time as the stenosis height increases.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- \({x}^{*}\) :
-
Dimensional coordinate along the channel \(\left(m\right)\)
- \({y}^{*}\) :
-
Dimensional coordinate perpendicular to the channel \(\left(m\right)\)
- \(x, y\) :
-
Dimensionless distances
- \({t}^{*}\) :
-
Dimensional time \(\left(s\right)\)
- \(t\) :
-
Dimensionless time
- \({p}^{*}\) :
-
Pressure \(\left(kg/m{ .s}^{2}\right)\)
- \({u}^{*}\) :
-
Dimensional blood velocity \(\left(m/s\right)\)
- \(u\) :
-
Dimensionless blood velocity
- \({T}^{*}\) :
-
Temperature of the nanofluid \(\left(K\right)\)
- \({T}_{w}\) :
-
Temperature at the wall \(\left(K\right)\)
- \({C}^{*}\) :
-
Concentration of the nanofluid \(\left(kg/{m}^{3}\right)\)
- \({C}_{w}\) :
-
Concentration at the wall \(\left(kg/{m}^{3}\right)\)
- \({q}_{r}\) :
-
Heat flux \(\left(W/{m}^{2}\right)\)
- \({k}_{f}\) :
-
Thermal conductivity of the fluid \(\left(W/m .K\right)\)
- \({k}_{n}\) :
-
Thermal conductivity of the nanoparticles \(\left(W/m .K\right)\)
- \({k}_{nf}\) :
-
Thermal conductivity of the nanofluid \(\left(W/m .K\right)\)
- \(k\) :
-
Permeability of the porous media \(\left({m}^{2}\right)\)
- \(A\) :
-
Amplitude of the flow \(\left(m\right)\)
- \({B}_{0}\) :
-
Uniform magnetic field \(\left(T\right)\)
- \({D}_{m}\) :
-
Molecular diffusivity \(\left({m}^{2}/s\right)\)
- \({D}_{T}\) :
-
Thermal diffusivity of the fluid \(\left({m}^{2}/s\right)\)
- \({K}_{bt}\) :
-
Thermal diffusion ratio
- \({T}_{m}\) :
-
Mean temperature of the fluid \(\left(K\right)\)
- \({k}^{*}\) :
-
Rosseland mean absorption coefficient \(\left({m}^{-1}\right)\)
- \({C}_{\infty }\) :
-
Far field concentration of the fluid \(\left(kg/{m}^{3}\right)\)
- \({T}_{\infty }\) :
-
Far field temperature of the fluid \(\left(K\right)\)
- \(h(x)\) :
-
Height of an abnormal channel \(\left(m\right)\)
- \({H}_{0}\) :
-
Height of normal channel \(\left(m\right)\)
- \({d}_{0}\) :
-
Distance of Onset of stenosis \(\left(m\right)\)
- \({l}_{0}\) :
-
Length of stenosis \(\left(m\right)\)
- \(Da\) :
-
Darcy number of the porous media
- \(Ec\) :
-
Eckert number
- \(Ha\) :
-
Hartmann number
- \(Pr\) :
-
Prandtl number
- \(Rd\) :
-
Radiation parameter
- \(Sc\) :
-
Schmidt number
- \(Sr\) :
-
Soret number
- \(Q\) :
-
Volumetric flow rate \(\left({m}^{3}/s\right)\)
- \({\mu }_{nf}\) :
-
Dynamic viscosity of the nanofluid \(\left(kg/m.s\right)\)
- \({\rho }_{nf}\) :
-
Density of the nanofluid \(\left(kg/{m}^{3}\right)\)
- \({\upsilon }_{nf}\) :
-
Kinematic viscosity of the nanofluid \(\left({m}^{2}/s\right)\)
- \({\mu }_{f}\) :
-
Dynamic viscosity of the fluid \(\left(kg/m.s\right)\)
- \({\rho }_{f}\) :
-
The density of the fluid \(\left(kg/{m}^{3}\right)\)
- \({\upsilon }_{f}\) :
-
Kinematic viscosity of the fluid \(\left({m}^{2}/s\right)\)
- \({\rho }_{n}\) :
-
Density of the nanoparticles \(\left(kg/{m}^{3}\right)\)
- \({\left(\rho {C}_{p}\right)}_{f}\) :
-
Heat capacity of the fluid \(\left(kg/m.{s}^{2}.K\right)\)
- \({\left(\rho {C}_{p}\right)}_{n}\) :
-
Heat capacity of the nanoparticles \(\left(kg/m.{s}^{2}.K\right)\)
- \({\left(\rho {C}_{p}\right)}_{nf}\) :
-
Heat capacity of the nanofluid \(\left(kg/m.{s}^{2}.K\right)\)
- \(\alpha \) :
-
The Womersley parameter
- \(\delta \) :
-
The maximum height of the of the stenosis \(\left(m\right)\)
