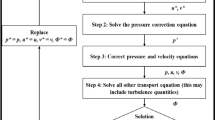
Abstract
In the human body, blood acts as a transporter of oxygen and other nutrients as well as carbon dioxide and other waste materials to and from all the organs. Therefore, continuous supply of blood to all the organs is critical for proper functioning of the human body. Blood is a complex fluid and has more than 40% flexible particles which include red blood cells, white blood cells, platelets and other proteins suspended in a water-like fluid, plasma. The dynamics of blood flow, known as haemodynamics, is critical in the development, diagnosis and treatment planning of vascular diseases and design and development of cardiovascular devices. Whilst the most advanced flow measurement techniques such as X-ray imaging, magnetic resonance imaging and ultrasound imaging are used in the diagnosis and treatment of vascular diseases, it is not possible to obtain the complete information of pressure and velocity field experimentally via in vivo methods. Therefore, in silico methods or computational modelling techniques are being increasingly employed not only to understand the haemodynamics but also for use in the clinical setting. Whilst blood is treated as a homogeneous, single-phase fluid in several studies, it is possible to capture several features of the flow of blood only by modelling it as a multiphase fluid. A number of approaches have been adopted to model multiphase flow of blood. A broad categorisation can be based on whether the cell boundary is captured explicitly, e.g. immersed boundary method, or the phases are treated as interpenetrating and two or more phases can exist simultaneously at a point, e.g. Euler–Euler method. In the literature, both the approaches have been adopted to model the flow of blood. Particle-based methods, such as smoothed particle hydrodynamics and dissipative particle dynamics have also been employed by researchers to study the complex interactions associated with the flow of blood. In this article, we discuss different multiphase modelling approaches and their application in the haemodynamics modelling.
Similar content being viewed by others
Notes
Inner cross-section of the vessel.
The space surrounding blood vessels and cells.
Erythrocytes: Erythors: red, cyte: cell.
Haematocrit: Hemato: blood; crit/krinein: to separate.
Leukocytes: Leuk: white; cyte: cell.
Plaque build-up in the arteries of legs or arms.
Formation of blood clot in the vein.
Circulating blood clot in the blood vessels (Thrombos: clot, emboli: foreign substance travelling through the blood stream).
Bulging of a weakened vessel wall.
By birth.
Narrowing of blood vessels.
Blood vessel rupture.
Eulerian description in fluid mechanics refers to flow of fluid in a control volume and the flow properties such as velocity and pressure are a function of time and space and any specific fluid particle.
Two phases can exist simultaneously at a location.
Abnormally constricted.
In the Lagrangian description of fluid flow, the motion of individual fluid particles is tracked.
Connection between two blood vessels.
Process of separation of one or more components of the blood.
Intracellular fluid or fluid present in the cell and does not include organelles.
References
Ottesen JT, Olufsen MS, Larsen JK (2004) Applied mathematical models in human physiology. Soc Ind Appl Math. https://doi.org/10.1137/1.9780898718287
Brust M, Schaefer C, Doerr R, Pan L, Garcia M, Arratia PE, Wagner C (2013) Rheology of human blood plasma: viscoelastic versus Newtonian behavior. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.110.078305
Rodrigues T, Mota R, Gales L, Campo-Deaño L (2022) Understanding the complex rheology of human blood plasma. J Rheol 66(4):761–774. https://doi.org/10.1122/8.0000442
Guyton A, Hall A (2020) Textbook of medical physiology, 3rd edn. Elsevier Health Science
Beris AN, Horner JS, Jariwala S, Armstrong MJ, Wagner NJ (2021) Recent advances in blood rheology: a review. Soft Matter 17(47):10591–10613. https://doi.org/10.1039/d1sm01212f
Fåhræus R, Lindqvist T (1931) The viscosity of the blood in narrow capillary tubes. Am J Physiol Legacy Content 96(3):562–568. https://doi.org/10.1152/ajplegacy.1931.96.3.562
Secomb TW, Pries AR (2013) Blood viscosity in microvessels: experiment and theory. C R Phys 14(6):470–478. https://doi.org/10.1016/j.crhy.2013.04.002
Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282(21):2035–2042. https://doi.org/10.1001/jama.282.21.2035
Baratchi S, Chen YC, Peter K (2020) Helical flow: a means to identify unstable plaques and a new direction for the design of vascular grafts and stents. Atherosclerosis 300:34–36. https://doi.org/10.1016/j.atherosclerosis.2020.03.002
