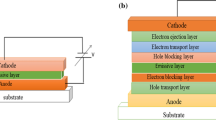
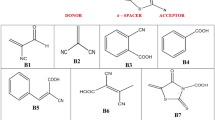
Abstract
Third-generation organic light-emitting diodes (OLEDs) based on metal-free thermally activated delayed fluorescent (TADF) materials have sparked tremendous interest in the last decade due to their nearly 100% exciton utilization efficiency, which can address the low-efficiency issue of the first-generation fluorescent emitters and the high-cost issue of the second-generation organometallic phosphorescent emitters. Construction of efficient and stable TADF-OLEDs requires utilizing TADF materials with a narrow singlet–triplet energy gap (ΔEST), high photoluminescence quantum yield (PLQY) and short TADF lifetime. A small ΔEST is necessary for an efficient reverse intersystem crossing (RISC) process, which can be achieved through the effective spatial separation of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). TADF emitters have been generally designed as intramolecular charge transfer (ICT) molecules with highly twisted donor–acceptor (D–A) molecular architectures. A wide variety of combinations of electron donors and acceptors have been explored. In this review, we shall focus on recent progress in organic TADF molecules incorporating strong electron-donor phenoxazine moiety and their application as emitting layer (EML) in OLEDs.
Graphical Abstract
















Similar content being viewed by others
References
Choi S, Kang C-m, Byun C-W, Cho H, Kwon B-H, Han J-H, Yang J-H, Shin J-W, Hwang C-S, Cho NS, Lee KM, Kim H-O, Kim E, Yoo S, Lee H (2020) Thin-film transistor-driven vertically stacked full-color organic light-emitting diodes for high-resolution active-matrix displays. Nat Commun 11:2732. https://doi.org/10.1038/s41467-020-16551-8
Ilmi R, Zhang D, Dutra JDL, Dege N, Zhou L, Wong W-Y, Raithby PR, Khan MS (2021) A tris β-diketonate europium(III) complex based OLED fabricated by thermal evaporation method displaying efficient bright red emission. Org Electron 96:106216. https://doi.org/10.1016/j.orgel.2021.106216
Ilmi R, Khan MS, Sun W, Zhou L, Wong W-Y, Raithby PR (2019) A single component white electroluminescent device fabricated from a metallo-organic terbium complex. J Mater Chem C 7:13966–13975. https://doi.org/10.1039/C9TC04653D
Pode R (2020) Organic light emitting diode devices: an energy efficient solid state lighting for applications. Renew Sustain Energy Rev 133:110043. https://doi.org/10.1016/j.rser.2020.110043
Ilmi R, Wang J, Dutra JDL, Zhou L, Wong W-Y, Raithby PR, Khan MS (2023) Efficient red organic light emitting diodes of nona coordinate europium Tris(β-diketonato) complexes bearing 4’-Phenyl-2,2’:6’,2’’-terpyridine. Chem Eur J. https://doi.org/10.1002/chem.202300376
Ilmi R, Li X, Al Rasbi NK, Zhou L, Wong WY, Raithby PR, Khan MS (2023) Two new red-emitting ternary europium(III) complexes with high photoluminescence quantum yields and exceptional performance in OLED devices. Dalton Trans 52:12885–12891. https://doi.org/10.1039/d3dt02147e
Murawski C, Gather MC (2021) Emerging biomedical applications of organic light-emitting diodes. Adv Opt Mater 9:2100269. https://doi.org/10.1002/adom.202100269
Graydon O (2023) OLEDs are more than just displays. Nat Photon 17:216–217. https://doi.org/10.1038/s41566-023-01160-w
Yang Z, Mao Z, Xie Z, Zhang Y, Liu S, Zhao J, Xu J, Chi Z, Aldred MP (2017) Recent advances in organic thermally activated delayed fluorescence materials. Chem Soc Rev 46:915–1016. https://doi.org/10.1039/C6CS00368K