- \(\sigma \) :
-
Electrical conductivity of the fluid \(\left(S/m\right)\)
- \({\sigma }^{*}\) :
-
Stefan- Boltzmann constant \(\left(W/{m}^{2}. {K}^{4}\right)\)
- \(\varphi \) :
-
Nanoparticle volume fraction
- \(\omega \) :
-
The angular frequency of the flow \(\left({s}^{-1}\right)\)
- \(\phi \) :
-
Dimensionless Concentration
- \(\theta \) :
-
Dimensionless Temperature
- \({\tau }_{w}\) :
-
Dimensionless Wall shear stress
- \(\zeta \) :
-
Dimensionless transverse coordinate
- \(f\) :
-
Fluid fraction
- \(n\) :
-
Nanoparticle
- \(nf\) :
-
Nanofluid fraction
References
Young DF (1979) Fluid mechanics of arterial stenosis. J Biomech Eng 101:157
Misra J, Chakravarty S (1986) Flow in arteries in the presence of stenosis. J Biomech 19(11):907–918
Chaturani P, Samy RP (1986) Pulsatile flow of Casson’s fluid through stenosed arteries with applications to blood flow. Biorheology 23(5):499–511
Chakravarty S, Mandal P (1994) Mathematical modelling of blood flow through an overlapping arterial stenosis. Math Comput Model 19(1):59–70
Buchanan J Jr, Kleinstreuer C, Comer J (2000) Rheological effects on pulsatile hemodynamics in a stenosed tube. Comput Fluids 29(6):695–724
Misra J, Shit GC (2006) Blood flow through arteries in a pathological state: a theoretical study. Int J Eng Sci 44(10):662–671
Misra J, Adhikary S, Shit G (2007) Multiphase flow of blood through arteries with a branch capillary: a theoretical study. J Mech Med Biol 7(04):395–417
Hung T-K, Tsai TM-C (1997) Kinematic and dynamic characteristics of pulsatile flows in stenotic vessels. J Eng Mech 123(3):247–259
Pedley TJ (1980) The fluid mechanics of large blood vessels. Cambridge University Press, Cambridge
Tu C, Deville M, Dheur L, Vanderschuren L (1992) Finite element simulation of pulsatile flow through arterial stenosis. J Biomech 25(10):1141–1152
Misra J, Ghosh S (2001) A mathematical model for the study of interstitial fluid movement vis-a-vis the non-Newtonian behaviour of blood in a constricted artery. Comput Math Appl 41(5–6):783–811
Ellahi R, Rahman S, Nadeem S, Akbar NS (2014) Blood flow of nanofluid through an artery with composite stenosis and permeable walls. Appl Nanosci 4:919–926
Ratchagar NP, Subasri S (2022) Study of multiple stenosed artery with hall current impact on MHD pulsatile blood fluid through porous channel unsteady wall suction/injection. Int J Mech Eng 7:2
Eldesoky IM (2012) Slip effects on the unsteady MHD pulsatile blood flow through porous medium in an artery under the effect of body acceleration. Int J Math Math Sci 2012:1–26
Eldesoky IM (2013) Effect of relaxation time on MHD pulsatile flow of blood through porous medium in an artery under the effect of periodic body acceleration. J Biol Syst 21(02):1350011
Bunonyo K, Amos E, Nwaigwe C (2021) Modeling the treatment effect on LDL-C and atherosclerotic blood flow through microchannel with heat and magnetic field. Int J Math Trends Technol 67(10):41–58
Shahzadi I, Nadeem S (2017) Stimulation of metallic nanoparticles under the impact of radial magnetic field through eccentric cylinders: a useful application in biomedicine. J Mol Liq 225:365–381
Shahzadi I, Nadeem S (2017) Inclined magnetic field analysis for metallic nanoparticles submerged in blood with convective boundary condition. J Mol Liq 230:61–73
