Abstract
While acute aerobic exercise has been found to have a facilitative effect on task components with varied cognitive control demands in school-age children, its effects on specific neural processes evoked by a cognitive control task remain underexplored. The objective of the current study was to examine the effects of acute aerobic exercise on task-evoked midfrontal theta event-related synchronization (ERS)—which plays a crucial role in supporting cognitive control. Thirty-three preadolescent children were recruited into this within-subjects, crossover study. Participants engaged in an aerobic exercise condition and a seated rest condition in a counterbalanced order. After both conditions, participants completed a flanker task, with concurrent EEG data collection. The results revealed no differences in midfrontal theta ERS following acute aerobic exercise as compared with following the control condition. However, midfrontal theta ERS was higher during congruent trials relative to incongruent trials following control condition but not following aerobic exercise. Collectively, acute bouts of aerobic exercise do not appear to modulate the control and behavioral monitoring processes indexed by midfrontal theta ERS during cognitive control in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A growing body of literature has supported the positive effects of acute bouts of exercise on brain and cognition in children (Drollette et al., 2014; Ludyga et al., 2017; Pontifex et al., 2013). The Physical Activity Guidelines for Americans in 2018 specifically indicate that a single bout of exercise can result in temporary facilitation of cognition and brain function (Erickson et al., 2019). Accordingly, such a single bout of exercise could be strategically implemented as a healthy, highly accessible, and low-threshold intervention throughout the school day to enhance brain and cognition, which, in turn, benefits academic performance and classroom-related behavior (Hillman et al., 2009; McGowan et al., 2020). Importantly, converging evidence has substantiated acute aerobic exercise at a moderate intensity as an effective intervention to facilitate children’s performance on task components that modulate cognitive control (e.g., flanker task) (Ludyga et al., 2017; Pontifex et al., 2013; Yu et al., 2020). Cognitive control refers to the ability to use internal goals to guide thoughts and behaviors (Egner., 2017). This includes the process of monitoring ongoing actions and performance outcomes (Ridderinkhof et al., 2004), which is necessary for day-to-day adaptive, goal‐directed behavior (Koechlin et al., 2003).
Beyond overt behavioral performance, research in this area has utilized neuroscientific measures to gain insights into the specific cognitive processes evoked by a cognitive control task (Pontifex et al., 2019). Although much of this literature has focused on neuroelectric activity associated with attentional resource allocation (e.g., the P3 component from event-related brain potential (ERP); Pontifex et al., 2019), considerable relevant cognitive-related information that may be contained in the electroencephalography (EEG) is lost because of the time-domain averaging approach utilized by ERPs. As such, it may be informative to utilize event-related EEG frequency dynamics to further inform on the covert neural changes induced by acute bouts of aerobic exercise. Here, we conducted a time–frequency analysis of EEG, which provides insights into the temporal dynamics of the magnitude of EEG, including neural synchronization and desynchronization (a state of synchrony/desynchrony in a population of neurons in support of specific mental processes) at specific frequency bands that relate to events of interest (Klimesch, 1999; Makeig et al., 2004). This approach affords investigation into cortical activities subserving cognitive control operations.
At a neural level, cognitive control is supported by a network of brain regions including the medial prefrontal cortex, the orbitofrontal cortex, and the anterior cingulate cortex (ACC), as revealed by functional neuroimaging and animal studies (Egner, 2017). In particular, the control function of medial-frontal circuit (i.e., medial prefrontal cortex and ACC) can be reflected in modulations of midfrontal theta oscillations (Cavanagh & Frank, 2014). Midfrontal theta is a neural marker of the frontally mediated, top-down control and monitoring processes (Cavanagh & Frank, 2014). It has been theorized that medial-frontal brain regions (e.g., medial prefrontal cortex, orbitofrontal cortex, and ACC) function as nodes for monitoring conflict and directing other distal brain regions for goal-oriented behavioral adaptations to conflict (Cohen & Donner, 2013). Theta event-related synchronizations (ERS) over the medial-frontal regions are sensitive to stimulus novelty and stimulus–response conflict and is evoked when individuals perceive a need for enhanced control processes to adaptively change behaviors (Cavanagh & Frank, 2014). Other studies also indicated that midfrontal theta ERS is associated with the monitoring and adaptation of behaviors. For example, midfrontal theta ERS is enhanced after individuals committed to a cognitive or behavior error (Cavanagh et al., 2012). In addition, midfrontal theta ERS is a relevant marker for the maturation of cognitive control during childhood. Specifically, studies in school-age children (Adam et al., 2020) have indicated that midfrontal theta ERS is a relevant marker for the functioning and maturation of the medial-frontal regions (Güntekin et al., 2020) and is implemented when facing upregulation of control process and conflicts resolution across various cognitive control tasks in children (Adam et al., 2020). These data, collectively, indicate the crucial role of midfrontal theta ERS in supporting maturation and functioning of cognitive control and its underlying cortical regions (e.g., medial prefrontal cortex and ACC) during childhood.
Accordingly, the objective of the current investigation was to determine the extent to which midfrontal theta ERS dynamics may alter in response to an acute bout of aerobic exercise as compared to a seated rest control condition in school-age children. The novelty of the current investigation lies in the study of the control and behavior monitoring processes indexed by midfrontal theta oscillations to provide an additional piece of evidence critical for understanding the effects of acute aerobic exercise at the neural level. As preadolescent children appear to exhibit the greatest post-exercise enhancement in cognitive control (Ludyga et al., 2016), the present investigation specifically focused on this population to increase the likelihood of observing potential post-exercise related differences in midfrontal theta ERS. We hypothesized that children would experience greater midfrontal theta ERS during a cognitive control task following aerobic exercise relative to a non-exercise condition, suggesting better ability to upregulate cortical control and behavior monitoring over task-relevant stimuli as a function of aerobic exercise. We also hypothesized that such neural modulations would be accompanied by better performance on a cognitive control task following aerobic exercise relative to a non-exercise condition.