Jiang H, Jin J, Wong W-Y (2023) High-performance multi-resonance thermally activated delayed fluorescence emitters for narrowband organic light-emitting diodes. Adv Funct Mater. https://doi.org/10.1002/adfm.202306880
Segal M, Baldo MA, Holmes RJ, Forrest SR, Soos ZG (2003) Excitonic singlet-triplet ratios in molecular and polymeric organic materials. Phys Rev B 68:075211. https://doi.org/10.1103/PhysRevB.68.075211
Baldo MA, O’Brien DF, You Y, Shoustikov A, Sibley S, Thompson ME, Forrest SR (1998) Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395:151–154. https://doi.org/10.1038/25954
Dirac PAM, Fowler RH (1997) The quantum theory of the electron. Proc R Soc Lond Ser A Contain Pap Math Phys Charact 117:610–624. https://doi.org/10.1098/rspa.1928.0023
Volz D, Wallesch M, Fléchon C, Danz M, Verma A, Navarro JM, Zink DM, Bräse S, Baumann T (2015) From iridium and platinum to copper and carbon: new avenues for more sustainability in organic light-emitting diodes. Green Chem 17:1988–2011. https://doi.org/10.1039/C4GC02195A
Xu Y, Xu P, Hu D, Ma Y (2021) Recent progress in hot exciton materials for organic light-emitting diodes. Chem Soc Rev 50:1030–1069. https://doi.org/10.1039/D0CS00391C
Kondakov DY (2015) Triplet–triplet annihilation in highly efficient fluorescent organic light-emitting diodes: current state and future outlook. Philos Trans R Soc A Math Phys Eng Sci 373:20140321. https://doi.org/10.1098/rsta.2014.0321
Parker CA, Hatchard CG (1961) Triplet-singlet emission in fluid solutions. Phosphorescence of eosin. Trans Faraday Soc 57:1894–1904. https://doi.org/10.1039/TF9615701894
Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C (2012) Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492:234–238. https://doi.org/10.1038/nature11687
Endo A, Sato K, Yoshimura K, Kai T, Kawada A, Miyazaki H, Adachi C (2011) Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl Phys Lett. https://doi.org/10.1063/1.3558906
Ravinson DSM, Thompson ME (2020) Thermally assisted delayed fluorescence (TADF): fluorescence delayed is fluorescence denied. Mater Horizons 7:1210–1217. https://doi.org/10.1039/D0MH00276C
Chen X-K, Kim D, Brédas J-L (2018) Thermally activated delayed fluorescence (TADF) path toward efficient electroluminescence in purely organic materials: molecular level insight. Acc Chem Res 51:2215–2224. https://doi.org/10.1021/acs.accounts.8b00174
Kim K-H, Kim J-J (2018) Origin and control of orientation of phosphorescent and TADF dyes for high-efficiency OLEDs. Adv Mater 30:1705600. https://doi.org/10.1002/adma.201705600
Wong MY, Zysman-Colman E (2017) Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv Mater 29:1605444. https://doi.org/10.1002/adma.201605444
Slater JC (2004) Atomic radii in crystals. J Chem Phys 41:3199–3204. https://doi.org/10.1063/1.1725697
Freitas VLS, Gomes JRB, Ribeiro da Silva MDMC (2014) Structural, energetic and reactivity properties of phenoxazine and phenothiazine. J Chem Thermodyn 73:110–120. https://doi.org/10.1016/j.jct.2013.11.013
Ionescu M, Mantsch H (1967). In: Katritzky AR, Boulton AJ (eds) Advances in heterocyclic chemistry. Academic Press, pp 83–113
Nguyen V-N, Kumar A, Lee MH, Yoon J (2020) Recent advances in biomedical applications of organic fluorescence materials with reduced singlet–triplet energy gaps. Coord Chem Rev 425:213545. https://doi.org/10.1016/j.ccr.2020.213545
Shukla S, Dwivedi J, Yaduvanshi N, Jain S (2021) Medicinal and biological significance of phenoxazine derivatives mini-reviews in medicinal. Chemistry 21:1541–1555. https://doi.org/10.2174/1389557520666201214102151