Kolin A (1936) An electromagnetic flowmeter. Principle of the method and its application to bloodflow measurements. Proc Soc Exp Biol Med 35(1):53–56
Korchevskii E, Marochnik L (1965) Magnetohydrodynamic version of movement of blood. Biophysics 10(2):411–414
Haik Y, Pai V, Chen C-J (2001) Apparent viscosity of human blood in a high static magnetic field. J Magn Magn Mater 225(1–2):180–186
Yadav R, Harminder S, Bhoopal S (2008) Experimental studies on blood flow in stenosis arteries in presence of magnetic field. Ultra Sci 20(3):499–504
Mekheimer KS, El Kot M (2008) Influence of magnetic field and Hall currents on blood flow through a stenotic artery. Appl Math Mech 29:1093–1104
Shit G, Roy M, Sinha A (2014) Mathematical modelling of blood flow through a tapered overlapping stenosed artery with variable viscosity. Appl Bionics Biomech 11(4):185–195
Cavalcanti S (1995) Hemodynamics of an artery with mild stenosis. J Biomech 28(4):387–399
Rathod V, Tanveer S (2009) Pulsatile flow of couple stress fluid through a porous medium with periodic body acceleration and magnetic field. Bull Malays Math Sci Soc 32:2
Sankar D, Lee U (2011) FDM analysis for MHD flow of a non-Newtonian fluid for blood flow in stenosed arteries. J Mech Sci Technol 25:2573–2581
Mirza IA, Abdulhameed M, Vieru D, Shafie S (2016) Transient electro-magneto-hydrodynamic two-phase blood flow and thermal transport through a capillary vessel. Comput Methods Programs Biomed 137:149–166
Tanveer A, Ashraf MB, Masood M (2023) Entropy analysis of peristaltic flow over curved channel under the impact of MHD and convective conditions. Numer Heat Trans Part B: Fundam. https://doi.org/10.1080/10407790.2023.2224507
Tanveer A, Ashraf MB (2023) Analysis of entropy generation and Joule heating effects for MHD peristaltic flow over an asymmetric channel with mixed convective conditions. ZAMM-J Appl Math Mech/Zeitschrift für Angewandte Mathematik und Mechanik. https://doi.org/10.1002/zamm.202300089
Shahzadi I, Nadeem S (2017) Impinging of metallic nanoparticles along with the slip effects through a porous medium with MHD. J Braz Soc Mech Sci Eng 39:2535–2560
Shahzadi I, Nadeem S, Rabiei F (2017) Simultaneous effects of single wall carbon nanotube and effective variable viscosity for peristaltic flow through annulus having permeable walls. Res Phys 7:667–676
Shahzadi I, Nadeem S (2017) Role of inclined magnetic field and copper nanoparticles on peristaltic flow of nanofluid through inclined annulus: application of the clot model. Commun Theor Phys 67(6):704
Irshad N, Saleem A, Nadeem S, Shahzadi I (2018) Endoscopic analysis of wave propagation with Ag-nanoparticles in curved tube having permeable walls. Curr Nanosci 14(5):384–402
Shahzadi I, Nadeem S (2019) A comparative study of Cu nanoparticles under slip effects through oblique eccentric tubes, a biomedical solicitation examination. Can J Phys 97(1):63–81
Ayub M, Shahzadi I, Nadeem S (2019) A ballon model analysis with Cu-blood medicated nanoparticles as drug agent through overlapped curved stenotic artery having compliant walls. Microsyst Technol 25:2949–2962
Shahzadi I, Ijaz S (2019) On model of hybrid Casson nanomaterial considering endoscopy in a curved annulas: a comparative study. Phys Scr 94(12):125215