Method
The current study was a secondary analysis of data combined from two subsets of children engaged in highly similar research protocols involving acute aerobic exercise, cognitive task performance, and EEG assessment (Hillman et al., 2009; Pontifex et al., 2013). One study sample (Pontifex et al., 2013) consisted of both neurotypical children and children with ADHD, and only data from non-ADHD children were sampled. Thus, all participants in the current study were free of an ADHD diagnosis. The novel aim of this secondary analysis was to investigate the modulatory effects of acute aerobic exercise on midfrontal theta ERS in children. The portion of the task performance (i.e., response accuracy and mean reaction times) data reported herein that overlap with previously published studies (Hillman et al., 2009; Pontifex et al., 2013) were presented only to better inform the neural oscillations findings.
Participants
The study protocols were approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign and conformed to the Declaration of Helsinki. All participants and their legal guardian signed informed assent and consent forms prior to the study. A total of 40 healthy preadolescent children (20 in the Hillman et al. dataset and 20 in the Pontifex et al. dataset) between the ages of 8–11 years were recruited from East-Central Illinois for this analysis. Of the original 40 participants, data from 7 children (4 from Hillman et al. dataset and 3 from Pontifex et al. dataset) were excluded because of poor EEG data quality or insufficient trials (< 10 trials per congruency) for averaged EEG data. As such, information and data reported in this investigation are based upon 33 children. Figure 1 depicts the flowchart of data selection from both studies. This sample size (n = 33) is sufficient for detecting an exercise effect exceeding f = 0.32 (Hillman et al., 2009) for behavioral outcomes (assuming correlation between repeated measures ≥ 0.5) with a repeated-measure design and a moderate effect size in paired t-tests on EEG outcomes (Kao et al., 2020), as computed using G*Power 3.1.9 (Faul et al., 2007). Legal guardians were asked to complete a health history and demographics questionnaire as well as other documentation indicating that their child (a) had normal or corrected-to-normal vision, (2) was free of neurological diseases (e.g., attention-deficit/hyperactivity disorders [ADHD]) and/or did not have an individual education plan related to developmental or attention disorders based on parental disclosure, and (c) had no physical disabilities that could be exacerbated by exercise participation, as indicated by the Physical Activity Readiness Questionnaire (PARQ) (Thomas et al., 1992).
Demographic information included age, sex, pubertal timing, socioeconomic status (SES), intelligence quotient (IQ), and body mass index (BMI). For pubertal stage, legal guardians provided ratings on a 5-point Tanner scale, with 1 indicating a prepubertal state and 5 indicating the full mature state (Taylor et al., 2001). SES was determined using a trichotomous index based on the following: (1) participation in free or reduced-price meal program at school, (2) the highest level of education obtained by the parents, and (3) number of parents who worked full-time (Birnbaum et al., 2002). Children also completed the Kaufman Brief Intelligence Test (K-BIT) to assess IQ (Kaufman, 2004). BMI was calculated as an individual’s weight divided by their height in meters squared, and BMI percentiles were further computed using Centers for Disease Control and Prevention growth charts (Kuczmarski et al., 2002). Table 1 summarizes demographic, anthropometric, and fitness data of participants.
Aerobic Fitness Assessment
Aerobic fitness was assessed using a test of maximal oxygen consumption (VO2peak) measured on a motor-driven treadmill following a modified Balke protocol (American College of Sports Medicine, 2010). This test employed a computerized indirect calorimetry system (TrueMax 2400; Parvo Medics, Sandy, UT, USA) while participants walked/ran on a motor-driven treadmill at a constant speed with a 2.5% incremental grade increase every 2 min until volitional exhaustion. A Polar heart rate (HR) monitor (Model A1; Polar Electro, Finland) was used to measure HR throughout the test. Ratings of perceived exertion (RPE) were assessed every 2 min with the children’s OMNI scale (Utter et al., 2002). The OMNI is a pictorial 10-point Likert scale ranging from “not at all tired” to “very, very tired”) (Utter et al., 2002). Relative peak oxygen consumption (VO2peak) was expressed in mL.kg−1.min−1 and was based upon maximal effort as evidenced by: (1) a VO2 plateau corresponding to an increase of less than 2 mL.kg−1.min−1 despite an increase in workload, (2) a peak heart rate ≥ 185 beats per minute (ACSM, 2010) or a HR plateau (P. S. Freedson & Goodman, 1993), (3) respiratory exchange ratio ≥ 1.0 (Bar-Or, 1983), and/or (4) ratings on the children’s OMNI scale of perceived exertion ≥ 8 (Utter et al., 2002). Participants had to either reach a VO2 plateau or had to meet at least two of the three remaining confidence criteria when VO2 plateau was not reached. To standardize scores, VO2peak percentile (VO2peak%) was determined based on individuals’ age, gender, and relative scores from normative data (Shvartz & Reibold, 1990).
