Abstract
Calcium Oxide (CaO) nanoparticles have been synthesized from waste eggshells (ES) as a source of calcium by solution combustion technique using muffle furnace and microwave oven. CaO nanoparticles obtained using muffle furnace (CaO-F) and via microwave oven (CaO-M) were characterized using X-ray diffraction, scanning electron microscopy, energy dispersive spectroscopy, AC conductivity and dielectric studies to correlate the physico-chemical behavior. The frequency dependence of AC conductivity, the permittivity (ε′) and dielectric loss tangent (D) studies have been undertaken in the frequency range 50 Hz–5 MHz at room temperature. AC conductivity was found to enhance with an increase in the frequency. The ε′ was found to decrease initially with the frequency and finally reaching a constant value at higher frequencies. The frequency dependence of D was found to exhibit a resonance peak in the studied frequency region. Catalytic activity of CaO-M was correlated with its dielectric behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Eggshell (ES) waste, is listed among the top 10 waste obtained from food processing industry. Disposal of ES is creating a huge problem due to difficulty associated with land fillings. Therefore, it is economical in transforming waste biomaterial into material of commercial importance. Shells from sea shore, bird’s shells, Mollusca shells, snail shells, duck and chicken ES are main important naturally occurring source of CaCO3. Calcium oxide (CaO) is an important industrial compound, which finds a huge application as catalyst for heterogeneous base catalysed reactions, as CO2 adsorbent, as a calcium supplement. The consumption of eggs on a manufacturing level leads to a substantial magnitude of residual ES waste, which is painstaking as a dissipate. Accordingly, with time, huge waste eggshells bio-material is accumulated. It may lead to environmental harms, if ES are not disposed properly. Thereby it is striking the company holders in solving the problem of getting rid of the waste generated. Food dispensation industry pays out an enormous sum of capital towards waste ES clearance [1]. ES waste is a good bio-ceramic material consisting of 96 % of CaCO3[2]. Waste ES are continuously utilized to reduce the accumulation of wastage. Effective utilization of ES has opened up a broad view of waste management issues. As per the literature studies, waste ES have been subjected to pyrolysis/calcination above 900 ºC to obtain CaO [3]. Obtained CaO is used as a catalyst towards biodiesel manufacture [4, 5].
In the similar streamline, endorsement of biomass as a substitute resource for energy and chemicals is an imperative prerequisite towards attaining sustainable development [6]. In this context, biomass is the main source for biodiesel production thereby resulting in tons of glycerol as the by-product [7]. Glycerol is colorless, viscous, safe to handle and common polyhydric alcohol with a high degree of functionality [8]. High degree of functionality of glycerol; resulted in glycerol valorization [9,10,11,12]. Amongst the various fine commodity chemicals obtained from glycerol; Glycerol carbonate (GC) is one of the important engineering green chemical [13]. GC finds its application as a green solvent in the manufacture of surfactant, polymers and detergents. CaO catalyst has been employed for the transesterification of glycerol to GC [5].
In the present studies, CaO nanoparticles have been synthesized from waste ES as a source of calcium by using solution combustion technique using muffle furnace and microwave oven. Synthesized samples were characterized using XRD, SEM and EDX analysis. AC conductivity and dielectric studies on the samples have been carried out to correlate the reported catalytic activity of CaO with its dielectric properties.
2 Materials and methods
2.1 Chemicals
Nitric acid (HNO3) and Urea (CH4N2O) were of the analytical grade and purchased from SD Fine Chemicals, India. Chemicals were used without additional purification as they were of research grade.
2.2 Synthesis of CaO
ES were washed with water to remove impurities and soaked in hot water for 2 h so that egg membrane gets separated from eggshells. Further, ES were repeatedly washed under running water; dried out and then pulverized using mortar and pestle. To obtain the fine powder, ES were ball milled. The well-grinded ES powder was heated to 200 °C for 6 h.
For the synthesis of CaO, calculated quantity of ES powder (CaCO3) was dissolved in nitric acid to get a yellow colour solution of calcium nitrate (Ca(NO3)2). To the above yellow solution, a stoichiometric amount of urea dissolved in 50 ml of double distilled water was added. Combustion reaction is as shown in scheme 1. The redox mixture obtained was taken in a Pyrex dish and kept in a preheated muffle furnace at 450 ± 10 ˚C. The obtained sample was labelled as CaO-F. A similar solution was prepared and combustion synthesis was carried out in modified domestic microwave oven (Koryo, KMC 2523, 900 W), the obtained product was highly porous and foamy. Non-flammable dense fumes were observed during MW combustion. The obtained sample was labelled as CaO-M. Both CaO-F and CaO-M were heated at 450 °C for 4 h in a muffle furnace. Figure 1. shows a representation of the catalyst preparation process.