Shahzadi I, Kousar N (2019) Hybrid mediated blood flow investigation for atherosclerotic bifurcated lesions with slip, convective and compliant wall impacts. Comput Methods Programs Biomed 179:104980
Sus C (1995) Enhancing thermal conductivity of fluids with nanoparticles, developments and applications of non-Newtonian flows. ASME, FED, MD, 1995 231:99-I05
Sandeep N, Sugunamma V, Mohankrishna P (2013) Effects of radiation on an unsteady natural convective flow of a EG-Nimonic 80a nanofluid past an infinite vertical plate. Adv Phys Theor Appl 23:36–43
Akbar NS, Nadeem S, Haq RU, Khan Z (2013) Radiation effects on MHD stagnation point flow of nano fluid towards a stretching surface with convective boundary condition. Chin J Aeronaut 26(6):1389–1397
Gireesha B, Manjunatha S, Bagewadi C (2012) Unsteady hydromagnetics boundary layer flow and heat transfer of dusty fluid over a stretching sheet. Afr Mat 23:229–241
Krishna PM, Sugunamma V, Sandeep N (2014) Radiation and magnetic field effects on unsteady natural convection flow of a nanofluid past an infinite vertical plate with heat source. Chem Process Eng Res 25:39–52
Sahoo S, Parveen S, Panda J (2007) The present and future of nanotechnology in human health care. Nanomed: Nanotechnol Biol Med 3(1):20–31
Nadeem S, Ijaz S, Akbar NS (2013) Nanoparticle analysis for blood flow of Prandtl fluid model with stenosis. Int Nano Lett 3:1–13
Akbar NS, Tripathi D, Bég OA (2017) Variable-viscosity thermal hemodynamic slip flow conveying nanoparticles through a permeable-walled composite stenosed artery. Eur Phys J Plus 132:1–11
Elnaqeeb T, Shah NA, Mekheimer KS (2019) Hemodynamic characteristics of gold nanoparticle blood flow through a tapered stenosed vessel with variable nanofluid viscosity. BioNanoScience 9:245–255
Zaman A, Ali N, Khan AA (2020) Computational biomedical simulations of hybrid nanoparticles on unsteady blood hemodynamics in a stenotic artery. Math Comput Simul 169:117–132
Abumandour R, Eldesoky IM, Abumandour M, Morsy K, Ahmed MM (2022) Magnetic field effects on thermal nanofluid flowing through vertical stenotic artery: analytical study. Mathematics 10(3):492
Algehyne EA et al (2023) Enhancing heat transfer in blood hybrid nanofluid flow with A g–TiO2 nanoparticles and electrical field in a tilted cylindrical W-shape stenosis artery: a finite difference approach. Symmetry 15(6):1242
Shahzadi I, Suleman S, Saleem S, Nadeem S (2020) Utilization of Cu-nanoparticles as medication agent to reduce atherosclerotic lesions of a bifurcated artery having compliant walls. Comput Methods Programs Biomed 184:105123
Shahzadi I, Bilal S (2020) A significant role of permeability on blood flow for hybrid nanofluid through bifurcated stenosed artery: drug delivery application. Comput Methods Programs Biomed 187:105248
Mekheimer KS, Shahzadi I, Nadeem S, Moawad A, Zaher A (2020) Reactivity of bifurcation angle and electroosmosis flow for hemodynamic flow through aortic bifurcation and stenotic wall with heat transfer. Phys Scr 96(1):015216
Sadaf H, Shahzadi I (2021) Physiological transport of Rabinowitsch fluid model with convective conditions. Int Commun Heat Mass Transfer 126:105365
Nazir U, Saleem S, Al-Zubaidi A, Shahzadi I, Feroz N (2022) Thermal and mass species transportation in tri-hybridized Sisko martial with heat source over vertical heated cylinder. Int Commun Heat Mass Transfer 134:106003