Cognitive Control Task
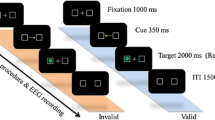
All participants completed a modified version of the Eriksen flanker task (Eriksen & Eriksen, 1974) to assess cognitive control. All stimuli were presented focally on a computer screen at ~ 1 m using Neuroscan Stim software (Compumedics, Charlotte, NC). Participants were instructed to attend to the center target stimulus amid four identical flanking stimuli that were either congruent (i.e., facing the same direction) or incongruent (i.e., facing the opposite direction) (Hillman et al., 2009; Pontifex et al., 2013) and respond as quickly and accurately as possible with a thumb press in accordance with the directionality of the target using a response pad. Two blocks of 100 trials were presented with equiprobable congruency and directionality. Differences between the combined protocols included stimulus type (3-cm tall goldfish on a blue background vs. 2.5-cm tall white arrows on a black background), duration (200 ms vs. 120 ms), and inter-stimulus interval (fixed 1700 ms vs. variable 1100 ms, 1300 ms, and 1500 ms). Given the differences in stimulus type, duration, and inter-trial interval across flanker tasks in the two studies (Hillman et al., 2009; Pontifex et al., 2013), we ran preliminary analyses to test whether these differences confounded the experimental effects. Please see the statistical and results sections for details. Performance on the flanker task for each experimental condition was indexed by response accuracy, mean response times (mean RT) of correctly responded trials, and the interference scores of response accuracy (AccuracyCongruent – AccuracyIncongruent) and mean RT (RTIncongruent – RTCongruent).
Neuroelectric Assessment
Electroencephalographic (EEG) activity was recorded from 64 electrode sites arranged according to the international 10–10 system using a Neuroscan EEG Quik-Cap (Compumedics, Charlotte, NC). All electrodes maintained an impedance < 10 kΩ prior to EEG recordings. Continuous data were referenced online using CCPz with Afz electrode serving as the ground. Additional electrodes were placed above and below the left orbit and outer canthus of each eye to monitor electrooculographic (EOG) activity. Continuous data were digitalized at a sampling rate of 500 Hz, amplified 500 times with a DC-to-70 Hz filter, and a 60-Hz notch filter was applied using a Neuroscan SynAmps2 amplifier. Matlab (R2019a, MathWorks Inc.), EEGLAB toolbox (version 2019.0) (Delorme & Makeig, 2004), and ERPLAB toolbox (version 7.0.0) (Lopez-Calderon & Luck, 2014) were used for offline data processing. Continuous data were re-referenced to averaged mastoids (M1, M2), followed by independent component analysis (ICA) using the infomax algorithm as this algorithm results in greater reduction in the eyeblink artifact compared to other popular options and eyeblink-related artifacts were identified and removed from the data using the icablinkmetrics method (Pontifex et al., 2017a, 2017b; Pontifex et al., 2017a, 2017b). Afterward, continuous EEG data were segmented into epochs from − 1500 to 1500 ms time-locked to stimulus onset (Hsieh et al., 2020), which were baseline corrected using the entire sweep (Chang et al., 2015; Hsieh et al., 2020). Trials with incorrect responses and those containing artifacts with amplitudes exceeding ± 150 μV were discarded. The average number of epochs included in the analyses were as follows: exercise-congruent 65.4 ± 18.1, exercise-incongruent 59.2 ± 18.0, rest-congruent 62.3 ± 18.0, and rest-incongruent 57.4 ± 18.0.
Time–Frequency Analysis
The EEGLAB toolbox was utilized to analyze the event-locked EEG responses. Data analysis and reporting of time–frequency processing comply with the latest recommendation and publication guidelines by Keli and colleagues (Keil et al., 2022). Oscillatory EEG power was computed by a Morlet-based wavelet transform with a width of 3 cycles of single trial data for a frequency band between 4 to 30 Hz using the newtimef() function in EEGLAB (EEGLAB) (Delorme & Makeig, 2004). Event-related spectral permutations were computed on the wavelet‐transformed epochs for each stimulus condition at each time point and wavelet frequency to yield time‐frequency maps. Oscillatory power (the magnitude of the analyzed signal) was then averaged across trials. The averaged oscillatory power of each task condition for each participant was rescaled by the baseline values from − 300 to − 100 ms relative to stimulus onset and taking the log10 transform of this quotient (dB) (dB power = 10 × 10 [power/baseline]), allowed a direct comparison of results of interest across frequencies. The − 300 to − 100 ms baseline was chosen to avoid edge artifacts and exclude activity evoked by the stimulus (Keil et al., 2022). The mean power in the time intervals between 100 and 500 ms at 4–7 Hz for midfrontal theta ERS was extracted. Time intervals and frequency bands were determined based on our time–frequency plots across experimental conditions and task trials from all participants (Fig. 2) as well as upon previous research using similar approach (Hsieh et al., 2020; Kao et al., 2020, 2021; van Noordt et al., 2022). For statistical analysis, we collapsed data from the frontal cluster (i.e., Fz and FCz) for midfrontal theta oscillations (Hsieh et al., 2020; Kao et al., 2020). Interference scores of midfrontal theta ERS (ThetaIncongruent – ThetaCongruent) were further calculated as supplementary data.
Procedure
The experimental protocol occurred over three separate days for each participant, roughly 5–8 days apart, at the same time of day. Participants were instructed to avoid structured physical education or athletic activities (i.e., practice or games) on the day of testing while trying to adhere as closely as possible to their normal routine. On the first visit, following provision of informed consent/assent described above, legal guardians completed a series of questionnaires on health history, demographic profiles (e.g., age, sex, pubertal timing, and SES), and ADHD symptoms (details described above) on behalf of participants. Simultaneously, participants completed the K-BIT to assess IQ, were measured for height and weight to calculate BMI, and completed practice blocks of the flanker task. Lastly, participants completed a graded maximal exercise test on a motorized treadmill to assess aerobic fitness (VO2max).
