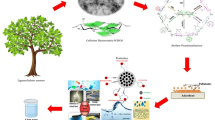
Abstract
Introduction
Organic pollutants have attracted great interest due to their toxic effects on human beings and the environment. Developing innovative sustainable and environmentally friendly efficient adsorbents are required for water purification.
Objectives
This article describes the immobilization of magnetite (Fe3O4) nanoparticles on TEMPO-oxidized cellulose nanofiber (TEMPO-CNF) through the co-precipitation of iron salts by the alkaline medium.
Experiments
TEMPO-CNF and TEMPO-CNF/Fe3O4 were prepared, and their structure was confirmed by XRD, TGA, SEM, TEM, BET, and AFM. The ability of the synthesized nanobiocomposite for methylene blue (MB) adsorption, as a cationic dye model, was examined. In addition, the thermodynamics parameters have been evaluated from direct measurement based on Isothermal Titration Calorimetry as well as the recyclability of the material.
Results
Characterization of the samples successfully showed the preparation of cellulose nanofibers with a diameter ranging from 10-15 nm and homogenous and uniform magnetic nanoparticles with a diameter of ~ 10 nm. At pH 7, the highest adsorption efficiency was observed. The isotherm, kinetic models, and enthalpic contributions were calculated to evaluate adsorption experiments. With an adsorption capacity of around 303 mg/g, pseudo-second-order and Langmuir isotherm provided the best interpretation adsorption process. The adsorption process was endothermic and spontaneous, with weak interactions (5.2 kJ/mol). The material exhibits a good desorption ability with a mixture of 5% (v/v) acetic acid and methanol up to the fifth regeneration cycle.
Conclusion
The current results showed that TEMPO-CNF/Fe3O4 nanocomposite is a promising green bioadsorbent for organic dye removal for water purification.












Similar content being viewed by others
Data Availability
The research data associated with a paper is available.
References
Wang P, Yan T, Wang L (2013) Removal of congo red from aqueous solution using magnetic chitosan composite microparticles. BioResources 8:6026–6043. https://doi.org/10.15376/biores.8.4.6026-6043
Salama A (2017) New sustainable hybrid material as adsorbent for dye removal from aqueous solutions. J Colloid Interface Sci 487:348–353. https://doi.org/10.1016/j.jcis.2016.10.034
Qian H, Wang J, Yan L (2020) Synthesis of lignin-poly(N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J Bioresour Bioprod 5:204–210. https://doi.org/10.1016/j.jobab.2020.07.006
Jjagwe J, Olupot PW, Menya E, Kalibbala HM (2021) Synthesis and application of Granular activated Carbon from Biomass Waste materials for Water Treatment: a review. J Bioresour Bioprod 6:292–322. https://doi.org/10.1016/j.jobab.2021.03.003
Darmograi G, Prelot B, Geneste A, De Menorval L-C, Zajac J (2016) Removal of three anionic orange-type dyes and cr(VI) oxyanion from aqueous solutions onto strongly basic anion-exchange resin. The effect of single-component and competitive adsorption. Colloids Surf Physicochem Eng Asp 508:240–250. https://doi.org/10.1016/j.colsurfa.2016.08.063
Darmograi G, Kus M, Martin-Gassin G, Zajac J, Cavaliere S, Prelot B (2017) How competitive species such as buffer solutions influence the adsorption of dyes onto photocatalyst TiO 2 particles. Mater Res Bull 94:70–76. https://doi.org/10.1016/j.materresbull.2017.05.025