Zhu X-Q, Dai Z, Yu A, Wu S, Cheng J-P (2008) Driving forces for the mutual conversions between phenothiazines and their various reaction intermediates in acetonitrile. J Phys Chem B 112:11694–11707. https://doi.org/10.1021/jp8041268
Im Y, Kim M, Cho YJ, Seo J-A, Yook KS, Lee JY (2017) Molecular design strategy of organic thermally activated delayed fluorescence emitters. Chem Mater 29:1946–1963. https://doi.org/10.1021/acs.chemmater.6b05324
Al-Ghamdi SN, Al-Ghamdi HA, El-Shishtawy RM, Asiri AM (2021) Advances in phenothiazine and phenoxazine-based electron donors for organic dye-sensitized solar cells. Dyes Pigm 194:109638. https://doi.org/10.1016/j.dyepig.2021.109638
Buene AF, Almenningen DM (2021) Phenothiazine and phenoxazine sensitizers for dye-sensitized solar cells – an investigative review of two complete dye classes. J Mater Chem C 9:11974–11994. https://doi.org/10.1039/D1TC03207K
Liu Y, Li C, Ren Z, Yan S, Bryce MR (2018) All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat Rev Mater 3:18020. https://doi.org/10.1038/natrevmats.2018.20
Shen Y-F, Li M, Zhao W-L, Wang Y-F, Lu H-Y, Chen C-F (2021) Quinoline-based TADF emitters exhibiting aggregation-induced emission for efficient non-doped organic light-emitting diodes. Mater Chem Front 5:834–842. https://doi.org/10.1039/D0QM00628A
Hall D, Rajamalli P, Duda E, Suresh SM, Rodella F, Bagnich S, Carpenter-Warren CL, Cordes DB, Slawin AMZ, Strohriegl P, Beljonne D, Köhler A, Olivier Y, Zysman-Colman E (2021) Substitution effects on a new pyridylbenzimidazole acceptor for thermally activated delayed fluorescence and their use in organic light-emitting diodes. Adv Opt Mater 9:2100846. https://doi.org/10.1002/adom.202100846
Krotkus S, Matulaitis T, Diesing S, Copley G, Archer E, Keum C, Cordes DB, Slawin AM, Gather MC, Zysman-Colman E (2021) Fast delayed emission in new pyridazine-based compounds. Front Chem 8:572862
Gudeika D, Bezvikonnyi O, Volyniuk D, Grazulevicius JV (2020) Differently substituted benzonitriles for non-doped OLEDs. Dyes Pigm 172:107789. https://doi.org/10.1016/j.dyepig.2019.107789
Kim H, Lee Y, Lee H, Hong J-I, Lee D (2021) Click-To-twist strategy to build blue-to-green emitters: bulky triazoles for electronically tunable and thermally activated delayed fluorescence. ACS Appl Mater Interfaces 13:12286–12295. https://doi.org/10.1021/acsami.1c00278
Ji S-C, Zhao T, Wei Z, Meng L, Tao X-D, Yang M, Chen X-L, Lu C-Z (2022) Manipulating excited states via Lock/Unlock strategy for realizing efficient thermally activated delayed fluorescence emitters. Chem Eng J 435:134868. https://doi.org/10.1016/j.cej.2022.134868
Chen J-X, Xiao Y-F, Wang K, Fan X-C, Cao C, Chen W-C, Zhang X, Shi Y-Z, Yu J, Geng F-X, Zhang X-H, Lee C-S (2020) Origin of thermally activated delayed fluorescence in a donor–acceptor type emitter with an optimized nearly planar geometry. J Mater Chem C 8:13263–13269. https://doi.org/10.1039/D0TC03747H
Wang P, Yu J, Chen S, Yu H, Yan X, Guan Y, Chen J, Li L (2020) 3-Benzoyl-4H-chromen-4-one: a novel twisted acceptor for highly efficient thermally activated delayed fluorescence emitters. Dyes Pigm 183:108744. https://doi.org/10.1016/j.dyepig.2020.108744
Chen H, Liu H, Xiong Y, He J, Zhao Z, Tang BZ (2022) New aggregation-induced delayed fluorescent materials for efficient OLEDs with high stabilities of emission color and efficiency. Mater Chem Front 6:924–932. https://doi.org/10.1039/D1QM01625C