Esmaily Moghadam M, Vignon-Clementel IE, Figliola R, Marsden AL (2013) A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. J Comput Phys 244:63–79. https://doi.org/10.1016/j.jcp.2012.07.035
Figueroa CA, Vignon-Clementel IE, Jansen KE, Hughes TJR, Taylor CA (2006) A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput Methods Appl Mech Eng 195(41–43):5685–5706. https://doi.org/10.1016/j.cma.2005.11.011
Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA (2010) Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng 38(10):3195–3209. https://doi.org/10.1007/s10439-010-0083-6
Marsden AL, Esmaily-Moghadam M (2015) Multiscale modeling of cardiovascular flows for clinical decision support. Appl Mech Rev. https://doi.org/10.1115/1.4029909
Shang JK, Esmaily M, Verma A, Reinhartz O, Figliola RS, Hsia T-Y, Feinstein JA, Marsden AL (2019) Patient-specific multiscale modeling of the assisted bidirectional Glenn. Ann Thorac Surg 107(4):1232–1239. https://doi.org/10.1016/j.athoracsur.2018.10.024
Vignon-Clementel IE, Figueroa CA, Jansen KE, Taylor CA (2010) Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput Methods Biomech Biomed Engin 13(5):625–640. https://doi.org/10.1080/10255840903413565
Apostolidis AJ, Beris AN (2014) Modeling of the blood rheology in steady-state shear flows. J Rheol 58(3):607–633. https://doi.org/10.1122/1.4866296
Bird B, Stewart W, Lightfoot E (2006) Transport phenomena. Wiley
Anand M, Kwack J, Masud A (2013) A new generalized Oldroyd-B model for blood flow in complex geometries. Int J Eng Sci 72:78–88. https://doi.org/10.1016/j.ijengsci.2013.06.009
Horner JS, Armstrong MJ, Wagner NJ, Beris AN (2018) Investigation of blood rheology under steady and unidirectional large amplitude oscillatory shear. J Rheol 62(2):577–591. https://doi.org/10.1122/1.5017623
Johnston BM, Johnston PR, Corney S, Kilpatrick D (2004) Non-Newtonian blood flow in human right coronary arteries: steady state simulations. J Biomech 37(5):709–720. https://doi.org/10.1016/j.jbiomech.2003.09.016
Boyd J, Buick JM, Green S (2007) Analysis of the Casson and Carreau-Yasuda non-Newtonian blood models in steady and oscillatory flows using the lattice Boltzmann method. Phys Fluids. https://doi.org/10.1063/1.2772250
Casson N (1959) Flow equation for pigment-oil suspensions of the printing ink-type. Rheol Disperse Syst, 84–104
Chandran KB, Rittgers SE, Yoganathan AP (2012) Biofluid Mechanics. CRC Press. https://doi.org/10.1201/b11709
Doost SN, Zhong L, Su B, Morsi YS (2016) The numerical analysis of non-Newtonian blood flow in human patient-specific left ventricle. Comput Methods Programs Biomed 127:232–247. https://doi.org/10.1016/j.cmpb.2015.12.020
Johnston BM, Johnston PR, Corney S, Kilpatrick D (2006) Non-Newtonian blood flow in human right coronary arteries: transient simulations. J Biomech 39(6):1116–1128. https://doi.org/10.1016/j.jbiomech.2005.01.034
Morbiducci U, Gallo D, Massai D, Ponzini R, Deriu MA, Antiga L, Redaelli A, Montevecchi FM (2011) On the importance of blood rheology for bulk flow in hemodynamic models of the carotid bifurcation. J Biomech 44(13):2427–2438. https://doi.org/10.1016/j.jbiomech.2011.06.028
Quemada D (1981) A rheological model for studying the hematocrit dependence of red cell-red cell and red cell-protein interactions in blood1. Biorheology 18(3–6):501–516. https://doi.org/10.3233/BIR-1981-183-615
Fry BC, Roy TK, Secomb TW (2013) Capillary recruitment in a theoretical model for blood flow regulation in heterogeneous microvessel networks. Physiol Rep. https://doi.org/10.1002/phy2.50
Pries AR, Reglin B, Secomb TW (2001) Structural adaptation of microvascular networks: functional roles of adaptive responses. www.ajpheart.org
Arciero JC, Carlson BE, Secomb TW (2008) Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am J Physiol Heart Circ Phys-Iol 295:1562–1571. https://doi.org/10.1152/ajpheart.00261.2008.-A
Carlson BE, Arciero JC, Secomb TW (2008) Theoretical model of blood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses. Am J Physiol Heart Circul Physiol. https://doi.org/10.1152/ajpheart.00262.2008
Cornelissen AJM, Dankelman J, Vanbavel ED, Spaan JAE, Vanbavel E, Spaan JAE (2002) Balance between myogenic, flow-dependent, and metabolic flow control in coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol 282:2224–2237. https://doi.org/10.1152/ajpheart.00491.2001.-Myogenic
Ursino, M., Colantuoni, A., & Bertuglia, S. (1998). Vasomotion and Blood Flow Regulation in Hamster Skeletal Muscle Microcirculation: A Theoretical and Experimental Study.