Darmograi G, Prelot B, Layrac G, Tichit D, Martin-Gassin G, Salles F, Zajac J (2015) Study of Adsorption and Intercalation of Orange-Type Dyes into Mg–Al Layered double hydroxide. J Phys Chem C 119:23388–23397. https://doi.org/10.1021/acs.jpcc.5b05510
Darmograi G, Prelot B, Geneste A, Martin-Gassin G, Salles F, Zajac J (2016) How does competition between Anionic Pollutants affect adsorption onto Mg–Al Layered double hydroxide? Three competition schemes. J Phys Chem C 120:10410–10418. https://doi.org/10.1021/acs.jpcc.6b01888
Salama A, Hesemann P (2018) New N-guanidinium chitosan/silica ionic microhybrids as efficient adsorbent for dye removal from waste water. Int J Biol Macromol 111:762–768. https://doi.org/10.1016/j.ijbiomac.2018.01.049
Hassan H, Salama A, El-ziaty AK, El-Sakhawy M (2019) New chitosan/silica/zinc oxide nanocomposite as adsorbent for dye removal. Int J Biol Macromol 131:520–526. https://doi.org/10.1016/j.ijbiomac.2019.03.087
Salama A, Abou-Zeid RE (2021) Ionic chitosan/silica nanocomposite as efficient adsorbent for organic dyes. Int J Biol Macromol 188:404–410. https://doi.org/10.1016/j.ijbiomac.2021.08.021
Zheng J, Yan B, Feng L, Zhang Q, Zhang C, Yang W, Han J, Jiang S, He S (2022) Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diam Relat Mater 128:109247. https://doi.org/10.1016/j.diamond.2022.109247
Abou-Zeid RE, Kamal KH, Abd El-Aziz ME, Morsi SM, Kamel S (2021) Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int J Biol Macromol 167:1091–1101. https://doi.org/10.1016/j.ijbiomac.2020.11.063
Bagheri E, Rahnama H, Hassannia MA, Behzad T, Mosaddegh P (2021) Oriented polylactic acid/graphene oxide nanocomposites with high mechanical and thermal properties. J Thermoplast Compos Mater 54:089270572110386. https://doi.org/10.1177/08927057211038625
Salama A, Etri S, Mohamed SAA, El-Sakhawy M (2018) Carboxymethyl cellulose prepared from mesquite tree: New source for promising nanocomposite materials. Carbohydr Polym 189:138–144. https://doi.org/10.1016/j.carbpol.2018.02.016
Salama A (2018) Preparation of CMC-g-P(SPMA) super adsorbent hydrogels: exploring their capacity for MB removal from waste water. Int J Biol Macromol 106:940–946. https://doi.org/10.1016/j.ijbiomac.2017.08.097
Abouzeid RE, Salama A, El-Fakharany EM, Guarino V (2022) Mineralized polyvinyl Alcohol/Sodium Alginate Hydrogels incorporating cellulose nanofibrils for bone and Wound Healing. Molecules 27:697. https://doi.org/10.3390/molecules27030697
Hassan ML, Zeid REA, Fadel SM, El Sakhawy M, Khiari R (2014) Cellulose nanocrystals and carboxymethyl cellulose from olive stones and their use to improve paper sheets properties. Int J Nanoparticles 7:261. https://doi.org/10.1504/IJNP.2014.067613
Abouzeid RE, Khiari R, El-Wakil N, Dufresne A (2019) Current state and New Trends in the Use of Cellulose Nanomaterials for Wastewater Treatment. Biomacromolecules 20:573–597. https://doi.org/10.1021/acs.biomac.8b00839
Salama A, Hesemann P (2020) Recent Trends in Elaboration, Processing, and derivatization of cellulosic materials using ionic liquids. ACS Sustain Chem Eng 8:17893–17907. https://doi.org/10.1021/acssuschemeng.0c06913
El-Sayed NS, Salama A, Guarino V (2022) Coupling of 3-Aminopropyl Sulfonic Acid to cellulose nanofibers for efficient removal of Cationic Dyes. Mater (Basel) 15:6964. https://doi.org/10.3390/ma15196964
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173. https://doi.org/10.1016/j.watres.2016.01.008
Abouzeid RE, Khiari R, Salama A, Diab M, Beneventi D, Dufresne A (2020) In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int J Biol Macromol 160:538–547. https://doi.org/10.1016/j.ijbiomac.2020.05.181