Liu H, Liu H, Fan J, Guo J, Zeng J, Qiu F, Zhao Z, Tang BZ (2020) An effective design strategy for robust aggregation-induced delayed fluorescence luminogens to improve efficiency stability of nondoped and doped OLEDs. Adv Opt Mater 8:2001027. https://doi.org/10.1002/adom.202001027
Zhang D, Wei H, Wang Y, Dai G, Zhao X (2020) Synthesis and properties of ipsilateral double substituted diphenylsulfone thermally activated delayed fluorescent materials. Dyes Pigm 174:108028. https://doi.org/10.1016/j.dyepig.2019.108028
Guo R, Leng P, Zhang Q, Wang Y, Lv X, Sun S, Ye S, Duan Y, Wang L (2021) Donor engineering for diphenylsulfone derivatives with both thermally activated delayed fluorescence and aggregation-induced emission properties. Dyes Pigm 184:108781. https://doi.org/10.1016/j.dyepig.2020.108781
Gao S, Chen X, Ge X, Chen Z, Zhao J, Chi Z (2022) Asymmetric thermally activated delayed fluorescence materials rendering high-performance OLEDs through both thermal evaporation and solution-processing. Chem Res Chin U 38:1526–1531. https://doi.org/10.1007/s40242-022-2111-0
Leng P, Sun S, Guo R, Zhang Q, Liu W, Lv X, Ye S, Wang L (2020) Modifying the AIE-TADF chromophore with host-substituents to achieve high efficiency and low roll-off non-doped OLEDs. Org Electron 78:105602. https://doi.org/10.1016/j.orgel.2019.105602
Li H-z, Zhang D, Xie F-m, Zeng X-Y, Li Y-Q, Wei H-x, Dai G-l, Tang J-X, Zhao X (2021) Thermally activated delayed fluorescence materials based on 3, 3′-position substituted bis(phenylsulfonyl)benzene. Dyes Pigm 188:109210. https://doi.org/10.1016/j.dyepig.2021.109210
Xiao R, Xiang Y, Cao X, Li N, Huang T, Zhou C, Zou Y, Xie G, Yang C (2020) Star-shaped thermally activated delayed fluorescence emitters with a tri-armed arylsulfonic acceptor for efficient solution processed organic light emitting diodes. J Mater Chem C 8:5580–5586. https://doi.org/10.1039/C9TC07049D
Zhong D, Yu Y, Yue L, Yang X, Ma L, Zhou G, Wu Z (2021) Optimizing molecular rigidity and thermally activated delayed fluorescence (TADF) behavior of phosphoryl center π-conjugated heterocycles-based emitters by tuning chemical features of the tether groups. Chem Eng J 413:127445. https://doi.org/10.1016/j.cej.2020.127445
Chen Y-W, Tsai C-C, Chih H-Y, Tsai H-Y, Wang W-Y, Liu G-Y, Wu M-Y, Chang C-H, Lu C-W (2022) Realizing performance improvement of borylated TADF materials for OLEDs. Dyes Pigm 197:109892. https://doi.org/10.1016/j.dyepig.2021.109892
Yang D, Kim J-M, Huh J-S, Kim J-J, Hong J-I (2021) The effect of the electron-donor ability on the OLED efficiency of twisted donor-acceptor type emitters. Org Electron 95:106187. https://doi.org/10.1016/j.orgel.2021.106187
Huang T, Chen Z, Zou Y, Gong S, Yang C (2021) Novel tetracoordinated organoboron emitters for thermally activated delayed fluorescence organic light-emitting diodes. Dyes Pigm 188:109192. https://doi.org/10.1016/j.dyepig.2021.109192
Chen J-X, Wang H, Zhang X, Xiao Y-F, Wang K, Zhou L, Shi Y-Z, Yu J, Lee C-S, Zhang X-H (2022) Using fullerene fragments as acceptors to construct thermally activated delayed fluorescence emitters for high-efficiency organic light-emitting diodes. Chem Eng J 435:134731. https://doi.org/10.1016/j.cej.2022.134731
Tian X, Yao M, Liang X, Zhou C, Xiao S, Gao Y, Liu H, Zhang S-T, Yang B (2022) Excited-state regulated electroluminescence performance from thermally-activated delayed fluorescence (TADF) to hybridized local and charge-transfer (HLCT) emission. Dyes Pigm 205:110463. https://doi.org/10.1016/j.dyepig.2022.110463