Participants were, then, counterbalanced into two different experimental conditions for days 2 and 3, such that half of the participants received the resting session on day 2 and the aerobic exercise session on day 3 whereas the other half received the aerobic exercise session on day 2 and the resting session on day 3. This within-subjects, counterbalanced design was used to ensure that the observed effects were not related to the specific order in which participants received the exercise and rest conditions. Upon arrival of day 2/3, participants were fitted with a Polar HR monitor and their resting HR was recorded after 5 min of seated rest. Afterward, HR and RPE were measured every 2 min throughout the experimental procedure. Next, participants attended either an aerobic exercise condition or a seated rest condition, depending on their session randomization. During the aerobic exercise condition, participants had 20 min of moderate-intensity treadmill walking at an intensity of 60–70% of maximal HR (mean HR during exercise = 128.5 ± 8.2 beats per minute, 61.1 ± 3.9% of maximal HR); during the seated rest, participants were allowed to read from a selection of books related to nature and science provided by the experimenters (mean HR during seated rest = 90.5 ± 11.6 beats per minute, 43.0 ± 5.5% of maximal HR). Following each experimental condition (i.e., exercise or seated rest), participants were fitted with an electrode cap in preparation for EEG recordings during flanker task performance. During this time (∼22.5 ± 3.4 min), participants’ HR was allowed to return to within 10% of baseline to avoid any general arousal effects on cognitive measures stemming from exercise participation. Once cap preparation was completed, participants were seated in a quiet testing chamber and provided with task instructions and practice trials prior to performing the flanker task. Upon completion of the study, participants were briefed on the purpose of the study and received $10/h remuneration.
Statistical Analysis
All statistical analyses were performed using SPSS 24.0 package (IBM Corporation, Armonk NY, USA), with a family-wise alpha of 0.05 set as the significance criteria. Preliminary analyses were performed to test whether the observed experimental effects on task performance and EEG outcomes were confounded by study samples. As such, we first included Study as a between-subjects factor into repeated-measure analyses of variance (RM ANOVAs) described below. If an interaction involving Study (e.g., condition by study samples and task congruency by study samples) was observed in the preliminary analyses, then repeated-measure analyses of covariance (RM ANCOVAs) adjusting for study samples were computed instead.
To examine the effects of exercise on task and EEG outcomes, 2 (Condition: exercise, seated rest) × 2 (Congruency: congruent trials, incongruent trials) RM ANOVAs were performed on response accuracy and mean RT, as well as midfrontal theta ERS. Supplementary analyses on interference scores of response accuracy and mean RT were performed with separate paired-sample t-tests between experimental conditions. Greenhouse–Geisser correction was utilized if the assumption of sphericity was violated. Post hoc comparisons were corrected with Bonferroni-corrected t-tests. Partial eta square (η2p) effect sizes were reported in addition to significance testing, with η2p of 0.01, 0.06, and 0.14 indicating small, medium, and large effect sizes, respectively (J. Cohen, 2013). Further, Cohen’s d with 95% confidence intervals (CI) was computed as effect size in post hoc comparisons, using appropriate variance corrections for repeated-measures comparisons (Cohen’s drm) (Lakens & Bakker, 2013). In addition, Bayesian statistics were computed using JASP (version 0.18.0.0; University of Amsterdam) to test the strength of evidence of F-tests and student t-tests wherever applicable to account for the small sample size. The classification scheme by Lee and Wagenmakers (Lee & Wagenmakers, 2014) provides descriptive labels for Bayes factors (BF10) and was used to interpret values. In brief, BF10 > 1 provides evidence in favor of the alternative hypothesis, whereas BF10 < 1 provides evidence in favor of the null hypothesis. Scores are labeled as modest (BF10 between 1 and 3 or 1 and 0.33), moderate (BF10 between 3 and 10 or 0.33 and 0.10), strong (BF10 between 10 and 30 or 0.10 and 0.03), very strong (BF10 between 30 and 100 or 0.03 and 0.01), or extreme (BF10 greater than 100 or lesser than 0.01).
Results
Preliminary analysis revealed a Congruency × Study interaction for mean RT, F(1, 31) = 19.17, p = < 0.001. Accordingly, study samples were adjusted for in RM ANCOVAs on mean RT.
Task Performance
Table 2 summarizes descriptive data of task performance across conditions and task congruencies. Replicating the previously reported findings for response accuracy, children exhibited higher accuracy rates following the exercise condition (91.2 ± 5.9%) as compared to following the seated rest condition (87.9 ± 7.4%), F(1, 32) = 5.93, p = 0.021, η2p = 0.16, BF10 = 3.22. Similarly, higher accuracy rates were observed for congruent trials (92.9 ± 4.8%) as compared to incongruent trials (86.3 ± 8.4%), F(1, 32) = 61.56, p = < 0.001, η2p = 0.66, BF10 > 100. Exploratory analysis examining accuracy interference (congruent – incongruent response accuracy) failed to observe any effects related to Condition (exercise 5.3 ± 5.0% vs. seated rest 7.8 ± 7.5%; t(32) = − 1.75, p = > 0.05, Cohen’s drm = − 0.31 [95% CI − 5.48–0.41], BF10 = 0.73). Figure 3 depicts changes in response accuracy as a function of condition.