Shayan M, Gwon J, Koo MS, Lee D, Adhikari A, Wu Q (2022) pH-responsive cellulose nanomaterial films containing anthocyanins for intelligent and active food packaging. Cellulose 1. https://doi.org/10.1007/s10570-022-04855-5
El-Gendy A, Abou-Zeid RE, Salama A, Diab MA, El-Sakhawy M (2017) TEMPO-oxidized cellulose nanofibers/polylactic acid/TiO2 as antibacterial bionanocomposite for active packaging. Egypt J Chem 60:1007–1014. https://doi.org/10.21608/ejchem.2017.1835.1153
Jaspal D, Malviya A (2020) Composites for wastewater purification: a review. Chemosphere 246:125788. https://doi.org/10.1016/j.chemosphere.2019.125788
Dutt MA, Hanif MA, Nadeem F, Bhatti HN (2020) A review of advances in engineered composite materials popular for wastewater treatment. J Environ Chem Eng 8:104073. https://doi.org/10.1016/j.jece.2020.104073
Yan B, Zheng J, Feng L, Du C, Jian S, Yang W, Wu YA, Jiang S, He S, Chen W (2022) Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 4:50. https://doi.org/10.1007/s42773-022-00176-9
Gutierrez AM, Dziubla TD, Hilt JZ (2017) Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev Environ Health 32:111–117. https://doi.org/10.1515/reveh-2016-0063
El-Nahas AM, Salaheldin TA, Zaki T, El-Maghrabi HH, Marie AM, Morsy SM, Allam NK (2017) Functionalized cellulose-magnetite nanocomposite catalysts for efficient biodiesel production. Chem Eng J 322:167–180. https://doi.org/10.1016/j.cej.2017.04.031
Abouzeid RE, Khiari R, Ali KA (2022) Activated Charcoal/Alginate Nanocomposite Beads for Efficient Adsorption of the Cationic Dye Methylene Blue: Kinetics and Equilibrium, Chem. Africa. https://doi.org/10.1007/s42250-022-00560-9
Alekseeva OV, Rodionova AN, Bagrovskaya NA, Agafonov AV, Noskov AV (2017) Hydroxyethyl cellulose/bentonite/magnetite hybrid materials: structure, physicochemical properties, and antifungal activity. Cellulose 24:1825–1836. https://doi.org/10.1007/s10570-017-1212-2
Kanmani P, Aravind J, Kamaraj M, Sureshbabu P, Karthikeyan S (2017) Environmental applications of chitosan and cellulosic biopolymers: a comprehensive outlook. Bioresour Technol 242:295–303. https://doi.org/10.1016/j.biortech.2017.03.119
Kloster GA, Marcovich NE, Mosiewicki MA (2015) Composite films based on chitosan and nanomagnetite. Eur Polym J 66:386–396. https://doi.org/10.1016/j.eurpolymj.2015.02.042
Morsi RE, Al-Sabagh AM, Moustafa YM, ElKholy SG, Sayed MS (2018) Polythiophene modified chitosan/magnetite nanocomposites for heavy metals and selective mercury removal. Egypt J Pet 27:1077–1085. https://doi.org/10.1016/j.ejpe.2018.03.004
Bezdorozhev O, Kolodiazhnyi T, Vasylkiv O (2017) Precipitation synthesis and magnetic properties of self-assembled magnetite-chitosan nanostructures. J Magn Magn Mater 428:406–411. https://doi.org/10.1016/j.jmmm.2016.12.048
Singh N, Yadav S, Mehta SK, Dan A (2022) In situ incorporation of magnetic nanoparticles within the carboxymethyl cellulose hydrogels enables dye removal. J Macromol Sci Part A 59:271–284. https://doi.org/10.1080/10601325.2022.2026788
Salama A, Abou-Zeid RE, Cruz-Maya I, Guarino V (2020) Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohydr Polym 229. https://doi.org/10.1016/j.carbpol.2019.115472
J.Ngenefeme F-T, Eko NJ, Mbom YD, Tantoh ND, Rui KWM (2013) A one Pot Green Synthesis and Characterisation of Iron Oxide-Pectin Hybrid Nanocomposite. Open J Compos Mater 03:30–37. https://doi.org/10.4236/ojcm.2013.32005