Kothavale S, Lee KH, Lee JY (2020) CN-modified imidazopyridine as a new electron accepting unit of thermally activated delayed fluorescent emitters. Chem Eur J 26:845–852. https://doi.org/10.1002/chem.201903877
Chen X-L, Tao X-D, Wei Z, Meng L, Lin F-L, Zhang D-H, Jing Y-Y, Lu C-Z (2021) Thermally activated delayed fluorescence amorphous molecular materials for high-performance organic light-emitting diodes. ACS Appl Mater Interfaces 13:46909–46918. https://doi.org/10.1021/acsami.1c12188
Huang Y, Zhang D-H, Tao X-D, Wei Z, Jiang S, Meng L, Yang M-X, Chen X-L, Lu C-Z (2022) Triptycene-derived thermally activated delayed fluorescence emitters with combined through-bond and through-space charge transfers. Dyes Pigm 204:110397. https://doi.org/10.1016/j.dyepig.2022.110397
Luo X-F, Li F-L, Zou J-W, Zou Q, Su J, Mao M-X, Zheng Y-X (2021) A series of fused carbazole/carbonyl based blue to yellow-green thermally activated delayed fluorescence materials for efficient organic light-emitting diodes. Adv Opt Mater 9:2100784. https://doi.org/10.1002/adom.202100784
Liu H, Song S, Chen H, Zhao Z, Xie G, Tang BZ (2021) Novel aggregation-induced delayed fluorescence luminogens for vacuum-deposited and solution-processed OLEDs with very small efficiency roll-offs. Org Electron 99:106339. https://doi.org/10.1016/j.orgel.2021.106339
Nasiri S, Macionis S, Gudeika D, Volyniuk D, Grazulevicius JV (2020) Facile structure-modification of xanthenone based OLED emitters exhibiting both aggregation induced emission enhancement and thermally activated delayed fluorescence. J Lumin 220:116955. https://doi.org/10.1016/j.jlumin.2019.116955
Zhang SL, Shi YZ, Wang K, Fan XC, Yu J, Ou XM, Zhang XH (2021) Pyridine-substituted triazine as an acceptor for thermally activated delayed fluorescence emitters showing high efficiency and low roll-off in organic light-emitting diodes. Mater Today Energy 20:100581. https://doi.org/10.1016/j.mtener.2020.100581
Shi Y-Z, Wang K, Fan X-C, Chen J-X, Ou X-M, Yu J, Jie J-S, Lee C-S, Zhang X-H (2021) High-performance nondoped organic light-emitting diode based on a thermally activated delayed fluorescence emitter with 1D intermolecular hydrogen bonding interactions. Adv Opt Mater 9:2100461. https://doi.org/10.1002/adom.202100461
Wang Z, Zhu X, Zhang S, Xu L, Zhao Z, He G (2021) Twisted biphenyl-diimide derivatives with aggregation-induced emission and thermally activated delayed fluorescence for high performance OLEDs. Adv Opt Mater 9:2001764. https://doi.org/10.1002/adom.202001764
Zhang L, Wang Y-F, Li M, Gao Q-Y, Chen C-F (2021) Quinoline-based aggregation-induced delayed fluorescence materials for highly efficient non-doped organic light-emitting diodes. Chin Chem Lett 32:740–744. https://doi.org/10.1016/j.cclet.2020.07.041
Wu X, Gong C, Jiang X, Gao J, Li M, He R, Chen P, Shen W (2022) Face-to-face order-packed mode promotes thermally activated delayed fluorescence to achieve stronger aggregation-induced emission. J Sci Adv Mater Devices 7:100432. https://doi.org/10.1016/j.jsamd.2022.100432
Xu J, Wu X, Li J, Zhao Z, Tang BZ (2022) Regulating photophysical property of aggregation-induced delayed fluorescence luminogens via heavy atom effect to achieve efficient organic light-emitting diodes. Adv Opt Mater 10:2102568. https://doi.org/10.1002/adom.202102568
Mei Y, Liu D, Li J, Li H, Wei W (2021) Acridin-9(10H)-one based thermally activated delayed fluorescence material: simultaneous optimization of RISC and radiation processes to boost luminescence efficiency. J Mater Chem C 9:5885–5892. https://doi.org/10.1039/D1TC00592H