For mean RT, after adjusting for study sample, RM ANCOVA revealed no differences in RT following exercise (528.9 ± 65.2 ms) as compared to following seated rest (522.4 ± 74.4 ms, F(1, 31) = 0.2, p > 0.05, η2p = 0.01, BF10 = 0.47, nor was RT interference (incongruent – congruent RT) observed to differ as a function of Condition (exercise 39.7 ± 26.2 ms vs. seated rest 46.0 ± 33.7 ms; t(32) = − 1.09, p > 0.05, Cohen’s drm = − 0.19 [95% CI − 18.22–5.51], BF10 = 0.32). However, a main effect of Congruency, F(1, 31) = 107.1, p < 0.001, η2p = 0.78, BF10 > 100, was observed, with congruent trials (509.1 ± 68.1 ms) having shorter mean RT than incongruent trials (547.1 ± 71.4 ms).
Midfrontal Theta ERS
Table 2 summarizes descriptive data of midfrontal theta performance across conditions and congruency trials, and Fig. 2 depicts the time–frequency plots of midfrontal theta oscillations relative to stimulus. For midfrontal theta ERS, RM ANOVA showed a significant Condition × Congruency interaction, F(1, 32) = 4.58, p = 0.040, η2p = 0.13, BF10 = 3.93. Decomposition of the interaction by investigating differences in Condition within each Congruency revealed that there was no difference as a function of Condition within either congruent trials (exercise-congruent 1.4 ± 0.9 dB vs. rest-congruent 1.7 ± 0.9 dB; t(32) = 1.88, p > 0.05, Cohen’s drm = − 0.20 [95% CI − 0.60–0.17], BF10 = 0.33) or incongruent trials (exercise-incongruent 1.5 ± 0.8 dB vs. rest-incongruent 1.2 ± 1.0 dB; t(32) = 1.88, p > 0.05, Cohen’s drm = 0.35 [95% CI − 0.03–0.62], BF10 = 0.89). Decomposition of the interaction by examining differences in Congruency within each Condition indicated that theta ERS was higher during congruent relative to incongruent trials following the seated rest condition (rest-congruent 1.7 ± 0.9 dB vs. rest-incongruent 1.2 ± 1.0 dB; t(32) = 2.64, p = 0.013, Cohen’s drm = 0.51 [95% CI 0.10–0.81], BF10 = 3.57), whereas no difference between task congruencies was observed following exercise condition (exercise-congruent 1.4 ± 0.9 dB vs. exercise-incongruent 1.5 ± 0.8 dB; t(32) = − 0.38, p > 0.05, Cohen’s drm = − 0.12 [95% CI − 0.35–0.24], BF10 = 0.20). Figure 4 depicts changes in frontal theta ERS. Exploratory analysis examining Theta ERS interference (incongruent – congruent) observed an effect related to Condition (exercise 0.1 ± 0.8 dB vs. seated rest − 0.5 ± 1.0 dB; t(32) = 2.14, p = 0.04, Cohen’s drm = 0.65 [95% CI 0.02–0.99], BF10 = 1.38).
Discussion
The focus of the current study was on determining the extent to which midfrontal theta ERS evoked by a cognitive control task in children might alter in response to a bout of aerobic exercise. While there was greater response accuracy across both congruent and incongruent trials following aerobic exercise (as reported previously by Hillman et al. and Pontifex et al.) (Hillman et al., 2009; Pontifex et al., 2013), midfrontal theta ERS was not observed to alter following a single bout of moderate-intensity aerobic exercise, as there was no difference in theta ERS between post-exercise and post-seated rest. Although such a finding was inconsistent with our a priori hypothesis, it provides insight into the spectrum of processes which subserve cognitive control operations. Specifically, increased midfrontal theta ERS reflects the implementation of control and behavior monitoring function mediated by the medial prefrontal cortex and ACC (Cavanagh & Frank, 2014; Cohen & Donner, 2013; Duprez et al., 2020). With this premise in mind, the current findings suggest that control and behavioral monitoring processes do not appear to be altered in response to a single bout of aerobic exercise. While a growing body of literature has supported the positive effects of acute aerobic exercise on attentional resources allocation (indexed by P3 component from ERP) during cognitive control in school-age children (Drollette et al., 2014; Ludyga et al., 2017; Yu et al., 2020), it appears that such modulatory effects may not be extended to the medial-frontal mediated control and behavior monitoring mechanism. Such a finding replicates recent findings in college-aged adults who similarly failed to observe aerobic exercise-related alterations in midfrontal theta ERS during a working memory task (Kao et al., 2020).
Alternatively, it may be that alterations in medial-frontal mediated control and behavior monitoring mechanisms are only elicited in situations where cognitive control operations are sufficiently taxed. Indeed, support for such an interpretation is provided by Pontifex et al. (2013), who observed acute aerobic exercise-induced alterations in action monitoring (indexed by the ERN component from ERP) and post-error adjustments in behavior only for children with ADHD who exhibited impaired cognitive control as compared to neurotypical children; as well as Pontifex et al. (Pontifex et al., 2021) who observed aerobic exercise induced alterations in action monitoring (indexed by the ERN component from ERP) in response to highly cognitive-demanding flanker task. Thus, from a strategic perspective, unless there is need to bring online resource-intensive medial-frontal mediated control and behavior monitoring mechanisms to ensure high levels of performance—such as in the case of children with ADHD or in response to highly-demanding tasks; then, potential alterations in such mechanisms may not be able to be observed.