Leighton D, Acrivos A (1987) The shear-induced migration of particles in concentrated suspensions. J Fluid Mech 181(1):415. https://doi.org/10.1017/S0022112087002155
Phillips RJ, Armstrong RC, Brown RA, Graham AL, Abbott JR (1992) A constitutive equation for concentrated suspensions that accounts for shear-induced particle migration. Phys Fluids A 4(1):30–40. https://doi.org/10.1063/1.858498
Mansour MH, Bressloff NW, Shearman CP (2010) Red blood cell migration in microvessels. Biorheology 47(1):73–93. https://doi.org/10.3233/BIR-2010-0560
Biasetti J, Spazzini PG, Hedin U, Gasser TC (2014) Synergy between shear-induced migration and secondary flows on red blood cells transport in arteries: considerations on oxygen transport. J R Soc Interface 11(97):20140403. https://doi.org/10.1098/rsif.2014.0403
Chandran K, Dalal IS, Tatsumi K, Muralidhar K (2020) Numerical simulation of blood flow modeled as a fluid- particulate mixture. J Nonnewton Fluid Mech 285:104383. https://doi.org/10.1016/j.jnnfm.2020.104383
Ranade VV, Utikar RP (eds) (2022) Multiphase flows for process industries. Wiley. https://doi.org/10.1002/9783527812066
White FM (2009) Fluid Mechanics, 7th edn. Mc-Graw Hill
Schiller L (1933) A drag coefficient correlation. Zeit Ver Deutsch Ing 77:318–320
Jung J, Hassanein A, Lyczkowski RW (2006) Hemodynamic computation using multiphase flow dynamics in a right coronary artery. Ann Biomed Eng 34(3):393–407. https://doi.org/10.1007/s10439-005-9017-0
Jung J, Lyczkowski RW, Panchal CB, Hassanein A (2006) Multiphase hemodynamic simulation of pulsatile flow in a coronary artery. J Biomech 39(11):2064–2073. https://doi.org/10.1016/j.jbiomech.2005.06.023
Berthier B, Bouzerar R, Legallais C (2002) Blood flow patterns in an anatomically realistic coronary vessel: influence of three different reconstruction methods. J Biomech 35(10):1347–1356. https://doi.org/10.1016/S0021-9290(02)00179-3
Jung J, Hassanein A (2008) Three-phase CFD analytical modeling of blood flow. Med Eng Phys 30(1):91–103. https://doi.org/10.1016/j.medengphy.2006.12.004
Qiao Y, Zeng Y, Ding Y, Fan J, Luo K, Zhu T (2019) Numerical simulation of two-phase non-Newtonian blood flow with fluid-structure interaction in aortic dissection. Comput Methods Biomech Biomed Engin 22(6):620–630. https://doi.org/10.1080/10255842.2019.1577398
Ling Y, Tang J, Liu H (2021) Numerical investigation of two-phase non-Newtonian blood flow in bifurcate pulmonary arteries with a flow resistant using Eulerian multiphase model. Chem Eng Sci 233:116426. https://doi.org/10.1016/J.CES.2020.116426
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26(3):240–248. https://doi.org/10.1136/thx.26.3.240
Rao MS, Bhan A, Talwar S, Sharma R, Choudhary SK, Airan B, Saxena A, Kothari SS, Juneja R, Venugopal P (2000) Modified blalock-taussig shunt in neonates: determinants of immediate outcome. Asian Cardiovasc Thorac Ann 8(4):339–343. https://doi.org/10.1177/021849230000800410
Lyras KG, Lee J (2022) A finite volume coupled level set and volume of fluid method with a mass conservation step for simulating two-phase flows. Int J Numer Meth Fluids 94(8):1027–1047. https://doi.org/10.1002/fld.5082
Gidaspow D, Huang J (2009) Kinetic theory based model for blood flow and its viscosity. Ann Biomed Eng 37(8):1534–1545. https://doi.org/10.1007/s10439-009-9720-3
Gidaspow D, Bacelos MS (2018) Kinetic theory based multiphase flow with experimental verification. Rev Chem Eng 34(3):299–318. https://doi.org/10.1515/revce-2016-0044
Huang J, Lyczkowski RW, Gidaspow D (2009) Pulsatile flow in a coronary artery using multiphase kinetic theory. J Biomech 42(6):743–754. https://doi.org/10.1016/j.jbiomech.2009.01.038
Chen T, Liu X, Si B, Feng Y, Zhang H, Jia B, Wang S (2021) Comparison between single-phase flow simulation and multiphase flow simulation of patient-specific total cavopulmonary connection structures assisted by a rotationally symmetric blood pump. Symmetry 13(5):912. https://doi.org/10.3390/sym13050912
Melka B, Gracka M, Adamczyk W, Rojczyk M, Golda A, Nowak AJ, Białecki RA, Ostrowski Z (2018) Multiphase simulation of blood flow within main thoracic arteries of 8-year-old child with coarctation of the aorta. Heat Mass Transf 54(8):2405–2413. https://doi.org/10.1007/s00231-017-2136-y