Wang J, Li N, Chen Q, Xiang Y, Zeng X, Gong S, Zou Y, Liu Y (2022) Triarylboron-cored multi-donors TADF emitter with high horizontal dipole orientation ratio achieving high performance OLEDs with near 39% external quantum efficiency and small efficiency Roll-off. Chem Eng J 450:137805. https://doi.org/10.1016/j.cej.2022.137805
Izumi S, Govindharaj P, Drewniak A, Crocomo PZ, Minakata S, de Sousa LE, de Silva P, Data P, Takeda Y (2022) Comparative study of thermally activated delayed fluorescent properties of donor–acceptor and donor–acceptor–donor architectures based on phenoxazine and dibenzo [a, j] phenazine. Beilstein J Organic Chem 18:459–468
Data P, Pander P, Okazaki M, Takeda Y, Minakata S, Monkman AP (2016) Dibenzo[a, j]phenazine-cored donor–acceptor–donor compounds as green-to-red/NIR thermally activated delayed fluorescence organic light emitters. Angew Chem Int Ed 55:5739–5744. https://doi.org/10.1002/anie.201600113
Cai Z, Chen H, Guo J, Zhao Z, Tang BZ (2020) Efficient aggregation-induced delayed fluorescence luminogens for solution-processed OLEDs with small efficiency roll-off. Front Chem 8:10. https://doi.org/10.3389/fchem.2020.00193
Yang Y, Xiao R, Cao X, Chen Z, Lv X, Zhang Y, Gong S, Zou Y, Yang C (2021) Phenoxazine-dibenzothiophene sulfoximine emitters featuring both thermally activated delayed fluorescence and aggregation induced emission. Molecules 26:5243
Ilmi R, Yin J, Dutra JDL, Al Rasbi NK, Oliveira WF, Zhou L, Wong WY, Raithby PR, Khan MS (2022) Single component white-OLEDs derived from tris(beta-diketonato) europium(III) complexes bearing the large bite angle N^N 2-(4-thiazolyl)benzimidazole ligand. Dalton Trans 51:14228–14242. https://doi.org/10.1039/D2DT01873J
Ilmi R, Sun W, Dutra JDL, Al-Rasbi NK, Zhou L, Qian P-C, Wong W-Y, Raithby PR, Khan MS (2020) Monochromatic red electroluminescence from a homodinuclear europium(iii) complex of a β-diketone tethered by 2,2′-bipyrimidine. J Mater Chem C 8:9816–9827. https://doi.org/10.1039/D0TC02181D
Shi Y-Z, Wang K, Fan X-C, Wu H, Ou X-M, Yu J, Jie J-S, Zhang X-H (2022) Applying intermolecular hydrogen bonding to exploit TADF emitters for high-performance orange-red non-doped OLEDs. J Mater Chem C 10:4717–4722. https://doi.org/10.1039/D1TC03803F
Zhou C, Chen W-C, Liu H, Cao X, Li N, Zhang Y, Lee C-S, Yang C (2020) Isomerization enhanced quantum yield of dibenzo[a, c]phenazine-based thermally activated delayed fluorescence emitters for highly efficient orange OLEDs. J Mater Chem C 8:9639–9645. https://doi.org/10.1039/D0TC01995J
Hong G, Si C, Gupta AK, Bizzarri C, Nieger M, Samuel IDW, Zysman-Colman E, Bräse S (2022) Fluorinated dibenzo[a, c]-phenazine-based green to red thermally activated delayed fluorescent OLED emitters. J Mater Chem C 10:4757–4766. https://doi.org/10.1039/D1TC04918F
Yang H-Y, Zhang M, Zhao J-W, Pu C-P, Lin H, Tao S-L, Zheng C-J, Zhang X-H (2022) Improving efficiency of red thermally activated delayed fluorescence emitter by introducing quasi-degenerate orbital distribution. Chin J Chem 40:911–917. https://doi.org/10.1002/cjoc.202100776
Balijapalli U, Lee Y-T, Karunathilaka BSB, Tumen-Ulzii G, Auffray M, Tsuchiya Y, Nakanotani H, Adachi C (2021) Tetrabenzo[a, c]phenazine backbone for highly efficient orange-red thermally activated delayed fluorescence with completely horizontal molecular orientation. Angew Chem Int Ed 60:19364–19373. https://doi.org/10.1002/anie.202106570