2.3 Characterization of synthesized CaO
The prepared catalyst samples were subjected to structural characterization. Crystal structure of the synthesized catalyst samples were determined using X-ray diffraction analysis, with a CuKα radiation source (λ = 1.5406 Å) using Bruker AXS D8 Advance diffractometer. Leica S440 Scanning electron microscope (SEM) attached with an Energy-Dispersive X-ray (EDX) spectroscopy was used to study morphology and chemical composition of the samples.
AC conductivity and dielectric behavior of the synthesized CaO-F and CaO-M samples have been undertaken using programmable impedance analyzer model HIOKI 3532-50 LCR HiTESTER. The measurements were carried out at room temperature in the range 50 Hz-5 MHz frequency. For the determination of AC conductivity and dielectric properties, the representative samples CaO-F and CaO-M were pressed into pellets with the diameter of 1 cm under a hydraulic pressure of 3.5 tons using polymer press automatic techno search instrument. After drying at 50 ºC the prepared pellets were coated with a silver paste to ensure good contacting.
3 Results and discussion
3.1 X-ray diffraction analysis
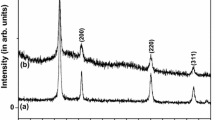
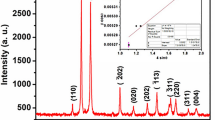
Figure 2 represents the XRD pattern of the CaO-F and CaO-M samples. XRD pattern indicates the presence of a cubic CaO phase and hexagonal Ca(OH)2 phases in the present samples. The diffraction peaks observed at 2θ angles 31.9°, 37.27°, 53.93°, 64.15°, and 67.41°, can be indexed entirely to a (space group: Fm-3 m) crystal cubic phase of CaO, with the lattice constant of a = 4.8105 Å, and which are well matched with the reported standard values (JCPDS card number 37-1497). Diffraction peaks observed at 17.8°, 28.3°, 34°, 46.9°, 50.6°, 62.4° corresponds to the crystalline phases of Ca(OH)2 are hexagonal having space group P3m1 with the lattice parameters a = 3.5899 Å, c = 4.916 Å, which are close to the reported values, (JCPDS card number 44-1481). In general, the broadening of XRD peaks may result from small grain sizes or structural micro distortions in the crystal. The average crystallite size of the CaO particles were determined by the Debye-Scherrer formula, \(D =\frac{ \text{k} {\uplambda }}{{\upbeta } \text{C}\text{o}\text{s} {\uptheta }}\), where D is the crystallite size, k = 0.9 is a correction factor to account for the particle shapes, β is the full width at a half maximum of the most intense diffraction peak (200) plane, λ is the wavelength of a Cu Kα radiation (1.5406 Å) and θ is the Bragg angle. The average crystallite size of the CaO-F and CaO-M particles are found to be 87 nm and 46 nm respectively. Thus, lower particle size of CaO-M indicates that the CaO-M particles possesses large surface to volume ratio than that of the CaO-F sample.
3.2 SEM and EDX analysis
Morphological studies have been performed on CaO-M and CaO-F samples using SEM analysis. SEM image (Fig. 3a) of CaO-M sample indicates that CaO-M sample possess a crystalline rod structures. Whereas, SEM image (Fig. 3b) of CaO-F indicates that the CaO-F sample is flaky and possess a fibrous structure with aggregates of irregular shapes.
Figure 4 displays the EDX pattern of CaO-M and CaO-F samples. The EDX pattern (Fig. 4a) shows the presence of 60.21 wt% of Ca and 39.79 wt% of O within the CaO-M sample. EDX pattern (Fig. 4b) shows the presence of 60.97 wt% of Ca and 39.03 wt% of O within the CaO-F sample. Further, histograms (Fig. 5) shows the grain size distributions of CaO-M and CaO-F samples.
3.3 A.C. conductivity and dielectric studies
The variation of AC conductivity (σac) with the frequency is shown in Fig. 6. The σac of the CaO-M and CaO-F is found to rise with the rise in the frequency. At low-frequency, the raise in σac is slower but as the frequency is raised, σac increases. This indicates that the conductive state is more active at high frequency region due to the hopping of charge carriers within the sample. The electrical conduction mechanism in the CaO is found to be in accordance with the electron hopping model. Inspection of the electrical conductivity as a function of frequency (below 1 kHz), the conductivity shows nearly no dispersion for the samples CaO-M and CaO-F. At higher frequency (above 1 K Hz), the conductivity of the sample displayed dependency on frequency. Thereby electrical property of CaO-M and CaO-F are the function of frequency of the applied field. The boost in conductivity is well thought-out as a fine gauge of a decline in activation energy (activation energy is always related to its catalytic activity) [14]. The observed increase in conductivity is in accordance with the increase of catalytic activity and mobility of charge carriers [15].