Shahzadi I, Duraihem FZ, Ijaz S, Raju C, Saleem S (2023) Blood stream alternations by mean of electroosmotic forces of fractional ternary nanofluid through the oblique stenosed aneurysmal artery with slip conditions. Int Commun Heat Mass Transfer 143:106679
Chow J, Soda K (1973) Laminar flow and blood oxygenation in channels with boundary irregularities. J Appl Mech 40:843
Abumandour RM, S. EL-Behery, M. H. Kamel, A. S. Dawood, and I. M. Eldesoky, (2020) Analysis of different stenotic geometries on two-phase blood flow. ERJ. Eng Res J 43(4):355–367
Hanvey R, Bunonyo K (2022) Effect of treatment parameter on oscillatory flow of blood through an atherosclerotic artery with heat transfer. J Niger Soc Phys Sci. https://doi.org/10.46481/jnsps.2022.682
Brewster MQ (1992) Thermal radiative transfer and properties. Wiley, Hoboken
Ali A, Bukhari Z, Amjad M, Ahmad S, Din TE, Hussain SM (2022) Newtonian heating effect in pulsating magnetohydrodynamic nanofluid flow through a constricted channel: a numerical study. Front Energy Res 10:1002672
P. Chaturani and R. Ponnalagarsamy, (1984) Analysis of pulsatile blood flow through stenosed arteries and its applications to cardiovascular diseases. In Proceedings of the 3rd National Conference on Fluid Mechanics and Fluid Power, pp. 463–468.
Shah Z, Kumam P, Selim MM, Alshehri A (2021) Impact of nanoparticles shape and radiation on the behavior of nanofluid under the Lorentz forces. Case Stud Ther Eng 26:101161
Tripathi J, Vasu B, Bég OA, Gorla RSR (2021) Unsteady hybrid nanoparticle-mediated magneto-hemodynamics and heat transfer through an overlapped stenotic artery: biomedical drug delivery simulation. Proc Inst Mech Eng [H] 235(10):1175–1196
G. Shit and M. Roy, (2012) Hydromagnetic pulsating flow of blood in a constricted porous channel: A theoretical study, In Proceedings of the World Congress on Engineering, London, UK, vol. 1.
Abu-Hamdeh NH, Bantan RA, Aalizadeh F, Alimoradi A (2020) Controlled drug delivery using the magnetic nanoparticles in non-Newtonian blood vessels. Alex Eng J 59(6):4049–4062
Singh R, Lillard JW Jr (2009) Nanoparticle-based targeted drug delivery. Exp Mol Pathol 86(3):215–223
Patra JK et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):1–33
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, F.K, and A.D.; methodology, F.K, and A.D.; software, F.K, and A.D., and R.A; formal analysis, F.K, A.D. and I. E.; investigation, F.K, A.D., and I.E., resources, F.K, A.D., I.E., and R.A.; data curation, F.K, A.D., I.E, and R.A.; writing—original draft preparation, F.K., and A.D.; writing—review and editing, F.K, A.D., I.E, and R.A.; visualization, F.K, A.D., I.E, and R.A.; supervision, F.K, A.D., I.E, and R.A.; project administration, F.K, A.D., I.E, and R.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
\(m_{1} = \sqrt {\left( {Ha^{2} (1 - \varphi )^{2.5} + \frac{1}{Da}} \right)} , m_{2} = - \sqrt {\left( {Ha^{2} (1 - \varphi )^{2.5} + \frac{1}{Da}} \right)} ,\)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dawood, A.S., Kroush, F.A., Abumandour, R.M. et al. Multi-effect analysis of nanofluid flow in stenosed arteries with variable pressure gradient: analytical study. SN Appl. Sci. 5, 382 (2023). https://doi.org/10.1007/s42452-023-05567-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05567-6