Chen J-X, Wang H, Zhou L, Wang K, Yu J, Zhang X-H (2022) Structure-property investigation of two red TADF isomers with different D-A conjugation for superior exciton utilization. Dyes Pigm 208:110801. https://doi.org/10.1016/j.dyepig.2022.110801
Yang W, Ning W, Gong S, Yang C (2021) Deep-red thermally activated delayed fluorescence emitters based on a phenanthroline-containing planar acceptor. Dyes Pigm 192:109474. https://doi.org/10.1016/j.dyepig.2021.109474
Wang B, Zheng Y, Wang T, Ma D, Wang Q (2021) 1,8-Naphthalimide-based hybrids for efficient red thermally activated delayed fluorescence organic light-emitting diodes. Org Electron 88:106012. https://doi.org/10.1016/j.orgel.2020.106012
Urban M, Marek-Urban PH, Durka K, Luliński S, Pander P, Monkman AP (2023) TADF invariant of host polarity and ultralong fluorescence lifetimes in a donor-acceptor emitter featuring a hybrid sulfone-triarylboron acceptor**. Angew Chemie IntEd 62:e202217530. https://doi.org/10.1002/anie.202217530
Fu Y, Liu J, Wu Z, Weigand JJ, Feng X (2020) Synthesis and characterization of AIE-active B-N-coordinated phenalene complexes. Organic Mater 02:240–247. https://doi.org/10.1055/s-0040-1715564
Tsiko U, Bezvikonnyi O, Volyniuk D, Minaev BF, Keruckas J, Cekaviciute M, Jatautiene E, Andruleviciene V, Dabuliene A, Grazulevicius JV (2022) TADF quenching properties of phenothiazine or phenoxazine-substituted benzanthrones emitting in deep-red/near-infrared region towards oxygen sensing. Dyes Pigm 197:109952. https://doi.org/10.1016/j.dyepig.2021.109952
Lin C, Wu Z, Liu J, Deng W-T, Zhuang Y, Xuan T, Xue J, Zhang L, Wei G, Xie R-J (2022) Extremely low efficiency roll-off in vacuum- and solution-processed deep-red/near-infrared OLEDs based on 1,8-naphthalimide TADF emitters. J Lumin 243:118683. https://doi.org/10.1016/j.jlumin.2021.118683
Kim K-H, Moon C-K, Lee J-H, Kim S-Y, Kim J-J (2014) Highly Efficient organic light-emitting diodes with phosphorescent emitters having high quantum yield and horizontal orientation of transition dipole moments. Adv Mater 26:3844–3847. https://doi.org/10.1002/adma.201305733
Luo X-F, Qu Z-Z, Han H-B, Su J, Yan Z-P, Zhang X-M, Tong J-J, Zheng Y-X, Zuo J-L (2021) Carbazole-based Iridium(III) complexes for electrophosphorescence with EQE of 32.2% and low efficiency roll-off. Adv Opt Mater 9:2001390. https://doi.org/10.1002/adom.202001390
Yang X, Guo H, Liu B, Zhao J, Zhou G, Wu Z, Wong W-Y (2018) Diarylboron-based asymmetric red-emitting Ir(III) complex for solution-processed phosphorescent organic light-emitting diode with external quantum efficiency above 28%. Adv Sci 5:1701067. https://doi.org/10.1002/advs.201701067
Acknowledgements
M.S.K. acknowledges His Majesty’s Trust Fund for Strategic Research (Grant no. SR/SQU/SCI/CHEM/21/01) and the Ministry of Higher Education, Research and Innovation (MoHERI), Oman (Grant no. RC/RG-SCI/CHEM/22/01) for funding. R.I. thanks HM’s Trust Fund for a postdoctoral fellowship. H.A.S. acknowledges the Ministry of Higher Education, Research & Innovation (MOHERI) for funding (Grant no. RC/GRG-SCI/CHEM/22/01) and the Ministry of Education, Oman, and SQU for a PhD scholarship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Sharji, H., Ilmi, R. & Khan, M.S. Recent Progress in Phenoxazine-Based Thermally Activated Delayed Fluorescent Compounds and Their Full-Color Organic Light-Emitting Diodes. Top Curr Chem (Z) 382, 5 (2024). https://doi.org/10.1007/s41061-024-00450-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-024-00450-3