Dielectric behavior of materials describes electrical energy storage, dissipation, and energy transfer as a result of dielectric polarization which causes rearrangement of molecular dipoles or charge displacement [16, 17]. Generally, high dielectric materials with optimized dielectric properties are suitable for energy transfer and energy storage devices. Whereas, low dielectric materials are suitable for substrate and packaging applications. It has been reported that the dielectric properties are also found to influence the catalytic activity of the sample [18]. The dielectric parameters such as dielectric permittivity (ε′) and dielectric loss tangent (D) are studied to know the effect of frequency on these parameters at room temperature in the frequency range 50 Hz-5 MHz. The variation of ε′ with frequency for the samples CaO-M and CaO-F are shown in Fig. 7. From the graph, it is observed that CaO-M has ε′ values of nearly 1650 and 1200 at frequencies 50 Hz and 100 Hz respectively. Similarly, CaO-F has ε′ values nearly 1100 and 950 at frequencies 50 Hz and 100 Hz respectively. It can be observed that for samples CaO-M and CaO-F, the ε′ initially decreases rapidly with increase in frequency but beyond 10 kHz remains fairly constant. Generally, the ε′ of any material depends on dipolar, electronic, ionic and interfacial polarizations [16]. At low frequencies, dipolar and interfacial polarizations are known to play a substantial role. While, at high frequencies, electronic and ionic polarizations are the key contributors. The dipoles can orient themselves with the electric field at the lower frequency range, but at higher frequency, the dipole response is restricted with applied voltage since the movement of contributing dipoles is seized. Therefore, with increasing frequency average polarization decreases generating from limited dielectric response that results in a lowering of dielectric constant at higher signal frequency.
The polarization of a catalyst increases its reactivity towards the hydrocarbons under conversion. This is achieved by inducing polarization of the hydrocarbons (which are usually weakly polar) and their subsequent attraction (adsorption); this is an important initial step in the catalytic process. From the previous studies, it has been clear that higher the ε′, higher is the catalytic activity [18, 19]. It is interesting note that the present CaO-M sample shows higher ε′ at a lower frequency up to 100 Hz implying high catalytic activity at lower frequency.
The electrical energy loss or dissipation in the material arises due to electrical charge transport, dielectric relaxation, resonant transitions and nonlinear dielectric effects. The loss tangent (D) is the energy dissipation in dielectrics, which is equal to the ratio of the imaginary part of dielectric permittivity to the real part and is largely dependent on random grain orientations and grain boundaries. Thus, the power of the dielectric loss of the absorbing material is characterized by the dielectric loss tangent, \(D={\epsilon }^{\prime\prime}/{\epsilon }^{\prime}\). Good dielectric loss material should possess a larger dielectric loss tangent independent of frequency. Figure 8 displays the variation of D with the log frequency for the CaO-M and CaO-F sample. It can be observed that D decreases with increasing frequency followed by the appearance of a resonance peak between frequency regions 50-300 Hz and 300 Hz-30 kHz. The observation of peak indicates the presence of resonance between the hopping frequency of the charge carriers and applied frequency. The relaxation polarization takes place in dielectric materials containing polar molecules, or molecules with polar radicals and weakly bound ions, as well as in materials in which electron defects are developed by thermal activation. Such relaxation polarization process requires long excitation and relaxation times [18]. Relaxation time reflects the rapidity with which dipoles or ions become oriented when an external field is applied. The condition for observing a maximum in the dielectric losses of a dielectric material is given by,
where \(\omega\) is the \(2\pi {f}_{max}\), here \({f}_{max}\) is the frequency at the maximum and \(\tau\) is the relaxation time.
\(\tau\) is found to be \(1.59\times 10^{-4} s\) and \(3.4 \times {10}^{-5} s\) for CaO-M and CaO-F samples respectively.
3.4 Relation between the catalytic activity and the dielectric behavior
Literature studies have shown that the catalytic activity assessment studies of the CaO synthesized by microwave combustion (CaO-M) had better catalytic activity as compared to CaO synthesized by muffle furnace (CaO-F) [5]. Where, the catalytic studies of the CaO-M and CaO-F were subjected to transesterification reaction of glycerol and dimethyl carbonate. Transesterification reaction was performed at different temperature, molar concentration and microwave irradiation time. In the literature, it has been reported that the catalytic activity of the materials is related to their dielectric permittivity [18]. Observed higher dielectric permittivity values of the CaO-M samples in the present studies and enhanced catalytic activity of the CaO-M samples reported in the literature also supports the observed relation between dielectric permittivity and catalytic activity. Similar results were obtained by Bahga Hanna and Fikry H Khalil for local clay samples [18].