Zajac JJ (2013) Calorimetry at the Solid–Liquid Interface, in: : pp. 197–270. https://doi.org/10.1007/978-3-642-11954-5_6
Yan B, Zheng J, Feng L, Chen W, Yang W, Dong Y, Jiang S, Zhang Q, He S (2022) All-cellulose-based high-rate performance solid-state supercapacitor enabled by nitrogen doping and porosity tuning. Diam Relat Mater 128:109238. https://doi.org/10.1016/j.diamond.2022.109238
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Ashrafi M, Arab Chamjangali M, Bagherian G, Goudarzi N (2017) Application of linear and non-linear methods for modeling removal efficiency of textile dyes from aqueous solutions using magnetic Fe3O4 impregnated onto walnut shell. Spectrochim Acta - Part A Mol Biomol Spectrosc 171:268–279. https://doi.org/10.1016/j.saa.2016.07.049
Monier M, Abdel-Latif DA (2012) Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of hg(II), cd(II) and zn(II) ions from aqueous solutions. J Hazard Mater 209–210. https://doi.org/10.1016/j.jhazmat.2012.01.015
Salama A (2016) Functionalized hybrid materials assisted organic dyes removal from aqueous solutions. Environ Nanatechnol Monit Manag 6:159–163. https://doi.org/10.1016/j.enmm.2016.10.003
Zhao R, Wang Y, Li X, Sun B, Wang C (2015) Synthesis of β-cyclodextrin-based electrospun nanofiber membranes for highly efficient adsorption and separation of methylene blue. ACS Appl Mater Interfaces 7:26649–26657. https://doi.org/10.1021/acsami.5b08403
Wang Q, Liu S, Chen H, Liu J, Zhu Q (2022) TEMPO-oxidized cellulose beads for cationic dye adsorption, BioResources. 176056–6066. https://doi.org/10.15376/biores.17.4.6056-6066
Chen L, Li Y, Hu S, Sun J, Du Q, Yang X, Ji Q, Wang Z, Wang D, Xia Y (2016) Removal of methylene blue from water by cellulose/graphene oxide fibres. J Exp Nanosci 11:1156–1170. https://doi.org/10.1080/17458080.2016.1198499
Mohammed N, Grishkewich N, Berry RM, Tam KC (2015) Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 22:3725–3738. https://doi.org/10.1007/s10570-015-0747-3
Liu H, Tian X, Xiang X, Chen S (2022) Preparation of carboxymethyl cellulose/graphene composite aerogel beads and their adsorption for methylene blue. Int J Biol Macromol 202:632–643. https://doi.org/10.1016/j.ijbiomac.2022.01.052
Li D, Hua T, Yuan J, Xu F (2021) Methylene blue adsorption from an aqueous solution by a magnetic graphene oxide/humic acid composite. Colloids Surf Physicochem Eng Asp 627:127171. https://doi.org/10.1016/j.colsurfa.2021.127171
Acknowledgements
The authors would like to thank the Academy of Scientific Research and Technology, Egypt, for providing financial support for the study efforts through the Egypt-France Scientific and Technological Cooperation Program PHC “IMHOTEP” 41898XD.
Author information
Authors and Affiliations
Contributions
Ahmed Salama and Benedicte Prelot shared equally in funding acquisition and project administration. A.S., B.P., R. A., M. D., M. A. investigation, methodology, validation, and writing—original draft. P. H. methodology, writing—review and editing. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Statement
In the manuscript, the authors stated that no informed consent was obtained for animal or human experiments.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salama, A., Abouzeid, R., Prelot, B. et al. Oxidized Cellulose Nanofibers Decorated with Magnetite as Efficient Bioadosrbent for Organic Dyes. Chemistry Africa 6, 2343–2356 (2023). https://doi.org/10.1007/s42250-023-00669-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00669-5