Wen CY, Yu YH (1966) A generalized method for predicting the minimum fluidization velocity. AIChE J 12(3):610–612. https://doi.org/10.1002/aic.690120343
Syamlal M (1987) The particle-particle drag term in a multiparticle model of fluidization. topical report DOE/MC 21353 2373 NTIS/DE 87006500
Bouchnita A, Belyaev AV, Volpert V (2021) Multiphase continuum modeling of thrombosis in aneurysms and recirculation zones. Phys Fluids. https://doi.org/10.1063/5.0057393
Longest PW, Kleinstreuer C, Buchanan JR (2004) Efficient computation of micro-particle dynamics including wall effects. Comput Fluids 33(4):577–601. https://doi.org/10.1016/j.compfluid.2003.06.002
Biglarian M, Firoozabadi B, Saidi MS (2021) Atheroprone sites of coronary artery bifurcation: Effect of heart motion on hemodynamics-dependent monocytes deposition. Comput Biol Med 133:104411. https://doi.org/10.1016/j.compbiomed.2021.104411
De Gruttola S, Boomsma K, Poulikakos D (2005) Computational simulation of a non-Newtonian model of the blood separation process. Artif Organs 29(12):949–959. https://doi.org/10.1111/j.1525-1594.2005.00164.x
Childress EM, Kleinstreuer C, Kennedy AS (2012) A new catheter for tumor-targeting with radioactive microspheres in representative hepatic artery systems—part ii: solid tumor-targeting in a patient-inspired hepatic artery system. J Biomech Eng. https://doi.org/10.1115/1.4006685
Kleinstreuer C, Basciano CA, Childress EM, Kennedy AS (2012) A new catheter for tumor targeting with radioactive microspheres in representative hepatic artery systems. Part I: impact of catheter presence on local blood flow and microsphere delivery. J Biomech Eng. https://doi.org/10.1115/1.4006684
Hirt CW, Nichols BD (1981) Volume of fluid (VOF) method for the dynamics of free boundaries. J Comput Phys 39(1):201–225. https://doi.org/10.1016/0021-9991(81)90145-5
Osher S, Fedkiw RP (2001) Level set methods: an overview and some recent results. J Comput Phys 169(2):463–502. https://doi.org/10.1006/jcph.2000.6636
Jacqmin D (1999) Calculation of two-phase Navier-Stokes flows using phase-field modeling. J Comput Phys 155(1):96–127. https://doi.org/10.1006/jcph.1999.6332
Tryggvason G, Bunner B, Esmaeeli A, Juric D, Al-Rawahi N, Tauber W, Han J, Nas S, Jan Y-J (2001) A front-tracking method for the computations of multiphase flow. J Comput Phys 169(2):708–759. https://doi.org/10.1006/jcph.2001.6726
Peskin CS (2002) The immersed boundary method. Acta Numer 11:479–517. https://doi.org/10.1017/S0962492902000077
Doddi SK, Bagchi P (2008) Lateral migration of a capsule in a plane Poiseuille flow in a channel. Int J Multiph Flow 34(10):966–986. https://doi.org/10.1016/j.ijmultiphaseflow.2008.03.002
Eggleton CD, Popel AS (1998) Large deformation of red blood cell ghosts in a simple shear flow. Phys Fluids 10(8):1834–1845. https://doi.org/10.1063/1.869703
Balogh P, Bagchi P (2017) A computational approach to modeling cellular-scale blood flow in complex geometry. J Comput Phys 334:280–307. https://doi.org/10.1016/j.jcp.2017.01.007
Krüger T, Kusumaatmaja H, Kuzmin A, Shardt O, Silva G, Viggen EM (2017) The lattice Boltzmann method. Springer International Publishing. https://doi.org/10.1007/978-3-319-44649-3
Hernández-Rodríguez M, C Rosales-Hernández M, E Mendieta-Wejebe J, Martínez-Archundia M, Correa Basurto J (2016) Current tools and methods in molecular dynamics (MD) simulations for drug design. Curr Med Chem 23(34):3909–3924. https://doi.org/10.2174/0929867323666160530144742
Bhatnagar PL, Gross EP, Krook M (1954) A model for collision processes in gases. I. Small amplitude processes in charged and neutral one-component systems. Phys Rev 94(3):511–525. https://doi.org/10.1103/PhysRev.94.511
Kumar K (1967) The chapman-enskog solution of the boltzmann equation: a reformulation in terms of irreducible tensors and matrices. Aust J Phys 20(3):205–252
Peskin CS (1977) Numerical analysis of blood flow in the heart. J Comput Phys 25(3):220–252. https://doi.org/10.1016/0021-9991(77)90100-0
Sun C, Munn LL (2008) Lattice-Boltzmann simulation of blood flow in digitized vessel networks. Comput Math Appl 55(7):1594–1600. https://doi.org/10.1016/j.camwa.2007.08.019