4 Conclusions
CaO nanoparticles have been successfully synthesized from waste ES as a source of calcium by solution combustion method using muffle furnace and microwave oven. CaO nanoparticles were characterized using XRD, SEM, EDX, AC conductivity and dielectric studies to correlate the physico-chemical behavior. AC conductivity was found to enhance with an increase in the frequency The ε′ was found to decrease initially with the frequency and finally reaching a constant value at higher frequencies. The frequency dependence of D was found to exhibit a resonance peak in the studied frequency region. Better catalytic activity of CaO-M sample as compared to CaO-F sample was attributed to its lower particle size and higher dielectric permittivity.
References
Mignon-Grasteau S, Beaumont C, Ricard FH (2001) Genetic analysis of a selection experiment on the growth curve of chickens. Poult Sci 80:849–854
Buasri A, Chaiyut N, Loryuenyong V, Wongweang C, Khamsrisuk S (2013) Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sus Energy 1:7–13
Kouzu M, Kasuno T, Tajika M, Sugimoto Y, Yamanaka S, Hidaka J (2008) Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel. Fuel 87:2798–2806
Viriya-empikul N, Krasae P, Puttasawat B, Yoosuk B (2010) Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour Technol 101:3765–3767
Bhagyalakshmi H, Veerabhadraswamy M, Venkatesha N (2018) Microwave assisted synthesis of highly crystalline nano CaO from waste egg shells—a low temperature record activity in glycerol carbonate. J Ultra Chem 14(4):115–125
Drożyner P, Rejmer W, Starowicz P, Klasa A, Skibniewska KA (2013) Biomass as a renewable source of energy. Tech Sci 16(3):211–220
Guerrero-Perez MO, Rosas JM, Bedia J, Rodriguez-Mirasol J, Cordero T (2009) “Recent Patents on Chemical Engineering” Green Chem 2(1):11–21
Pagliaro M, Rossi M (2008) The Future of Glycerol: New Usages for a Versatile Raw Material. RSC Green Chemistry Book Series. https://doi.org/10.1039/9781847558305
Nanda MR, Zhang Y, Yuan Z, Qin W, Ghaziaskar HS, Xu C (2016) Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: a review. Sustain Energy Rev 56:1022–1031
Tasnadi-Asztalos Z, Agachi PS, Cormos CC (2015) Evaluation of energy efficient low carbon hydrogen production concepts based on glycerol residues from biodiesel production. Int J Hydrogen Energy 40:7017–7027
Liu L, Ye XP (2015) Simultaneous production of lactic acid and propylene glycol from glycerol using solid catalysts without external hydrogen. Fuel Process Technol 137:55–65
Li H, Gao D, Gao P, Wang F, Zhao N, Xiao F, Wei W, Sun Y (2013) The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3–ZnO catalysts. Catal Sci Technol 3:2801–2809
Ochoa-Go´mez JR, Go´mez-Jime´nez-Aberasturi O, Ramı´rez-Lo´pez C, Belsue M (2012) A brief review on industrial alternatives for the manufacturing of glycerol carbonate, a green chemical. Org Process Res Dev 16:389–399
Gobara HM, Gomaa MM (2009) Electrical properties of Ni/Silica Gel and Pt/-Alumina catalysts in relation to catalytic activity. Pet Sci Technol 27:1572–1591
Kreuer KD, Dippel T, Haynovsky NG, Maier J (1992) Proton conductivity: compounds and their structural and chemical peculiarities. Bunsenges Phys Chem 96:1736
Madhu BJ, Ashwini ST, Shruthi B, Divyashree BS, Manjunath A, Jayanna HS (2014) Structural, dielectric and electromagnetic shielding properties of Ni–Cu nanoferrite/PVP composites. Mater Sci Eng B 186:1–6
Madhu BJ, Gurusiddesh M, Kiran T, Shruthi B, Jayanna HS (2016) Structural, dielectric, ac conductivity and electromagnetic shielding properties of polyaniline/Ni0.5Zn0.5Fe2O4 composites. J Mater Sci Mater Electron 27:7760–7766
Hanna B, Khalil FH (1982) Relation between the electric properties and the catalytic activity of some egyptian clays. Surf Technol 17:61–68
Zhang X, Zhang F, Chan KY (2004) The synthesis of large mesopores alumina by microemulsion templating, their characterization and properties as catalyst support. Mater Lett 58:2872–2877
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Madhu, B.J., Bhagyalakshmi, H., Shruthi, B. et al. Structural, AC conductivity, dielectric and catalytic behavior of calcium oxide nanoparticles derived from waste eggshells. SN Appl. Sci. 3, 637 (2021). https://doi.org/10.1007/s42452-021-04607-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04607-3