Interestingly, in addition to the null effect of acute aerobic exercise, we also found a non-significant congruency effect following aerobic exercise and a significant congruency effect following seated rest, but in a reversed pattern. That is, midfrontal theta ERS was higher during congruent trials relative to incongruent trials following a seated rest condition. These patterns of midfrontal theta ERS, while unexpected, could be explained by (a) immaturity of the frontal cortex in children and (b) the nature of the cognitive control tasks. Specifically, school-age children may poorly deploy medial-frontal mediated control and behavior monitoring mechanisms in response to task conditions that are novel and engender uncertainty (Adam et al., 2020). Given that the two congruency trials in the flanker task were randomly presented and child participants only had a relatively short response window (i.e., 800–1000 ms), it is plausible that child participants were either unable to differentiate the needs for the control mechanism or failed to flexibly adjust the behavior monitoring process under task conditions with high uncertainty and a short response window, as reflected by our theta ERS findings. While the above explanations are hypothetical, the observed midfrontal theta ERS patterns are somewhat consistent with our published data from 171 school-age children, wherein we also found no difference in midfrontal theta ERS between congruency trials during a flanker task (Hsieh et al., 2020). Our finding can be supported by a recent study (Eisma et al., 2021) comparing midfrontal theta oscillations evoked during different inhibitory control tasks who revealed that task components with increased reactive control and response inhibition (e.g., Go/Nogo task), wherein individuals have to override an erroneous or dominant response, exert greater demand for midfrontal theta activation compared to task components tapping inhibitory control at a perceptual and attention level (e.g., flanker task). The design and nature of cognitive control tasks may play a crucial role in determining the modulation of midfrontal theta oscillations as a function of cognitive control demands.
Despite the strength of the present investigation, this study is not without limitations that should be acknowledged. First, although the within-subjects, crossover, post-test design was adequate for evaluating acute exercise effects on cognition, the lack of a pre-test in this study may pose a potential threat to the interpretability of the current findings because individual day-to-day variations were not captured (Pontifex et al., 2019). However, we argue that this competing explanation is mitigated, in part, by the use of counterbalancing in our research design, as well as the effort to test participants on the same day of the week, the same time of the day, and our instruction to participants to avoid vigorous physical activity before each visit. Second, the seated rest control condition employed in the current study may also be less relevant in practical settings compared to active and cognitively engaged control conditions (Pontifex et al., 2019). However, the use of these ecologically relevant control conditions may introduce variance such as individual differences in the degree of physical and/or cognitive engagement, which are rarely verified in the literature. Further, our use of a passive seated rest control does not stand alone, as there are numerous relevant studies using the same approach (Hsieh et al., 2018; Kao et al., 2020, 2021; Yu et al., 2020). Nevertheless, future research is necessary incorporating pre-test assessments as well as considering alternative control conditions to better understand if and how medial-frontal mediated control and behavior monitoring mechanisms may alter in response to exercise. Finally, it is noteworthy that our results might be underpowered, partly because of a repeated measured design and relatively small sample size, and should be interpreted with caution. However, we consider that the risks of type 1 or 2 error in our results could be mitigated, as the Bayes factors were in favor of the F-tests and t-tests on whether a null effect was rejected or accepted.
In conclusion, the focus of the current study was on the examination of the effects of acute exercise on task-evoked midfrontal theta oscillations to provide additional piece of evidence on the modulatory effects of acute bouts of aerobic exercise on the control and behavior monitoring processes during cognitive control. Specifically, our data suggest that acute bouts of aerobic exercise do not appear to modulate the control and behavior monitoring processes indexed by midfrontal theta ERS during cognitive control in children. At the task level, acute aerobic exercise has a general facilitative effect on task components with varied cognitive control demands. Despite a null effect of acute aerobic exercise on midfrontal theta oscillations, the current data still add to the literature regarding the modulatory effects of acute bouts of exercise on different cognitive processes during cognitive control. Future research seeking the modulatory effects of acute bouts of exercise on specific cognitive and neural processes can use the current findings as an empirical foundation.
Data Availability
The data that support the findings of this study are available from the corresponding author, S. S. Hsieh, upon reasonable request.
References
American College of Sports Medicine. (2010). ACSM’s guidelines for exercise testing and prescription (8th ed.). Loppincott Williams & Wilkins.
