Abstract
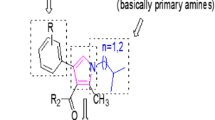
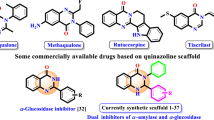
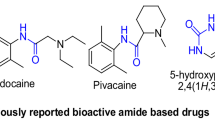
In a search for α-amylase and α-glucosidase inhibitors to treat type 2 diabetes in this study, a new series of thiazolidine-2,4-dione derivatives with azole heterocyclic compounds were designed and synthesized via Knoevenagel condensation for the first time. These synthesized compounds are characterized by IR, 1H NMR, 13C NMR, DEPT-135, and HRMS. All the synthesized compounds were evaluated for α-amylase and α-glucosidase inhibitory activity using acarbose as standard. The lipophilicity (C log P) and molar refractivity (MR) for the compounds were also studied. Structure–activity relationship study (SAR) revealed that compounds 14a–c exhibited maximum inhibition due to the presence of fluorine in a hydrophobic site of aromatic phenyl amine at the seventh position of azaindole. To further verify the effect of C log P of substituents and MR on biological activity, calculation of C log P and MR (molecular refractivity) parameter of all compounds have done using ChemDraw software. Compounds 14a–c found to show higher inhibition due to higher value of C log P (14a = 6.49, 14b = 5.63, 14c = 6.45) and MR (14a = 125, 14b = 117, 14c = 123). These properties make compounds 14a–c to have more inhibitory activity. Among the tested compounds, 14a–c showed inhibition in the range of standard acarbose at the concentration of 250 µg/ml against α-amylase and α-glucosidase.








Similar content being viewed by others
References
Alegaon SG, Alagawadi KR, Sonkusare PV, Chaudhary SM, Dadwe DH, Shah AS (2012) Novel imidazo[2,1-b][1,3,4]thiadiazole carrying rhodanine-3-acetic acid as potential antitubercular agents. Bioorg Med Chem Lett 22:1917–1921
American Diabetes Association (2009) Diabetes Care 32(Suppl 1):S62–S67
Ansari KF, Lal C (2009) Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur J Med Chem 44:4028–4033
Asati V, Mahapatra DK, Bharti SK (2014) Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry. Potential anticancer agents. Eur J Med Chem 87:814–833
AshokKumar J, Tiwari AK, Saidachary G, Kishor C, Anand Kumar D, Ali Z, Sridhar B, Addlagatta A, China Raju B (2014) Pancreatic a-amylase inhibition and free radical scavenging activity of substituted pyranochromenone derivatives. Med Chem Res 23:2821–2833
Azizmohammadi M, Khoobi M, Ramazani A, Emami S, Zarrin A, Firuzi O, Miri R, Shafiee A (2013) 2H-chromene derivatives bearing thiazolidine-2,4-dione, rhodanine or hydantoin moieties as potential anticancer agents. Eur J Med Chem 5:15–22
El-Sharief MAMS, Moussa Z, El-Sharief AMS (2011) Synthesis, characterization, and derivatization of some novel types of fluorinated mono and bis-imidazolidineiminothiones with antitumor, antiviral, antibacterial and antifungal activities. J Fluorine Chem 132:596–611
Guan A, Liu C, Huang G, Li H, Hao S, Xu Y, Xie Y, Li Z (2014) Synthesis and fungicidal activity of fluorine-containing chlorothalonil derivatives. J Fluor Chem 160:82–87
Guo S, Wang Y, Sun C, Li J, Zou D, Wu Y, Wu Y (2013) Efficient synthesis of 2-arylamino substituted pyridinyl nitriles by Buchwald-Hartwig amination. Tetrahedron Lett. 54:3233–3237
Jawale DV, Pratap UR, Rahuja N, Srivastava AK, Mane RA (2012a) Synthesis and anti hyperglycemic evaluation of new 2,4-thiazolidinediones having biodynamic aryl sulfonylurea moieties. Bioorg Med Chem Lett 22:436–439
Jawale DV, Pratap UR, Mane RA (2012b) An alternative synthetic route for anantidiabetic drug, rosiglitazone. Bioorg Med Chem Lett 22:924–928
Khazi MIA, Belavagi NS, Kim KR, Gong YD, Khazi IAM (2013) Synthesis, hypoglycaemic, hypolipidemic and PPARγ agonist activities of 5-(2-alkyl/aryl-6-Arylimidazo[2,1-b][1,3,4]thiadiazol-5-yl)methylene-1,3-thiazolidinediones. Chem Biol Drug Des 82:147–155
Kumar BRP, Soni M, Santhosh Kumar S, Singh K, Nasir Baig RB, Adhikary L (2011) Synthesis, glucose uptake activity and structureeactivity relationships of some novel glitazones incorporated with glycine, aromatic and alicyclic amine moieties via two carbon acyl linker. Eur J Med Chem 46:835–844
Lee HW, Yang JY, Lee HS (2014) Quinoline-2-carboxylic acid isolated from ephedrapachyclada and its structural derivatives show inhibitory effects against α-Glucosidase and α-amylase. J Korean Soc Appl Biol Chem 57:441–444
Liu XF, Zheng CJ, Sun LP, Liu XK, Piao HR (2011) Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur J Med Chem 46:3469–3473