Zhang J, Johnson PC, Popel AS (2007) An immersed boundary lattice Boltzmann approach to simulate deformable liquid capsules and its application to microscopic blood flows. Phys Biol 4(4):285–295. https://doi.org/10.1088/1478-3975/4/4/005
Zhang J, Johnson PC, Popel AS (2009) Effects of erythrocyte deformability and aggregation on the cell free layer and apparent viscosity of microscopic blood flows. Microvasc Res 77(3):265–272. https://doi.org/10.1016/j.mvr.2009.01.010
Enjalbert R, Hardman D, Krüger T, Bernabeu MO (2021) Compressed vessels bias red blood cell partitioning at bifurcations in a hematocrit-dependent manner: Implications in tumor blood flow. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2025236118
Zhou Q, Perovic T, Fechner I, Edgar LT, Hoskins PR, Gerhardt H, Krüger T, Bernabeu MO (2021) Association between erythrocyte dynamics and vessel remodelling in developmental vascular networks. J R Soc Interface 18(179):20210113. https://doi.org/10.1098/rsif.2021.0113
Rashidi Y, Simionato G, Zhou Q, John T, Kihm A, Bendaoud M, Krüger T, Bernabeu MO, Kaestner L, Laschke MW, Menger MD, Wagner C, Darras A (2023) Red blood cell lingering modulates hematocrit distribution in the microcirculation. Biophys J 122(8):1526–1537. https://doi.org/10.1016/j.bpj.2023.03.020
Karniadakis GE, Kevrekidis IG, Lu L, Perdikaris P, Wang S, Yang L (2021) Physics-informed machine learning. Nature Rev Phys 3(6):422–440. https://doi.org/10.1038/s42254-021-00314-5
Wang L, Dong D, Tian F-B (2022) Fast prediction of blood flow in stenosed arteries using machine learning and immersed boundary-lattice Boltzmann method. Front Physiol. https://doi.org/10.3389/fphys.2022.953702
Gingold RA, Monaghan JJ (1977) Smoothed particle hydrodynamics: theory and application to non-spherical stars. Mon Not R Astron Soc 181(3):375–389. https://doi.org/10.1093/mnras/181.3.375
Lucy LB (1977) A numerical approach to the testing of the fission hypothesis. Astron J 82:1013. https://doi.org/10.1086/112164
Monaghan JJ (1992) Smoothed particle hydrodynamics. Ann Rev Astron Astrophys 30(1):543–574. https://doi.org/10.1146/annurev.aa.30.090192.002551
Liu MB, Liu GR (2010) Smoothed particle hydrodynamics (SPH): an overview and recent developments. Arch Comput Methods Eng 17(1):25–76. https://doi.org/10.1007/s11831-010-9040-7
Violeau D, Rogers BD (2016) Smoothed particle hydrodynamics (SPH) for free-surface flows: past, present and future. J Hydraul Res 54(1):1–26. https://doi.org/10.1080/00221686.2015.1119209
Ye T, Pan D, Huang C, Liu M (2019) Smoothed particle hydrodynamics (SPH) for complex fluid flows: recent developments in methodology and applications. Phys Fluids. https://doi.org/10.1063/1.5068697
Lind SJ, Rogers BD, Stansby PK (2020) Review of smoothed particle hydrodynamics: towards converged Lagrangian flow modelling. Proc R Soc A: Math Phys Eng Sci. https://doi.org/10.1098/rspa.2019.0801
Pozorski J, Olejnik M (2023) Smoothed particle hydrodynamics modelling of multiphase flows: an overview. Acta Mech. https://doi.org/10.1007/s00707-023-03763-4
Becker M, Teschner M (2007) Weakly compressible SPH for free surface flows, Proceedings of the 2007 ACM SIGGRAPH/Eurographics symposium on computer animation, San Diego, CA, USA (2007), pp 1–8
Monaghan JJ (1994) Simulating free surface flows with SPH. J Comput Phys 110(2):399–406. https://doi.org/10.1006/jcph.1994.1034
Antuono M, Colagrossi A, Marrone S, Molteni D (2010) Free-surface flows solved by means of SPH schemes with numerical diffusive terms. Comput Phys Commun 181(3):532–549. https://doi.org/10.1016/j.cpc.2009.11.002
Cummins SJ, Rudman M (1999) An SPH projection method. J Comput Phys 152(2):584–607. https://doi.org/10.1006/jcph.1999.6246
Hosseini SM, Feng JJ (2009) A particle-based model for the transport of erythrocytes in capillaries. Chem Eng Sci 64(22):4488–4497. https://doi.org/10.1016/j.ces.2008.11.028
Chui Y-P, Heng P-A (2010) A meshless rheological model for blood-vessel interaction in endovascular simulation. Prog Biophys Mol Biol 103(2–3):252–261. https://doi.org/10.1016/j.pbiomolbio.2010.09.003