Adam, N., Blaye, A., Gulbinaite, R., Delorme, A., & Farrer, C. (2020). The role of midfrontal theta oscillations across the development of cognitive control in preschoolers and school-age children. Developmental Science, 23, 5. https://doi.org/10.1111/desc.12936
Bar-Or, O. (1983). Pediatric sports medicine for the practitioner: From physiologic principles to clinical applications. Springer-Verlag. https://doi.org/10.1007/978-1-4612-5593-2
Birnbaum, A. S., Lytle, L. A., Murray, D. M., Story, M., Perry, C. L., & Boutelle, K. N. (2002). Survey development for assessing correlates of young adolescents’ eating. American Journal of Health Behavior, 26(4), 284–295. https://doi.org/10.5993/AJHB.26.4.5
Cavanagh, J. F., & Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–421. https://doi.org/10.1016/J.TICS.2014.04.012
Cavanagh, J. F., Zambrano-Vazquez, L., & Allen, J. J. B. (2012). Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology, 49(2), 220–238. https://doi.org/10.1111/j.1469-8986.2011.01293.x
Chang, Y. K., Chu, C. H., Wang, C. C., Song, T. F., & Wei, G. X. (2015). Effect of acute exercise and cardiovascular fitness on cognitive function: An event-related cortical desynchronization study. Psychophysiology, 52(3), 342–351. https://doi.org/10.1111/psyp.12364
Cohen, M. X., & Donner, T. H. (2013). Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. Journal of Neurophysiology, 110(12), 2752–2763. https://doi.org/10.1152/JN.00479.2013/ASSET/IMAGES/LARGE/Z9K0241322250006.JPEG
Cohen, J. (2013). Statistical power analysis for the behavioral sciences (2nd ed.). Routledge. https://doi.org/10.4324/9780203771587
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Drollette, E. S., Scudder, M. R., Raine, L. B., Moore, R. D., Saliba, B. J., Pontifex, M. B., & Hillman, C. H. (2014). Acute exercise facilitates brain function and cognition in children who need it most: An ERP study of individual differences in inhibitory control capacity. Developmental Cognitive Neuroscience, 7, 53–64. https://doi.org/10.1016/j.dcn.2013.11.001
Duprez, J., Gulbinaite, R., & Cohen, M. X. (2020). Midfrontal theta phase coordinates behaviorally relevant brain computations during cognitive control. NeuroImage, 207, 116340. https://doi.org/10.1016/J.NEUROIMAGE.2019.116340
Egner, T. (Ed.). (2017). The Wiley handbook of cognitive control. Wiley. https://doi.org/10.1002/9781118920497
Eisma, J., Rawls, E., Long, S., Mach, R., & Lamm, C. (2021). Frontal midline theta differentiates separate cognitive control strategies while still generalizing the need for cognitive control. Scientific Reports, 11, 1. https://doi.org/10.1038/s41598-021-94162-z
Erickson, K. I., Hillman, C., Stillman, C. M., Ballard, R. M., Bloodgood, B., Conroy, D. E., Macko, R., Marquez, D. X., Petruzzello, S. J., & Powell, K. E. (2019). Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. In Medicine and Science in Sports and Exercise, 51(6), 1242–1251. https://doi.org/10.1249/MSS.0000000000001936. Lippincott Williams and Wilkins.
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics, 16(1), 143–149. https://doi.org/10.3758/BF03203267
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
Freedson, P. S., Goodman, T. L. (1993). Measurement of oxygen consumption. In P. S., G. T. L. Freedson (Ed.), Pediatric laboratory exercise testing: Clinical guidelines (pp 91–113). Human Kinetics.
Güntekin, B., Uzunlar, H., Çalışoğlu, P., Eroğlu-Ada, F., Yıldırım, E., Aktürk, T., Atay, E., Ceran, Ö. (2020). Theta and alpha oscillatory responses differentiate between six-to seven-year-old children and adults during successful visual and auditory memory encoding. Brain Research, 1747, 147042. https://doi.org/10.1016/J.BRAINRES.2020.147042
Hillman, C. H., Pontifex, M. B., Raine, L. B., Castelli, D. M., Hall, E. E., & Kramer, A. F. (2009). The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience, 159(3), 1044–1054. https://doi.org/10.1016/j.neuroscience.2009.01.057
Hsieh, S. S., Huang, C. J., Wu, C. T., Chang, Y. K., & Hung, T. M. (2018). Acute exercise facilitates the N450 inhibition marker and P3 attention marker during stroop test in young and older adults. Journal of Clinical Medicine, 7, 11. https://doi.org/10.3390/jcm7110391
Hsieh, S. S., Chueh, T. Y., Morris, T. P., Kao, S. C., Westfall, D. R., Raine, L. B., Hopman, R. J., Pontifex, M. B., Castelli, D. M., Kramer, A. F., & Hillman, C. H. (2020). Greater childhood cardiorespiratory fitness is associated with better top-down cognitive control: A midfrontal theta oscillation study. Psychophysiology, 57(12), e13678. https://doi.org/10.1111/psyp.13678
Kao, S. C., Wang, C. H., & Hillman, C. H. (2020). Acute effects of aerobic exercise on response variability and neuroelectric indices during a serial n-back task. Brain and Cognition, 138, 105508. https://doi.org/10.1016/j.bandc.2019.105508
Kao, S. C., Wang, C. H., Kamijo, K., Khan, N., & Hillman, C. (2021). Acute effects of highly intense interval and moderate continuous exercise on the modulation of neural oscillation during working memory. International Journal of Psychophysiology, 160, 10–17. https://doi.org/10.1016/j.ijpsycho.2020.12.003
Kaufman, A. S. (2004). Kaufman brief intelligence test. 2nd. AGS Publishing. https://doi.org/10.1002/9781118660584.ese1325
Keil, A., Bernat, E. M., Cohen, M. X., Ding, M., Fabiani, M., Gratton, G., Kappenman, E. S., Maris, E., Mathewson, K. E., Ward, R. T., & Weisz, N. (2022). Recommendations and publication guidelines for studies using frequency domain and time-frequency domain analyses of neural time series. Psychophysiology, 59(5), e14052. https://doi.org/10.1111/PSYP.14052
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2–3), 169–195. https://doi.org/10.1016/S0165-0173(98)00056-3
Koechlin, E., Ody, C., & Kouneiher, F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science, 302(5648), 1181–1185. https://doi.org/10.1126/science.1088545
Kuczmarski, R. J., Ogden, C. L., Guo, S. S., Grummer-Strawn, L. M., Flegal, K. M., Mei, Z., Wei, R., Curtin, L. R., Roche, A. F., & Johnson, C. L. (2002). 2000 CDC growth charts for the United States: Methods and development. Vital and Health Statistics. Series 11. Data from the National Health Survey, 246, 1–190.