Lukmantara AY, Kalinowski DS, Kumar N, Richardson DR (2013) Structure–activity studies of 4-phenylsubstituted 2’-benzoylpyridine thiosemicarbazones with potent and selective anti-tumour activity. Org Biomol Chem 11(37):6414–6425
Maccari R, Ciurleo R, Paoli P, Manao G, Vigorita MG, Ottana R, Steindl T, Jacomelli M, Camici G (2007) 5-Arylidene-2,4-thiazolidinediones as inhibitors of protein tyrosine phosphatases. Bioorg Med Chem 15:5137–5149
Maccari R, Ottanà R, Ciurleo R, Rakowitz D, Matuszczak B, Laggner C, Langer T (2008) Synthesis, induced-fit docking investigations, and in vitro aldose reductase inhibitory activity of non-carboxylic acid containing 2,4-thiazolidinedione derivatives. Bioorg Med Chem 16:5840–5850
Magdole P, Meciarova M, Toma S (2001) Ultrasound effect on the synthesis of 4-alkyl-(aryl) amino benzaldehyde. Tetrahedron 57:4781–4785
Matysiak J (2007) Evaluation of electronic, lipophilic and membrane affinity effects on antiproliferative activity of 5-substituted-2-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles against various human cancer cells. Eur J Med Chem 42(7):940–947
Nazreen S, Alam MS, Hamid H, Yar MS, Dhulap A, Alam P, Pasha MAQ, Bano S, Alam MM, Haider S, Kharbanda C, Ali Y, Pillai KK (2014) Thiazolidine-2,4-diones derivatives as PPAR-γ agonists: synthesis, molecular docking, in vitro and in vivo antidiabetic activity with hepatotoxicity risk evaluation and effect on PPAR-γ gene expression. Bioorg Med Chem Lett 24:3034–3042
Neogi P, Cheng J, Lakner FJ, Dey D, Medicherla S, Gowri M, Nag B, Sharma SD, Pickford LB, Gross C (2003) Synthesis and structure—activity relationship studies of cinnamic acid-based novel thiazolidinedione antihyperglycemic agents. Bioorg Med Chem 11:4059–4067
Nowak M, Malinowski Z, Jozwiak A, Fornal E, Blaszczyk A, Kontek R (2014) Substituted benzoquinazolinones. Part 1: Synthesis of 6-aminobenzo[h] quinazolinones via BuchwaldeHartwig amination from 6-bromobenzo[h] quinazolinones. Tetrahedron 70:5153–5160
Panahi F, Yousefi R, Mehraban MH, Nezhad AK (2013) Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic α-glucosidase inhibitors. Carbohydr Res 380:81–91
Ponnuchamy S, Kanchithalaivan S, Ranjith Kumar R, Ali MA, Choon TS (2014) Antimycobacterial evaluation of novel hybrid arylidene thiazolidine-2,4-diones. Bioorg Med Chem Lett 24:1089–1093
Purohit SS, Alman A, Shewale J (2012) Synthesis and antimicrobial activity of a new series of 3,5-disubstitued thiazolidine-2,4-diones. Int J Pharm Pharm Sci 4:273–276
Rakowitz D, Maccari R, Ottana R, Vigorita MG (2006) In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones. Bioorg Med Chem 14:567–574
Seifert A, Ladewig K, Schoherr P, Hofmann K, Lungwitz R, Roth I, Pohlers A, Hoyer W, Baumann G, Schulze S, Hietschold M, Moszner N, Burtscher P, Spange S (2010) Synthesis of dye functionalized xerogels via nucleophilic aromatic substitution of fluoro aromatic compounds with aminosilanes. J Sol-Gel Sci Technol 53:328–341
Sunduru N, Srivastava K, Rajakumar S, Puri SK, Saxena JK, Chauhan PMS (2009) Synthesis of novel thiourea, thiazolidinedione and thioparabanic acid derivatives of 4-aminoquinoline as potent antimalarials. Bioorg Med Chem Lett 19:2570–2573
Swathi N, Ananda Kumar TD, Subrahmanyam CVS, Satyanarayana K (2013) Synthesis and in silico drug likeness evaluation of N-5-disubstituted-1,3-thiazolidine-2,4-dione analogues. J Pharm Res 6:107–111
Wilcox G (2005) Insulin and insulin resistance. Clin Biochem Rev 26:19–39
Wu Y, Tai HH, Cho H (2010) Synthesis and SAR of thiazolidinedione derivatives as 15-PGDH inhibitors. Bioorg Med Chem 18:1428–1433
Yonemoto R, Shimada M, Gunawan-Puteri MDPT, Kato E, Kawabata J (2014) α-Amylase inhibitory triterpene from abrus precatorius leaves. J Agric Food Chem 62:8411–8414
Youssef AM, White MS, Villanueva EB, El-Ashmawy IM, Klegeris A (2010) Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorg Med Chem 18:2019–2028
Acknowledgements
The authors thank the management of VIT University, Vellore, for all the support and encouragement. In addition, the support from SIF-Chemistry, School of Advanced Sciences, VIT University, Vellore, and DST-FIST is greatly acknowledged for the spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthilkumar, N., Vijayakumar, V., Sarveswari, S. et al. Synthesis of New Thiazolidine-2,-4-dione-azole Derivatives and Evaluation of Their α-Amylase and α-Glucosidase Inhibitory Activity. Iran J Sci Technol Trans Sci 43, 735–745 (2019). https://doi.org/10.1007/s40995-018-0593-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-018-0593-x