Gholami B, Comerford A, Ellero M (2014) A multiscale SPH particle model of the near-wall dynamics of leukocytes in flow. Int J Numer Methods Biomed Eng 30(1):83–102. https://doi.org/10.1002/cnm.2591
Polwaththe-Gallage H-N, Saha SC, Sauret E, Flower R, Senadeera W, Gu Y (2016) SPH-DEM approach to numerically simulate the deformation of three-dimensional RBCs in non-uniform capillaries. Biomed Eng Online 15(S2):161. https://doi.org/10.1186/s12938-016-0256-0
Soleimani M, Sahraee S, Wriggers P (2019) Red blood cell simulation using a coupled shell–fluid analysis purely based on the SPH method. Biomech Model Mechanobiol 18(2):347–359. https://doi.org/10.1007/s10237-018-1085-9
Topalovic M, Nikolic A, Milovanovic V, Vulovic S, Ivanovic M (2022) Smoothed particle hydrodynamics for blood flow analysis: development of particle lifecycle algorithm. Comput Part Mech 9(6):1119–1135. https://doi.org/10.1007/s40571-021-00454-6
Monteleone A, Viola A, Napoli E, Burriesci G (2023) Modelling of thrombus formation using smoothed particle hydrodynamics method. PLoS ONE 18(2):e0281424. https://doi.org/10.1371/journal.pone.0281424
Wang F, Xu S, Jiang D, Zhao B, Dong X, Zhou T, Luo X (2021) Particle hydrodynamic simulation of thrombus formation using velocity decay factor. Comput Methods Progr Biomed 207:106173. https://doi.org/10.1016/j.cmpb.2021.106173
Hosseini SM, Feng JJ (2012) How Malaria parasites reduce the deformability of infected red blood cells. Biophys J 103(1):1–10. https://doi.org/10.1016/j.bpj.2012.05.026
Wu T, Feng JJ (2013) Simulation of malaria-infected red blood cells in microfluidic channels: passage and blockage. Biomicrofluidics. https://doi.org/10.1063/1.4817959
Español P, Revenga M (2003) Smoothed dissipative particle dynamics. Phys Rev E 67(2):026705. https://doi.org/10.1103/PhysRevE.67.026705
Müller K, Fedosov DA, Gompper G (2015) Smoothed dissipative particle dynamics with angular momentum conservation. J Comput Phys 281:301–315. https://doi.org/10.1016/j.jcp.2014.10.017
Fedosov DA, Gompper G (2014) White blood cell margination in microcirculation. Soft Matter 10(17):2961–2970. https://doi.org/10.1039/C3SM52860J
Müller K, Fedosov DA, Gompper G (2014) Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci Rep 4(1):4871. https://doi.org/10.1038/srep04871
Müller K, Fedosov DA, Gompper G (2016) Understanding particle margination in blood flow: a step toward optimized drug delivery systems. Med Eng Phys 38(1):2–10. https://doi.org/10.1016/j.medengphy.2015.08.009
Caballero A, Mao W, Liang L, Oshinski J, Primiano C, McKay R, Kodali S, Sun W (2017) Modeling left ventricular blood flow using smoothed particle hydrodynamics. Cardiovasc Eng Technol 8(4):465–479. https://doi.org/10.1007/s13239-017-0324-z
Shahriari S, Kadem L, Rogers BD, Hassan I (2012) Smoothed particle hydrodynamics method applied to pulsatile flow inside a rigid two-dimensional model of left heart cavity. Int J Numer Methods Biomed Eng 28(11):1121–1143. https://doi.org/10.1002/cnm.2482
Shahriari S, Maleki H, Hassan I, Kadem L (2012) Evaluation of shear stress accumulation on blood components in normal and dysfunctional bileaflet mechanical heart valves using smoothed particle hydrodynamics. J Biomech 45(15):2637–2644. https://doi.org/10.1016/j.jbiomech.2012.08.009
Biffi B, Gritti M, Grasso A, Milano EG, Fontana M, Alkareef H, Davar J, Jeetley P, Whelan C, Anderson S, Lorusso D, Sauvage E, Maria Bosi G, Schievano S, Capelli C (2019) A workflow for patient-specific fluid–structure interaction analysis of the mitral valve: a proof of concept on a mitral regurgitation case. Med Eng Phys 74:153–161. https://doi.org/10.1016/j.medengphy.2019.09.020
Caballero A, Mao W, McKay R, Primiano C, Hashim S, Sun W (2018) New insights into mitral heart valve prolapse after chordae rupture through fluid–structure interaction computational modeling. Sci Rep 8(1):17306. https://doi.org/10.1038/s41598-018-35555-5
Mao W, Caballero A, McKay R, Primiano C, Sun W (2017) Fully-coupled fluid-structure interaction simulation of the aortic and mitral valves in a realistic 3D left ventricle model. PLoS ONE 12(9):e0184729. https://doi.org/10.1371/journal.pone.0184729