Lakens, D., & Bakker, M. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Article, 4, 1. https://doi.org/10.3389/fpsyg.2013.00863
Lee, M. D., & Wagenmakers, E.-J. (2014). Bayesian cognitive modeling. Cambridge University Press. https://doi.org/10.1017/CBO9781139087759
Lopez-Calderon, J., Luck, S. J. (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. https://doi.org/10.3389/fnhum.2014.00213
Ludyga, S., Gerber, M., Brand, S., Holsboer-Trachsler, E., & Pühse, U. (2016). Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology, 53(11), 1611–1626. https://doi.org/10.1111/psyp.12736
Ludyga, S., Brand, S., Gerber, M., Weber, P., Brotzmann, M., Habibifar, F., & Pühse, U. (2017). An event-related potential investigation of the acute effects of aerobic and coordinative exercise on inhibitory control in children with ADHD. Developmental Cognitive Neuroscience, 28, 21–28. https://doi.org/10.1016/j.dcn.2017.10.007
Makeig, S., Debener, S., Onton, J., & Delorme, A. (2004). Mining event-related brain dynamics. Trends in Cognitive Sciences, 8(5), 204–210. https://doi.org/10.1016/J.TICS.2004.03.008
McGowan, A. L., Ferguson, D. P., Gerde, H. K., Pfeiffer, K. A., & Pontifex, M. B. (2020). Preschoolers exhibit greater on-task behavior following physically active lessons on the approximate number system. Scandinavian Journal of Medicine and Science in Sports, 30(9), 1777–1786. https://doi.org/10.1111/sms.13727
Pontifex, M. B., Saliba, B. J., Raine, L. B., Picchietti, D. L., & Hillman, C. H. (2013). Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. Journal of Pediatrics, 162(3), 543–551. https://doi.org/10.1016/j.jpeds.2012.08.036
Pontifex, M. B., Gwizdala, K. L., Parks, A. C., Billinger, M., & Brunner, C. (2017a). Variability of ICA decomposition may impact EEG signals when used to remove eyeblink artifacts. Psychophysiology, 54(3), 386–398. https://doi.org/10.1111/psyp.12804
Pontifex, M. B., Miskovic, V., & Laszlo, S. (2017b). Evaluating the efficacy of fully automated approaches for the selection of eyeblink ICA components. Psychophysiology, 54(5), 780–791. https://doi.org/10.1111/PSYP.12827
Pontifex, M. B., McGowan, A. L., Chandler, M. C., Gwizdala, K. L., Parks, A. C., Fenn, K., & Kamijo, K. (2019). A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychology of Sport and Exercise, 40, 1–22. https://doi.org/10.1016/j.psychsport.2018.08.015
Pontifex, M. B., Parks, A. C., Delli Paoli, A. G., Schroder, H. S., & Moser, J. S. (2021). The effect of acute exercise for reducing cognitive alterations associated with individuals high in anxiety. International Journal of Psychophysiology, 167, 47–56. https://doi.org/10.1016/j.ijpsycho.2021.06.008
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443–447. https://doi.org/10.1126/science.1100301
Shvartz, E., & Reibold, R. C. (1990). Aerobic fitness norms for males and females aged 6 to 75 years: A review. Aviation, Space, and Environmental Medicine, 61(1), 3–11.
Taylor, W., Hindmarsh, L., & Odoki, C. (2001). Performance of a new pubertal self-assessment questionnaire: A preliminary study. Paediatric and Perinatal Epidemiology, 15(1), 88–94. https://doi.org/10.1046/j.1365-3016.2001.00317.x
Thomas, S., Reading, J., & Shephard, R. J. (1992). Revision of the physical activity readiness questionnaire (PAR-Q). Canadian Journal of Sport Sciences, 17(4), 338–345.
Utter, A. C., Robertson, R. J., Nieman, D. C., & Kang, J. (2002). Children’s OMNI scale of perceived exertion: Walking/running evaluation. Medicine and Science in Sports and Exercise, 34(1), 139–144. https://doi.org/10.1097/00005768-200201000-00021
van Noordt, S., Heffer, T., & Willoughby, T. (2022). A developmental examination of medial frontal theta dynamics and inhibitory control. NeuroImage, 246, 118765. https://doi.org/10.1016/J.NEUROIMAGE.2021.118765
Yu, C. L., Hsieh, S. S., Chueh, T. Y., Huang, C. J., Hillman, C. H., & Hung, T. M. (2020). The effects of acute aerobic exercise on inhibitory control and resting state heart rate variability in children with ADHD. Scientific Reports, 10(1), 19958. https://doi.org/10.1038/s41598-020-76859-9
Acknowledgements
We thank the participants who participated in the study and their parents and teachers for their collaboration.
Funding
The current study was financially supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) grant R01 HD055352 to C. Hillman, and a Postdoctoral Research Abroad Fellowship by the National Science and Technology Council, under grant 109–2917-I-564–034, to S. Hsieh.
Author information
Authors and Affiliations
Contributions
M.B.P. and C.H.H. designed the experiments; M.B.P. and L.B.R. collected the data; S.S.H. and S.C.K. reduced the data; S.S.H. performed statistical analyses; S.S.H. wrote the manuscript; M.B.P., C.H.H., and K.M.D. critically reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Approval was obtained from the ethics committee of the Institutional Review Board of the University of Illinois at Urbana-Champaign. The procedures used in this study adhere to the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
Written informed consent was obtained from the parents/legal guardians of all children. In addition, all children provided written informed assent.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsieh, SS., Kao, SC., Raine, L.B. et al. Acute Bouts of Aerobic Exercise Do Not Modulate Task-Evoked Midfrontal Theta Oscillations in School-Age Children. J Cogn Enhanc 8, 9–20 (2024). https://doi.org/10.1007/s41465-023-00281-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-023-00281-y