Mao W, Li K, Sun W (2016) Fluid-structure interaction study of transcatheter aortic valve dynamics using smoothed particle hydrodynamics. Cardiovasc Eng Technol 7(4):374–388. https://doi.org/10.1007/s13239-016-0285-7
Toma M, Jensen MØ, Einstein DR, Yoganathan AP, Cochran RP, Kunzelman KS (2016) Fluid-structure interaction analysis of papillary muscle forces using a comprehensive mitral valve model with 3D chordal structure. Ann Biomed Eng 44(4):942–953. https://doi.org/10.1007/s10439-015-1385-5
Hoogerbrugge PJ, Koelman JMVA (1992) Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys Lett (EPL) 19(3):155–160. https://doi.org/10.1209/0295-5075/19/3/001
Español P, Warren P (1995) Statistical mechanics of dissipative particle dynamics. Europhys Lett (EPL) 30(4):191–196. https://doi.org/10.1209/0295-5075/30/4/001
Español P, Warren PB (2017) Perspective: dissipative particle dynamics. J Chem Phys. https://doi.org/10.1063/1.4979514
Groot RD, Warren PB (1997) Dissipative particle dynamics: bridging the gap between atomistic and mesoscopic simulation. J Chem Phys 107(11):4423–4435. https://doi.org/10.1063/1.474784
Liu MB, Liu GR, Zhou LW, Chang JZ (2015) Dissipative particle dynamics (DPD): an overview and recent developments. Arch Comput Methods Eng 22(4):529–556. https://doi.org/10.1007/s11831-014-9124-x
Moeendarbary E, Ng TY, Zangeneh M (2009) Dissipative particle dynamics: introduction, methodology and complex fluid applications: a review. Int J Appl Mecha 01(04):737–763. https://doi.org/10.1142/S1758825109000381
Santo KP, Neimark AV (2021) Dissipative particle dynamics simulations in colloid and Interface science: a review. Adv Coll Interface Sci 298:102545. https://doi.org/10.1016/j.cis.2021.102545
Pivkin IV, Caswell B, Karniadakis GE (2010) Dissipative particle dynamics. In: Lipkowitz KB (ed) Reviews in computational chemistry, vol 27. https://doi.org/10.1002/9780470890905.ch2
Allen MP, Tildesley DJ (2017) Computer simulation of liquids. Oxford University Press, Oxford. https://doi.org/10.1093/oso/9780198803195.001.0001
Dzwinel W, Boryczko K, Yuen DA (2003) A discrete-particle model of blood dynamics in capillary vessels. J Colloid Interface Sci 258(1):163–173. https://doi.org/10.1016/S0021-9797(02)00075-9
Español P (1998) Fluid particle model. Phys Rev E 57(3):2930–2948. https://doi.org/10.1103/PhysRevE.57.2930
Boryczko K, Dzwinel W, YuenD A (2003) Dynamical clustering of red blood cells in capillary vessels. J Mol Model 9(1):16–33. https://doi.org/10.1007/s00894-002-0105-x
Boryczko K, Dzwinel W, Yuen DA (2004) Modeling fibrin aggregation in blood flow with discrete-particles. Comput Methods Progr Biomed 75(3):181–194. https://doi.org/10.1016/j.cmpb.2004.02.001
Pivkin IV, Karniadakis GE (2008) Accurate coarse-grained modeling of red blood cells. Phys Rev Lett 101(11):118105. https://doi.org/10.1103/PhysRevLett.101.118105
Fedosov DA, Caswell B, Karniadakis GE (2010) A multiscale red blood cell model with accurate mechanics, rheology, and dynamics. Biophys J 98(10):2215–2225. https://doi.org/10.1016/j.bpj.2010.02.002
Pan W, Caswell B, Karniadakis GE (2010) A low-dimensional model for the red blood cell. Soft Matter 6(18):4366. https://doi.org/10.1039/c0sm00183j
Fedosov DA, Caswell B, Suresh S, Karniadakis GE (2011) Quantifying the biophysical characteristics of Plasmodium-falciparum -parasitized red blood cells in microcirculation. Proc Natl Acad Sci 108(1):35–39. https://doi.org/10.1073/pnas.1009492108
Fedosov DA, Lei H, Caswell B, Suresh S, Karniadakis GE (2011) Multiscale modeling of red blood cell mechanics and blood flow in malaria. PLoS Comput Biol 7(12):e1002270. https://doi.org/10.1371/journal.pcbi.1002270
Fedosov DA, Pan W, Caswell B, Gompper G, Karniadakis GE (2011) Predicting human blood viscosity in silico. Proc Natl Acad Sci 108(29):11772–11777. https://doi.org/10.1073/pnas.1101210108
Peng Z, Li X, Pivkin IV, Dao M, Karniadakis GE, Suresh S (2013) Lipid bilayer and cytoskeletal interactions in a red blood cell. Proc Natl Acad Sci 110(33):13356–13361. https://doi.org/10.1073/pnas.1311827110
Gao C, Zhang P, Marom G, Deng Y, Bluestein D (2017) Reducing the effects of compressibility in DPD-based blood flow simulations through severe stenotic microchannels. J Comput Phys 335:812–827. https://doi.org/10.1016/j.jcp.2017.01.062
Hoque SZ, Anand DV, Patnaik BSV (2022) A dissipative particle dynamics simulation of a pair of red blood cells in flow through a symmetric and an asymmetric bifurcated microchannel. Comput Part Mech 9(6):1219–1231. https://doi.org/10.1007/s40571-021-00453-7
Ye T, Phan-Thien N, Khoo BC, Lim CT (2014) Dissipative particle dynamics simulations of deformation and aggregation of healthy and diseased red blood cells in a tube flow. Phys Fluids. https://doi.org/10.1063/1.4900952
Chang H-Y, Li X, Li H, Karniadakis GE (2016) MD/DPD multiscale framework for predicting morphology and stresses of red blood cells in health and disease. PLoS Comput Biol 12(10):e1005173. https://doi.org/10.1371/journal.pcbi.1005173
Hoque SZ, Anand DV, Patnaik BSV (2018) The dynamics of a healthy and infected red blood cell in flow through constricted channels: a DPD simulation. Int J Numer Methods Biomed Eng. https://doi.org/10.1002/cnm.3105
Lei H, Karniadakis GE (2012) Quantifying the rheological and hemodynamic characteristics of sickle cell anemia. Biophys J 102(2):185–194. https://doi.org/10.1016/j.bpj.2011.12.006
Lei H, Karniadakis GE (2013) Probing vasoocclusion phenomena in sickle cell anemia via mesoscopic simulations. Proc Natl Acad Sci 110(28):11326–11330. https://doi.org/10.1073/pnas.1221297110
Li X, Du E, Lei H, Tang Y-H, Dao M, Suresh S, Karniadakis GE (2016) Patient-specific blood rheology in sickle-cell anaemia. Interface Focus 6(1):20150065. https://doi.org/10.1098/rsfs.2015.0065
Chang H-Y, Li X, Karniadakis GE (2017) Modeling of biomechanics and biorheology of red blood cells in type 2 diabetes mellitus. Biophys J 113(2):481–490. https://doi.org/10.1016/j.bpj.2017.06.015
Chang H-Y, Yazdani A, Li X, Douglas KAA, Mantzoros CS, Karniadakis GE (2018) Quantifying platelet margination in diabetic blood flow. Biophys J 115(7):1371–1382. https://doi.org/10.1016/j.bpj.2018.08.031
Deng Y, Papageorgiou DP, Li X, Perakakis N, Mantzoros CS, Dao M, Karniadakis GE (2020) Quantifying fibrinogen-dependent aggregation of red blood cells in type 2 diabetes mellitus. Biophys J 119(5):900–912. https://doi.org/10.1016/j.bpj.2020.07.026
Han K, Ma S, Sun J, Xu M, Qi X, Wang S, Li L, Li X (2023) In silico modeling of patient-specific blood rheology in type 2 diabetes mellitus. Biophys J 122(8):1445–1458. https://doi.org/10.1016/j.bpj.2023.03.010
Hareendranath S, Sathian SP (2023) Dynamic response of red blood cells in health and disease. Soft Matter 19(6):1219–1230. https://doi.org/10.1039/D2SM01090A
Filipovic N, Kojic M, Tsuda A (2008) Modelling thrombosis using dissipative particle dynamics method. Philos Trans R Soci A Math Phys Eng Sci 366(1879):3265–3279. https://doi.org/10.1098/rsta.2008.0097
Zhang P, Gao C, Zhang N, Slepian MJ, Deng Y, Bluestein D (2014) Multiscale particle-based modeling of flowing platelets in blood plasma using dissipative particle dynamics and coarse grained molecular dynamics. Cell Mol Bioeng 7(4):552–574. https://doi.org/10.1007/s12195-014-0356-5
Yazdani A, Karniadakis GE (2016) Sub-cellular modeling of platelet transport in blood flow through microchannels with constriction. Soft Matter 12(19):4339–4351. https://doi.org/10.1039/C6SM00154H
Han C, Zhang P, Zhu Y, Cong G, Kozloski JR, Yang CC, Zhang L, Deng Y (2022) Scalable multiscale modeling of platelets with 100 million particles. J Supercomput 78(18):19707–19724. https://doi.org/10.1007/s11227-022-04648-4
Acknowledgements
RG acknowledges the financial support provided by Indian Council of Medical Research (ICMR) vide Grant No. 2021-13210. MS acknowledges the Prime Minister’s Research Fellowship (ID: 1901276) from Ministry of Education.
Funding
The funding information was funded by the Indian Council of Medical Research, 2021-13210 and Prime Ministers' Research Fellowship (PMRF) India, 1901276.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, R., Kumar, A. & Singhal, M. A Critical Review of Multiphase Modelling of Blood Flow in Human Cardiovascular System. J Indian Inst Sci (2024). https://doi.org/10.1007/s41745-024-00430-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41745-024-00430-y